Abstract
Cervical cord compression from cervical root neurofibromas represents an important clinical problem in patients with neurofibromatosis type 1 (NF1), but is rarely reported. The aim of this study was to describe the clinical presentation and follow‐up of children and adults with NF1 and cervical cord compression. A retrospective review of clinical records and neuroimaging studies from two large tertiary care centres between 1996 and 2006 was performed. 13 patients with NF1 and cervical cord compression were identified. Age at presentation ranged from 9 to 61 years. The most common presentation was progressive quadriparesis. 11 of 13 patients underwent cervical decompression and subtotal resection of the associated neurofibroma. The majority of patients had recovery of neurological function and no further clinical progression. Progressive neurological deficit (typically quadriparesis), rather than neuroimaging appearances, should dictate the need for surgery.
Neurofibromatosis type 1 (NF1) is a common autosomal dominant disorder in which affected individuals develop both benign and malignant tumours. Neurofibromas are benign tumours arising from the endoneurium and are characteristic of NF1. They develop as discrete focal cutaneous or subcutaneous tumours or more diffuse plexiform neurofibromas that grow along the length of nerves frequently involving multiple nerve fascicles, branches and plexuses. Spinal nerve root neurofibromas can arise throughout the spine and at multiple levels, and likely represent plexiform tumours based on the involvement of multiple nerve fascicles. One neuroimaging study detected cervical nerve root tumours in 52% of 54 patients, but none developed symptoms of cord compression.1 There are infrequent single case reports and limited information in the literature about clinical presentation, management and prognosis of patients with NF1 with cervical cord compression from plexiform neurofibromas.2,3,4,5,6,7,8,9 The largest series in the literature, focused entirely on children, identified 10 patients with clinical features of cord compression due to intraspinal extension of a plexiform neurofibroma.10 Six of these individuals had neurofibromas involving the cervical nerve roots.
In this report, we present 13 adults and children with NF1 and symptomatic spinal cord compression arising from cervical root neurofibromas. We discuss the age at presentation, duration of symptoms, clinical and radiological presentation, and functional outcome at follow‐up.
Materials and methods
Thirteen individuals with NF1 and cervical spinal cord compression due to plexiform neurofibromas were identified from a retrospective review of clinical records and neuroimaging. Approximately 1500 clinical records were assessed from patients with NF1 cared for in two large tertiary care centres between 1996 and 2006 (Washington University, St Louis, Missouri, USA and Guy's and St Thomas' Hospitals, London, UK). The diagnosis of NF1 was made using established diagnostic criteria.11 This study was approved by the Human Studies Committee at Washington University and as a clinical audit project at Guy's and St Thomas' Hospitals, London.
Results
There were nine females and four males, with an age range of 9–61 years (median 25) (table 1). The presenting symptoms were progressive quadriparesis (seven patients), paraparesis involving the lower extremities (two patients) or upper extremities (one patient), incontinence (one patient) and neck pain (three patients). One patient presented with a sensory level at C2 (case No 11) and one individual experienced tingling without neurological deficit (case No 6). None of our patients presented with radiographic evidence of a kyphotic deformity, including those who presented with neck pain.
Table 1 Clinical features and course of patients with neurofibromatosis type 1and cervical cord compression*.
| Case No | Age (y) | Sex | Symptoms | Location | Follow‐up and clinical outcome |
|---|---|---|---|---|---|
| 1 | 17 | F | Quadriparesis | C2, C3, C4 | 5 months—weakness resolved |
| 2 | 10 | F | Quadriparesis | C2, C3 | 10 months—weakness resolved |
| 3 | 24 | F | LE weakness | C1, C2 | 120 months–quadriparesis and incontinence, required second operation; 70 months—weakness improved |
| 4 | 14 | F | Quadriparesis | C2, C3 | 21 months–weakness resolved |
| 5 | 27 | M | Quadriparesis | C1, C2 | 43 months–weakness resolved |
| 6 | 25 | F | Neck pain, tingling in hands | C2, C3, C4, C5 | 111 months–no progression (no surgery) |
| 7 | 50 | M | Quadriparesis, urinary hesitancy | C2 | 13 months–died from MPNST thigh and metastatic disease |
| 8 | 9 | F | Neck pain, UE weakness | C2, C3, C4,C5 | 159 months—no further progression |
| 9 | 24 | M | Paraparesis | C3, C4,C5 | 36 months—quadriparesis and incontinence, required second operation; 70 months—no further progression |
| 10 | 48 | F | Neck pain | C2, C3, C4, C5, C6 | 30 months—no progression (no surgery) |
| 11 | 46 | M | Sensory level C2 | C2 | 13 months—died from MPNST buttock and metastatic disease |
| 12 | 61 | F | Quadriparesis | C2,C4,C5 | 13 months—persistent dorsal column sensory loss |
| 13 | 28 | F | Quadriparesis | C2 C3 | 170 months—weakness resolved; 132 months—MPNST thigh and buttock |
LE, lower extremity; MPNST, malignant peripheral nerve sheath tumour; UE, upper extremity.
