Abstract
We applied optimised voxel based morphometry (VBM) to brain MRIs from autopsy proven cases of tau positive frontotemporal lobar degeneration (FTLD‐T, n = 6), ubiquitin and TDP‐43 positive/tau negative FTLD (FTLD‐U, n = 8) and cognitively normal controls (n = 61). The analysis revealed that FTLD‐T and FTLD‐U both show atrophy in the frontal cortex and striatum, but striatal atrophy is more severe in FTLD‐T. Manual region of interest tracing of caudate and putamen volumes confirmed the VBM findings. These anatomical differences may help distinguish between FTLD spectrum pathological subtypes in vivo.
The pathology of frontotemporal lobar degeneration (FTLD) is heterogeneous, with two main divisions based on immunohistochemical staining of intracellular inclusions. One group shows tau immunoreactive inclusions (FTLD‐T) while the other shows ubiquitin and TDP‐43 positive/tau negative pathology (FTLD‐U).1,2 As molecule specific therapies are developed for FTLD, it will become important to accurately predict pathological subtypes. Neuroimaging could serve this purpose.3,4,5,6
We applied voxel based morphometry (VBM) to the earliest brain MRIs of pathologically proven cases of FTLD‐T and FTLD‐U in order to search for distinct patterns of tissue loss that could assist in predicting the pathological subtype in vivo.
Methods
Subjects
Fourteen patients (six FTLD‐T and eight FTLD‐U) (table 1) seen at the UCSF Memory and Aging Center (MAC) between 1998 and 2006 who met pathological criteria for FTLD at autopsy1 and had good quality MRI scans with no strokes were included. Patients with a pathological diagnosis of dementia lacking distinctive histology, corticobasal degeneration or progressive supranuclear palsy were excluded.
Table 1 Group characteristics in FTLD‐T, FTLD‐U and NC.
| FTLD‐T (n) | FTLD‐U (n) | NC | p Value | |
|---|---|---|---|---|
| n | 6 | 8 | 61 | |
| Sex (M:F) | 5:1 | 6:2 | 26:35 | 0.055 |
| Age at MRI (y) | 67.7 (6.3) | 60.0 (10.0)* | 68.0 (8.0) | 0.038 |
| Onset to MRI (y) | 7.2 (4.9) | 5.4 (4.8) | NA | 0.520 |
| Survival (y) | 9.7 (4.8) | 5.4 (4.8) | NA | 0.406 |
| MMSE | 17.8 (9.9)*† | 22.9 (7.5)* | 29.5 (0.7) | <0.001 |
| CDR | 1.3 (0.5) (4) | 0.8 (0.6) (7) | 0.208 | |
| Clinical diagnosis | ||||
| FTD | 5 | 5‡ | ||
| SD | 1 | 2 | ||
| PNFA | 0 | 1 (w/MND) |
CDR, Clinical Dementia Rating; FTD, frontotemporal dementia; FTLD, frontotemporal lobar degeneration; FTLD‐T, FTLD with tau positive inclusions; FTLD‐U, FTLD with ubiquitin and TDP‐43 positive/tau negative inclusions; MMSE, Mini Mental State Examination; MND, motor neuron disease; n, number of patients whose data were available for analysis; NC, normal controls; PNFA, progressive non‐fluent aphasia; SD, semantic dementia.
*p<0.05 vs controls.
†p<0.05 vs FTLD‐U.
‡Four of these patients also had motor neuron disease.
Sixty‐one cognitively normal controls with no history of neurological or psychiatric illness were selected from a cohort of volunteers followed at the MAC. All patients and controls had undergone at least one clinical evaluation at the MAC, including a neurological examination, informant interview and neuropsychological testing, reviewed elsewhere.7 Clinical diagnosis was based on standard research criteria,8 while controls were determined to be “cognitively normal” after a comprehensive clinical assessment. Clinical diagnosis was blinded to imaging findings.
Neuropathology
Twelve of 14 autopsies were performed at UCSF or the University of Pennsylvania using a previously described protocol.9 One autopsy was performed at the University of California, Irvine, and the other at Stanford University. All reports were reviewed by a neurologist to ensure adherence to a standard protocol. Six of eight FTLD‐U patients were re‐stained for TDP‐43 and found to show TDP‐43 immunoreactive inclusions, which have been reported to be absent in neuronal intermediate filament inclusion disease.2 Therefore, a significant contribution of patients with neuronal intermediate filament inclusion disease to the FTLD‐U group is unlikely.
