Neurological side effects from calcineurin inhibitors (ciclosporin, tacrolimus) are most commonly mild, such as headaches, dysarthria, visual changes or postural tremor.1 More severe side effects can include psychosis, opisthotonus with severe rigidity, cortical blindness and seizures.1 Neurotoxicity is more common early after initiation of tacrolimus, but may occur months or even years later.2
We report a patient who presented with refractory generalised status epilepticus and prolonged coma associated with extensive leukoencephalopathy caused by tacrolimus neurotoxicity.
Case report
A 55‐year‐old woman with a history of insulin dependent diabetes mellitus and pancreatic transplant 3 months prior to presentation consulted for a 3 week history of daily headaches, dizziness and ataxia. The patient denied cognitive changes, episodic confusion, diplopia, dysarthria or fever. Examination revealed subjective diplopia on right lateral gaze without oculoparesis or papilloedema, truncal ataxia and sensory loss in stocking and glove distribution. As the patient was receiving tacrolimus as part of her immunosuppressive regimen, neurotoxicity from this agent was suspected. Brain MRI was normal. Tacrolimus trough level was 10.3 ng/ml (therapeutic range 5.0–15.0). Spinal fluid studies were unremarkable. The patient improved over the subsequent 2 weeks and failed to return for a 3 month follow‐up.
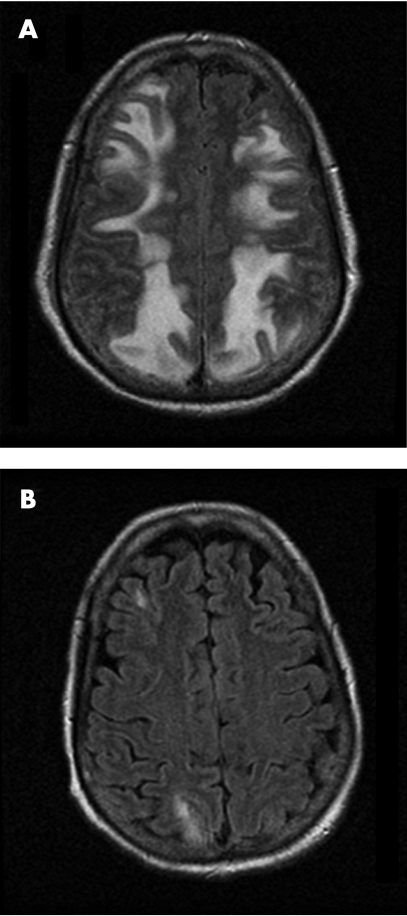
Eight months later, the patient developed worsening headache and nausea for 2 days followed by altered mental status progressing to unresponsiveness. She required intubation for airway protection. On examination, the patient was comatose and exhibited rhythmic head turning and clonic eye movements to the right. Emergent EEG showed multifocal epileptiform discharges intermixed with generalised seizures. Diffuse slowing and loss of the normal posterior background activity were also noted. After administration of lorazepam and loading with fosphenytoin, the recording showed less frequent partial seizures, but propofol infusion was needed to control the electrographic seizures. Head CT revealed diffuse white matter changes, predominantly in the posterior head regions, consistent with the pattern of posterior reversible encephalopathy syndrome (PRES), then confirmed by MRI (fig 1A). Incidentally, the patient's blood pressure had been elevated (systolic blood pressure >200 mm Hg), and hypertensive encephalopathy was thought to be the most likely explanation for the patient's clinical presentation. Lumbar puncture was not performed, but the patient was empirically treated with broad spectrum antibiotics with adequate blood–brain barrier penetration. Blood pressure was lowered, but the patient remained comatose. Tacrolimus trough level was elevated (27 ng/ml), and the dose of the medication was adjusted. The level of tacrolimus had been therapeutic 2 days before (5.4 ng/ml), and it was deemed possible that administration of metoclopramide for nausea might have been responsible for the sudden raise by improving gastric motility and intestinal absorption of the medication.3 The patient did not have hypomagnesaemia at the time of acute symptom onset.
Figure 1 Fluid attenuated inversion recovery (FLAIR) MRI of the brain, demonstrating increased T2 signal involving the white matter of the occipital and parietal lobes at the onset of coma (A), with significant improvement 11 days after discontinuation of tacrolimus (B).
The patient was initially continued on treatment with fosphenytoin (up to 430 mg per day), clonazepam (up to 1.5 mg per day) and propofol (up to 141.6 μg/kg/min), but continued to have frequent electrographic seizure discharges on continuous video EEG monitoring. As seizures became better controlled over the next few days, propofol was tapered and then discontinued. However, the patient remained comatose with only preserved brainstem reflexes and minimal responsiveness to pain. Lumbar puncture was then performed and showed no CSF abnormalities. Despite appropriate levels of antiepileptics, the patient then experienced further generalised seizures without recurrence of status. Repeat brain MRI showed only minimal improvement. After the patient had been comatose for 11 days, tacrolimus was discontinued. Serum trough tacrolimus levels had remained within or below the therapeutic range since its dose had been adjusted (levels ranged between 2.0 and 13 ng/ml).