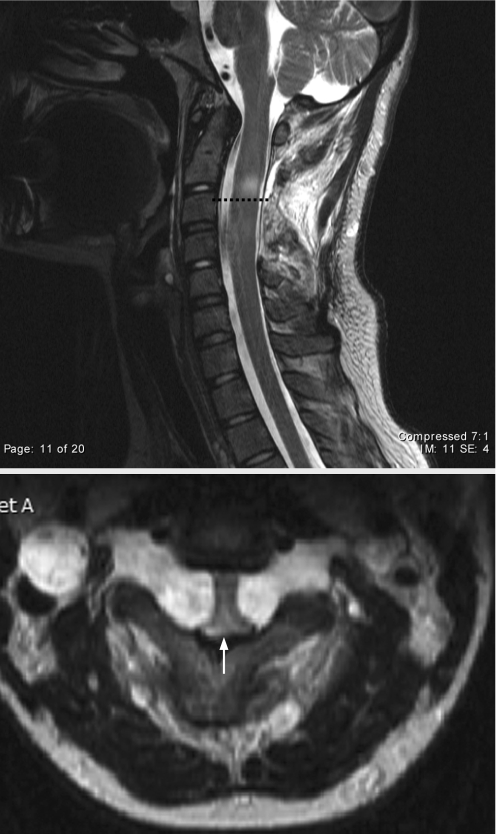
*All patients, except case Nos 6 and 10, underwent cervical laminectomies and subtotal resection of the plexiform neurofibroma. Neuroimaging identified spinal nerve root neurofibromas at multiple levels in 11 of 13 patients. A representative MRI scan of cervical cord compression is shown in fig 1. The most common levels affected were C2 and C3. Histology confirmed that the tumours were benign neurofibromas in all 11 patients who underwent surgery.
Eleven of the 13 patients underwent cervical laminectomy at one or multiple levels, with subtotal resection of the neurofibroma for progressive weakness, and were followed for an additional 5–170 months (median 28). The decision to operate was based primarily on the presence of progressive neurological deficits. In cases where multiple levels demonstrated neurofibromas on MRI, every level showing cord compression was decompressed at the time of surgery. Two patients required additional surgery for symptom progression due to tumour regrowth. Histological examination of the resected specimens in the cervical spine showed that all were neurofibromas and not malignant peripheral nerve sheath tumours (MPNSTs). One of these patients developed quadriparesis and incontinence at 120 months (case No 3; age 24 years), but has experienced no further clinical progression after 70 months. The second patient developed quadriparesis and incontinence at 36 months (case No 9; age 24 years), but has remained stable for 70 months. The remainder of the patients had no further clinical progression of their cervical cord disease, and the majority experienced improvement or resolution of their weakness.
Three patients developed MPNSTs in the buttock (case Nos 11 and 13) and thigh (case Nos 7 and 13). Cervical cord compression occurred 6 months (case No 7) after the diagnosis of MPNST in one individual and 1 month and 136 months, respectively, before the development of MPNST in two patients (case Nos 11 and 13.) Two of these patients died 10 and 13 months after MPNST diagnosis (case Nos 7 and 11), and one patient with a low grade MPNST was asymptomatic 36 months after diagnosis. Post‐laminectomy kyphosis was noted in case No 2, 1 month following surgery, and in case No 1, 3 months following decompression. Case No 2 underwent 3 months of halo immobilisation with correction of the deformity. At the last follow‐up, flexion/extension cervical spine imaging showed no angulation. In addition, one patient developed persistent dorsal column sensory deficits following surgery, which is now improving (case No 12).