Image acquisition and voxel based morphometry
MRI scans and optimised VBM were performed using previously described protocols10,11 and the SPM2 software package (http://www.fil.ion.ucl.ac.uk/spm). Comparisons were made using the following contrasts: (1) FTLD‐T versus controls, (2) FTLD‐U versus controls; (3) FTLD‐T versus FTLD‐U; and (4) FTLD‐U versus FTLD‐T, with total intracranial volume, age, sex and Mini‐Mental State Examination (MMSE) as covariates. A conjunction analysis of contrasts (1) and (2) was performed to identify voxels where both patient groups showed tissue loss compared with controls. The conjunction results were inclusively masked with each individual contrast to ensure that the effect was present in each contrast.12
Voxels were considered significant at p<0.05 after family‐wise error correction for multiple comparisons. Results are displayed on a study specific template brain as t‐maps thresholded at p<0.001 uncorrected for multiple comparisons.
Results
Clinicopathological correlations
Mean MMSE score in FTLD‐T was significantly lower than in FTLD‐U (table 1). FTLD‐T patients were also more impaired on a modified version of the Trails‐B task than FTLD‐U patients (p<0.05, uncorrected for multiple comparisons) but there were no other differences in cognitive performance. The majority of patients in both groups presented clinically as frontotemporal dementia, and five patients in the FTLD‐U group had comorbid motor neuron disease.
VBM
Gray matter
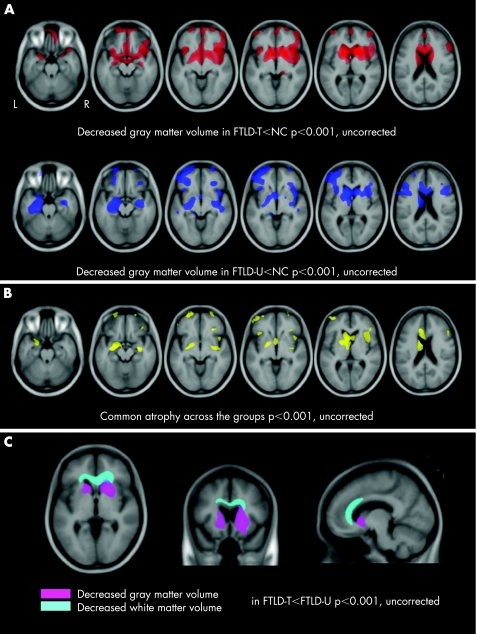
Compared with controls, patients with both pathological subtypes demonstrated decreased gray matter volumes in frontal lobes (predominantly in the ventromedial and striatal regions) and anterior temporal lobes (fig 1A). After correction for multiple comparisons, FTLD‐T patients had atrophy in the right inferior frontal gyrus, left head of caudate/anterior putamen and right putamen, while FTLD‐U patients had greater atrophy than controls in the left inferior frontal gyrus, left hippocampus, left caudate and left globus pallidus (table 2).
Figure 1 Patterns of gray matter loss in frontotemporal lobar degeneration (FTLD) pathological subtypes. T score maps are displayed on multiple axial sections on a study specific template. L, left; R, right. (A) Gray matter loss in FTLD with tau positive inclusions (FTLD‐T, upper row) and FTLD with ubiquitin and TDP‐43 positive/tau negative inclusions (FTLD‐U, bottom row) compared with normal controls (NC). (B) Common regions of gray matter loss found in both groups compared with controls. (C) Regions of greater gray matter loss (displayed in pink) and white matter loss (displayed in sky blue) in FTLD‐T compared with FTLD‐U. T score maps are presented on axial (z = −1), coronal (y = 13) and sagittal (x = −10) sections of a study specific template.
Table 2 Regions of gray matter loss in FTLD‐T and FTLD‐U compared with controls and compared with each other.
| Region | BA | Common atrophic area | FTLD‐T vs NC | FTLD‐U vs NC | |||
|---|---|---|---|---|---|---|---|
| X, Y, Z | T | X, Y, Z | T | X, Y, Z | T | ||
| L head of caudate/anterior putamen | – | −6, 9, 10 | 4.30* | −13, 15, 3 | 9.01* | −9, 13, 10 | 6.83* |
| R putamen | – | 20, 14, −1 | 8.57*† | ||||
| R inferior frontal gyrus | 47 | 42, 16, 28 | 3.81* | 37, 24, −12 | 6.37* | 42, 16, 28 | 5.12 |
| L pallidum | – | −17, 4, 0 | 5.66* | ||||
| L hippocampus | 20 | −27, −11, −17 | 5.62* | ||||
| L amygdala | – | −23, −5, −16 | 3.82* | −23, −5, −16 | 3.83 | −23, −5, −16 | 5.08 |
| R hippocampus | 20 | 30, −9, −13 | 3.74* | 30, −9, −13 | 3.74 | 32, −10, −15 | 5.21 |
| L middle OF gyrus | 11 | −30, 61, −6 | 3.34* | −44, 58, −6 | 3.76 | −32, 61, −5 | 4.53 |
| L inferior frontal gyrus | 47 | −28, 33, −13 | 3.33* | −52, 44, −7 | 3.30 | −40, 11, 27 | 5.42* |
BA, Brodmann area; FTLD‐T, FTLD with tau positive inclusions; FTLD‐U, FTLD with ubiquitin and TDP‐43 positive/tau negative inclusions; L, left; NC, normal controls; OF, orbitofrontal; R, right; T, T score at given voxel.