Over the next few days, the patient's mental status improved markedly. She regained alertness 3 days after stopping the drug, began following commands consistently 2 days later and was then successfully extubated. She experienced no further seizures. Follow‐up MRI 10 days after discontinuation of tacrolimus (fig 1B) showed remarkable improvement of the leukoencephalopathy. Her functional recovery 4 months later was partial despite recovery of lucidity, mostly because of an episode of sepsis from an intra‐abdominal source requiring prolonged rehospitalisation. The patient died 5 months later at an outside institution secondary to complications of sepsis.
Discussion
Symptoms of tacrolimus neurotoxicity vary depending on the organ transplanted, and range from tremor and dysarthria to cortical blindness and psychosis.1 Refractory generalised status epilepticus and prolonged coma are not well recognised manifestations of tacrolimus toxicity in the literature.
Although the mechanism of tacrolimus neurotoxicity is not fully understood, some have postulated that it may be similar to hypertensive encephalopathy. However, not all patients with immunosuppressive induced leukoencephalopathy have hypertension.1 Hypertensive encephalopathy is the most widely recognised cause of PRES, a condition characterised by the rapid development of vasogenic oedema in the posterior head regions. Our patient was initially hypertensive and had neuroimaging findings consistent with PRES. However, she failed to improve after her blood pressure was controlled and the radiological changes persisted much longer than those observed with hypertensive encephalopathy. Clinical and frank radiological improvement only occurred after discontinuation of tacrolimus.
The incidence of MRI changes in tacrolimus induced leukoencephalopathy is not known. Changes may also involve the cortex, cerebellum and basal ganglia. Fluid attenuated inversion recovery (FLAIR) is the optimal sequence to identify the leukoencephalopathy.4 Diffusion weighted images usually show increased diffusion, consistent with vasogenic oedema.4 Pathological studies have shown evidence of extracellular oedema with endothelial damage, typically in the absence of infarctions or demyelination.5 Endothelial toxicity may result from damage to a drug efflux pump, p‐glycoprotein, and would be responsible for the disruption of the blood–brain barrier.1 Although vasogenic oedema predominates, intracellular (cytotoxic) oedema can occur in patients with more severe neurotoxicity.1 In our patient, the prolonged exposure to tacrolimus probably caused cytotoxic oedema, leading to slower and incomplete resolution of white matter changes and neurological deficits.
Prompt recognition of tacrolimus neurotoxicity may be challenging. In our patient, tacrolimus neurotoxicity was suspected months before her presentation with status epilepticus and coma, but the diagnosis could not be proven and the medication was continued. Hence it is important to be mindful of certain diagnostic considerations when the possibility of tacrolimus neurotoxicity is entertained: (1) absence of the characteristic leukoencephalopathy on MRI does not exclude the possibility of tacrolimus neurotoxicity, especially early in the course of the disorder; (2) serum drug trough levels of tacrolimus have poor correlation with the occurrence of neurotoxicity1; (3) once alternative diagnoses have been excluded, particularly infections (such as JC virus), discontinuation of tacrolimus needs to be strongly considered since the diagnosis can only be firmly established by neurological improvement following removal of the drug. Reduction of the dose may be insufficient, as noted in our patient; (4) even extremely severe neurotoxicity may be mostly reversible with discontinuation of the drug.
Footnotes
Competing interests: None.
References
- 1.Wijdicks E F. Neurotoxicity of immunosuppressive drugs. Liver Transpl 20017937–942. [DOI] [PubMed] [Google Scholar]
- 2.Reinohs M, Straube T, Baum P.et al Recurrent reversible cerebral edema after long term immunosuppression with tacrolimus. J Neurol 2002249780–781. [DOI] [PubMed] [Google Scholar]
- 3.Prescott W A, Jr, Callahan B L, Park J M. Tacrolimus toxicity associated with concomitant metoclopramide therapy. Pharmacotherapy 200424532–537. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa M, Terae S, Chu B C.et al MRI in seven cases of tacrolimus (FK‐506) encephalopathy: utility of FLAIR and diffusion‐weighted imaging. Neuroradiology 200143615–621. [DOI] [PubMed] [Google Scholar]
- 5.Lavigne C M, Shrier D A, Ketkar M.et al Tacrolimus leukoencephalopathy: a neuropathologic confirmation. Neurology 2004631132–1133. [DOI] [PubMed] [Google Scholar]