Figure 1 T2 weighted sagittal (top) and axial (bottom) MRI of the upper cervical spine showed a 1–2 mm reduction in the size of the spinal canal (arrow) in a 17‐year‐old female (case No 1) who presented with progressive quadriparesis, hyperreflexia and up‐going toes. The broken line denotes abnormal signal intensity in the area of cord compression.
Discussion
Cervical cord compression arising from cervical nerve root neurofibromas is a recognised complication of NF1, but has been reported infrequently. Multiple case series have described the surgical management of isolated cervical cord neurofibromas and associated spine deformities.12,13 In contrast, the management of plexiform neurofibromas in individuals with NF1 has received less attention in the literature. Adequate documentation of clinical presentation, management and follow‐up is reported in only 12 cases.2,3,4,5,6,7,8,9,10 Our study of 13 patients with NF1 and cervical cord compression is the largest series to date and raises several important points regarding age at presentation, presenting symptoms, role of neuroimaging and long‐term outcome.
The age at presentation ranged from the first decade to the seventh decade of life in both our series (ages 9–61 years) and in the published literature (ages 1–35 years). However, in contrast with other manifestations of NF1 (eg, optic glioma), the risk of becoming symptomatic from a cervical cord neurofibroma does not decrease with age, and patients at any age are prone to developing signs or symptoms of cervical cord compression.
In both our series (seven cases) and in the published literature (seven cases), the most common presenting symptom was progressive quadriparesis. The majority of patients presented with progressive quadriparesis, and paraparesis, incontinence and neck pain were observed less frequently. Three of our patients developed an MPNST in other locations, but there was no evidence of malignant transformation of the cervical root neurofibromas and the neck pain was attributed to pressure on the upper cervical nerve roots. A larger study will be required to determine whether the presence of cervical nerve root neurofibromas and cord compression is a risk factor for the development of MPNST.
While neurofibromas can involve any of the cervical nerve roots, in our patients cord compression occurred most commonly in the upper cervical spine at C2 and C3. The reason underlying the predilection for high cervical cord compression is not known; it is possible that it reflects increased vulnerability of the C2 nerve root to repeated low grade trauma as it exits from the neural foramen and runs over the superior aspect of the C2 lamina.
Cervical cord compression was identified in the majority of our patients when they developed symptoms (eg, progressive quadriparesis). However, in one patient (case No 1), cord compression was identified on neuroimaging 11 months prior to the development of quadriparesis and she subsequently developed progressive weakness, despite minimal progression on MRI. Another patient, a 25‐year‐old woman (case No 6), remained clinically stable with only minor neurological deficit despite significant evidence of cord compression on neuroimaging. Furthermore, she had a normal pregnancy and delivery of a healthy child without worsening of her symptoms. These observations emphasise the fact that the appearance on neuroimaging does not always predict the need for surgical intervention. We recommend that the decision to undertake surgical decompression should be based on the progressive nature of neurological symptoms and deficits.
The majority of our patients were followed for over 12 months and only two patients exhibited clinical progression from cervical cord compression and required further surgery as a result of tumour regrowth. All but one patient (case No 9) remained ambulatory with minor sensory or motor deficits. One patient developed cervical cord compression when he presented with a metastatic MPNST (case No 7); however, surgical decompression was undertaken, which resulted in an improved quality of life. As with the other cases in our series, this cervical cord tumour was a benign neurofibroma.
Despite the increased incidence of kyphotic deformity reported in patients with cervical neurofibromas, none of the patients in our series presented with a kyphotic deformity. However, post‐laminectomy kyphosis has been described frequently after surgical treatment of individuals with NF1,14 perhaps due to an intrinsic abnormality of bone development and growth.15 In our series, one patient (case No 2) developed a kyphotic deformity at C2–3 which responded to 3 months of halo immobilisation, and another patient (case No 1) showed slight angulation at C4–5 which we are attempting to manage conservatively. Given our results achieved with limited laminectomies, we do not advocate cervical spine instrumentation at the time of decompression.