*p<0.05 after family‐wise error correction for multiple comparisons for this contrast.
†FTLD‐T versus FTLD‐U in p<0.05 after family‐wise error correction for multiple comparisons.
The conjunction analysis revealed significant volume loss in the left caudate, bilateral inferior frontal gyrii and right hippocampus (fig 1B, table 2).
FTLD‐T patients showed greater atrophy in the striatum compared with FTLD‐U (fig 1C), with voxels in the right putamen being significant after correction for multiple comparisons (table 2). The contrast of FTLD‐U versus FTLD‐T did not yield significant results.
White matter
FTLD‐T showed significant atrophy in the genu and anterior body of the corpus callosum and the white matter underlying the anterior cingulate cortex compared with both controls and FTLD‐U (fig 1C). Significant white matter atrophy was found underlying the left inferior temporal cortex in FTLD‐U compared with controls (data not shown).
Striatal volume
The striatal findings in FTLD‐T and FTLD‐U could have been confounded by tissue misclassification in periventricular structures—a potential problem in VBM.13 We therefore confirmed the VBM findings using manually traced striatal volumes. Using Brains 2 software (http://www.psychiatry.uiowa.edu/mhcrc/IPLpages/IPLhome.htm), a trained investigator blinded to clinical and pathological information drew regions of interest around the left and right caudate and putamen on the same T1 weighted MRIs used for VBM analysis for the six FTLD‐T patients, 7/8 FTLD‐U patients and nine 9/61 controls (selected at random). Landmarks used to identify regions of interest are listed in appendix 1 (appendix 1 can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental). Volumes were normalised to total intracranial volume and analysed with ANCOVA, using diagnosis as the grouping variable, and age, sex and MMSE as covariates.
Compared with controls, patients with FTLD‐T had significantly lower volumes in the bilateral caudate and putamen (left caudate: p = 0.017; right caudate: p = 0.011; left putamen: p = 0.027; right putamen: p = 0.001), and FTLD‐U patients showed significantly smaller volumes in the bilateral putamen (left putamen: p = 0.027; right putamen: p = 0.020). Mean right caudate volume was smaller in FTLD‐T than in FTLD‐U (p = 0.007), with a non‐significant trend in the right putamen (p = 0.056).
Parkinsonian motor features
Based on the finding of greater striatal atrophy in FTLD‐T, we retrospectively investigated whether there were differences in parkinsonian symptoms and signs between groups. A blinded neurologist rated the presence (1) or absence (0) of nine parkinsonian features (slowed speech, decreased facial expression, tremor at rest, action tremor, rigidity, postural instability, parkinsonian gait, bradykinesia, decreased leg or hand agility) based on the transcribed descriptions of the clinical history and neurological examination in each patient. Almost all patients (5/5 FTLD‐T, 7/8 FTLD‐U) had at least one parkinsonian feature at that time of the MRI. Only FTLD‐T patients (2/5) had three parkinsonian features. Mean score in each group was 1.8 (1.1) in FTLD‐T and 1.4 (0.7) in FTLD‐U (p = 0.419).
Discussion
We compared gray and white matter tissue content in the two major pathological variants of FTLD. Compared with healthy controls, both FTLD‐T and FTLD‐U had gray matter loss in the frontal cortices and striatum bilaterally. Striatal atrophy was more severe in FTLD‐T than in FTLD‐U. These findings highlight a stronger association between striatal damage and tau based pathology as opposed to ubiquitin/TDP‐43 based pathology.
There is no prior report of striatal atrophy in pathologically confirmed FTLD. The finding is consistent with the clinical data in this cohort indicating a slightly higher burden of parkinsonism in FTLD‐T, and with the literature relating atypical parkinsonism with tau positive pathology.9,14,15,16 The worse performance for FTLD‐T on our version of the Trails‐B task, which is timed, could also be a sign of motor slowing. As none of these patients were diagnosed with parkinsonian disorders, the fact that FTLD‐T only showed slightly more parkinsonism is not surprising. These data suggest that tau pathology has a special proclivity for the basal ganglia, even in patients who do not present with parkinsonian features among their chief symptoms.