Finally, we do not recommend routine spinal imaging for asymptomatic NF1 patients—neurological deficit rather than radiological detection of cord compression should dictate the need for surgical intervention. Patients who develop progressive paraparesis or quadriparesis require prompt neurological assessment and appropriate neuroimaging. Timely decompression of the cord affected by the spinal neurofibroma is recommended for patients with significant or progressive neurological deficit.
Acknowledgements
We acknowledge the generous support of Schnuck Markets, Inc. (to DHG) and our neurosurgical colleague Chris Chandler at King's College Hospital. We also thank Dr Vincent Riccardi for helpful discussions.
Abbreviations
MPNST - malignant peripheral nerve sheath tumours
NF1 - neurofibromatosis type 1
Footnotes
Competing interests: None.
References
- 1.Thakkar S D, Feigen U, Mautner V ‐ F. Spinal tumours in neurofibromatosis type 1: an MRI study of frequency, multiplicity, and variety. Neuroradiology 199941625–629. [DOI] [PubMed] [Google Scholar]
- 2.Ferner R E, Honavar M, Gullan R W. Spinal neurofibroma presenting as atlanto‐axial subluxation in von Recklinghausen neurofibromatosis. Neurofibromatosis 1989243–46. [PubMed] [Google Scholar]
- 3.Wise J B, Cryer J E, Belasco J B.et al Management of head and neck plexiform neurofibromas in pediatric patients with neurofibromatosis type 1. Arch Otolaryngol Head Neck Surg 2005131712–718. [DOI] [PubMed] [Google Scholar]
- 4.Creange A, Zeller J, Rostaing‐Rigattieri S.et al Neurological complications of neurofibromatosis type 1 in adulthood. Brain 2003122473–481. [DOI] [PubMed] [Google Scholar]
- 5.Barber D B, Quattrone B E, Lomba M E.et al Neurofibromatosis: an unusual cause of cervical myelopathy. J Spinal Cord Med 199821148–150. [DOI] [PubMed] [Google Scholar]
- 6.Carod‐Artal F J, Melo M, da Silva R T.et al Type I neurofibromatosis presenting as progressive cervical myelopathy. Rev Neurol 200031307–310. [PubMed] [Google Scholar]
- 7.Clarke D B, Farmer J P, Montes J L.et al Newborn apnea caused by a neurofibroma at the craniocervical junction. Can J Neurol Sci 19942164–66. [DOI] [PubMed] [Google Scholar]
- 8.Garg S, Hosalkar H, Dormans J P. Quadriplegia in a 10 year‐old boy due to multiple cervical neurofibromas. Spine 200328E339–E343. [DOI] [PubMed] [Google Scholar]
- 9.Chung C J, Armfield K B, Mukherji S K.et al Cervical neurofibromas in children with NF‐1. Pediatr Radiol 199929353–356. [DOI] [PubMed] [Google Scholar]
- 10.Pollack I F, Colak A, Fitz C.et al Surgical management of spinal cord compression from plexiform neurofibromas in patients with neurofibromatosis 1. Neurosurgery 199843248–255. [DOI] [PubMed] [Google Scholar]
- 11. National Institutes of Health Consensus Development Conference Statement: Neurofibromatosis Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 12.Lot G, George B. Cervical neuromas with extradural components: Surgical management in a series of 57 patients. Neurosurgery 199741813–822. [DOI] [PubMed] [Google Scholar]
- 13.Seppala M T, Haltia M J, Sankila R J.et al Long‐term outcome after removal of spinal neurofibroma. J Neurosurg 199582572–577. [DOI] [PubMed] [Google Scholar]
- 14.Craig J B, Govender S. Neurofibromatosis of the cervical spine. A report of eight cases. J Bone Joint Surg Br 199274575–578. [DOI] [PubMed] [Google Scholar]
- 15.Kolanczyk M, Kossler N, Kuhnisch J.et al Multiple roles for neurofibromin in skeletal development and growth. Hum Mol Genet 200716874–886. [DOI] [PubMed] [Google Scholar]