The pathogenesis of white matter atrophy in FTLD has not been fully defined. However, the loss of tissue in the anterior corpus callosum in FTLD‐T is consistent with previous MRI work showing anterior callosal atrophy in frontotemporal dementia relative to other neurodegenerative diseases.17 Our data suggest that this may be particularly true with tau pathology. In contrast with patients with corticobasal degeneration (which is associated with tau pathology), FTLD subjects in this study lacked abundant white matter pathology at autopsy (data not shown).18 Thus the white matter loss may also relate to demyelination or axon loss within tracts. This possibility suggests that approaches more appropriate for studying white matter tracts, such as diffusion tensor imaging, may be valuable for studying FTLD.19
The major limitation of our study and similar case series3,4,5,6 is the small number of patients, a frequent problem in imaging studies of patients with pathologically confirmed diagnoses. While our findings need to be confirmed in larger series, we detected significant differences in atrophy patterns between FTLD pathological subtypes that converge with clinical features, suggesting that these distinctive patterns are clinically relevant. Understanding how FTLD‐T and FTLD‐U target differing brain regions may shed light on the underlying pathogenesis of these closely related diseases.
Appendix 1 can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental
Copyright © 2007 BMJ Publishing Group Ltd
Supplementary Material
Acknowledgements
This study was supported by the John Douglas French Alzheimer's Foundation, the Larry L Hillblom Foundation, NIA grants P01‐AG1972403, R01‐AG022983, K08‐AG027086‐01, K08‐AG020760‐01, ADRC grant P50‐AG023501 and GCRC grant M01‐RR00079.
Abbreviations
FTLD - frontotemporal lobar degeneration
FTLD‐T - tau positive frontotemporal lobar degeneration
FTLD‐U - ubiquitin and TDP‐43 positive/tau negative frontotemporal lobar degeneration
MAC - Memory and Aging Center
MMSE - Mini Mental State Examination
VBM - voxel based morphometry
Footnotes
Competing interests: None.
Appendix 1 can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental
References
- 1.McKhann G M, Albert M S, Grossman M.et al Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 2001581803–1809. [DOI] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu D M, Kwong L K.et al Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006314130–133. [DOI] [PubMed] [Google Scholar]
- 3.Whitwell J L, Warren J D, Josephs K A.et al Voxel‐based morphometry in tau‐positive and tau‐negative frontotemporal lobar degenerations. Neurodegener Dis 20041225–230. [DOI] [PubMed] [Google Scholar]
- 4.Whitwell J L, Josephs K A, Rossor M N.et al Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol 2005621402–1408. [DOI] [PubMed] [Google Scholar]
- 5.Whitwell J L, Jack C R, Jr, Senjem M L.et al Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology 200666102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs K A, Whitwell J L, Dickson D W.et al Voxel‐based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. (in press) [DOI] [PMC free article] [PubMed]
- 7.Kramer J H, Jurik J, Sha S J.et al Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 200316211–218. [DOI] [PubMed] [Google Scholar]
- 8.Neary D, Snowden J S, Gustafson L.et al Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998511546–1554. [DOI] [PubMed] [Google Scholar]
- 9.Forman M S, Farmer J, Johnson J K.et al Frontotemporal dementia: clinicopathological correlations. Ann Neurol 200659952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Good C D, Scahill R I, Fox N C.et al Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage 20021729–46. [DOI] [PubMed] [Google Scholar]
- 11.Good C D, Johnsrude I S, Ashburner J.et al A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 20011421–36. [DOI] [PubMed] [Google Scholar]
- 12.Friston K J, Penny W D, Glaser D E. Conjunction revisited. Neuroimage 200525661–667. [DOI] [PubMed] [Google Scholar]
- 13.Senjem M L, Gunter J L, Shiung M M.et al Comparison of different methodological implementations of voxel‐based morphometry in neurodegenerative disease. Neuroimage 200526600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges J R, Davies R R, Xuereb J H.et al Clinicopathological correlates in frontotemporal dementia. Ann Neurol 200456399–406. [DOI] [PubMed] [Google Scholar]
- 15.Kertesz A, McMonagle P, Blair M.et al The evolution and pathology of frontotemporal dementia. Brain 20051281996–2005. [DOI] [PubMed] [Google Scholar]
- 16.Josephs K A, Petersen R C, Knopman D S.et al Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 20066641–48. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi H, Fukuyama H, Nagahama Y.et al Comparison of the pattern of atrophy of the corpus callosum in frontotemporal dementia, progressive supranuclear palsy, and Alzheimer's disease. J Neurol Neurosurg Psychiatry 200069623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman M S, Zhukareva V, Bergeron C.et al Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol 20021602045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson E M, Englund E, Sjobeck M.et al MRI with diffusion tensor imaging post‐mortem at 3.0 T in a patient with frontotemporal dementia. Dement Geriatr Cogn Disord 200417316–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.