Abstract
A woman with epilepsy died during a seizure and the event was recorded on ambulatory EEG. The circumstances were typical of sudden death in epilepsy (SUDEP). The EEG revealed that the patient had suffered a generalised seizure that abruptly ended with cessation of all cerebral electrical activity. Two other cases recorded on videotelemetry demonstrating similar EEG features were reported in the literature. We postulate that abrupt irreversible cerebral electrical shutdown during a seizure may be the primary mechanism of SUDEP.
Sudden death in epilepsy (SUDEP) is defined as a “sudden unexpected witnessed or unwitnessed, non‐traumatic and non‐drowning death in a patient with epilepsy, with or without evidence for a seizure and excluding documented status epilepticus where necropsy examination does not reveal a toxicological or an anatomical cause for death”.1
On the basis of this definition and available epidemiological data, approximately 400 people are expected to die each year in Britain from SUDEP,2 yet the exact cause of death remains an issue for speculation. Interictal EEGs and cardiac monitoring during seizures are frequently reported but only rarely has the death occurred while EEG monitoring is taking place.3,4 We report a case of SUDEP whose death occurred while undergoing ambulatory EEG recording.
Case report
A right‐handed woman in her fifties presented with poorly controlled epilepsy. She had no perinatal neurological problems. At the age of 4 years she developed her first spontaneous seizure. Details of her previous treatment were sketchy as she had only recently moved to the locality. One sibling and maternal twin had epilepsy. We have not been able to obtain a more detailed history, as we were unsuccessful in contacting her family.
The patient described simple partial and extratemporal seizures with occasional generalisation with tongue biting. The seizures were refractory to treatment for a range of antiepileptic medications.
On examination, her right upper and lower limbs were slightly smaller compared with the left. Despite the limb asymmetry, no neurological abnormality was found.
MRI scan of the brain was reported as showing left hippocampal atrophy on the basis of minor asymmetry. An interictal EEG revealed left anterior temporal theta activity without any paroxysmal epileptiform discharges. She had been on 350 mg of phenytoin daily since her youth. For the preceding 3 years she had also been taking sodium valproate 1200 mg/day. Phenytoin was withdrawn and lamotrigine was introduced but her seizure control remained poor. She developed a tremor and her epilim dose was reduced and maintained at the level of 1000 mg/day. She lived alone at home.
Because of the uncertainty over the frequency of seizures, an ambulatory EEG recording was arranged. She was due to be seen in the EEG department the next morning for review of the tape but did not attend. She had been found dead by a friend who called to drive her to the hospital. She had been found lying prone on the floor in her nightclothes with her arm outstretched towards the telephone.
The post mortem showed no signs of external injury. She had mild pulmonary congestion and the lungs weighed 348 g and 298 g, respectively. There was no evidence of aspiration, pulmonary embolism or myocardial damage. She had minimal coronary atheroma but there was no evidence of an infarction. Stomach contents showed no evidence of medication. Her brain was not swollen and was macroscopically normal. Toxicological studies showed a lamotrigine level of 7.6 μg/ml (normal range 12–15 μg/ml); sodium valproate was undetectable.
Ambulatory EEG
The ambulatory EEG was set up at 13:00 and it continued to record cerebral activity until the next morning when the patient was found dead. The recording was undertaken using a digital Micromed MS 40 brain spy 12 channel recorder. A good quality recording was obtained throughout and the EEG was not contaminated with artefacts.
The record showed bursts of irregular slow and sharp waves occurring intermittently during the day but with increasing frequency during the night. Prolonged bursts of high amplitude spikes began to appear towards midnight.
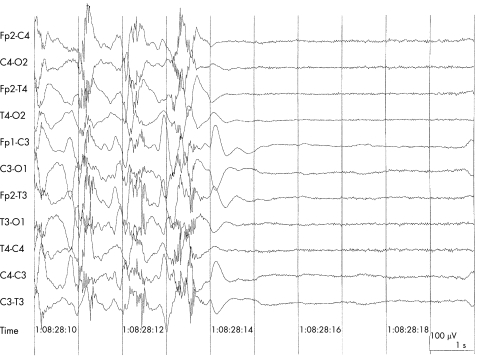
The patient fell asleep around midnight and normal sleep stages 1–4 were recognised in the recording but no REM sleep was noted. Paroxysmal activity became more frequent and prolonged around 08:00, eventually becoming continuous with spike wave discharges developing to a seizure at 08:27:18. Seizure activity then became polyspike (up to six spikes) and continued for 52 s. The seizure activity abruptly terminated at 08:28:14 and the EEG became a “flat line” (fig 1)
Figure 1 Sudden termination of polyspike and slow wave discharges with flattening of the EEG. (Ambulatory recordings using digital Micromed MS 40 BrainSpy 12 channel recorder: 10 s sample, 10 μV/mm amplitude, filter 70 Hzl/TC 0.3.)
Rhythmic movement artefact (possible head jerking) was detected involving the electrode T3, associated with muscle (EMG) activity that became less frequent and disappeared completely at 08:31, leaving a continuous flat EEG.
The exact time of death could not be determined, as respiration and heart rate were not recorded simultaneously. No pulse artefact could be identified or retrieved by further offline analysis.
The EEG did not show evidence of slow wave activity that could be attributed to hypotension or cerebral hypoxia.
In summary, the EEG changes consisted of a seizure with continuous spike wave discharges followed by an abrupt change to a “flat EEG” that failed to recover.
Discussion
Our patient died in the early hours when alone and was typical of SUDEP in all aspects of definition although a little older than most cases. It is likely that SUDEP occurs as a result of more than one mechanism.
Tachycardia5,6 is a frequent observation during seizures because of sympathetic overactivity. Bradycardia and asystole are also reported in association with seizures.7,8,9
Prolonged QT syndrome can present as seizures10 and sudden death may occur.11 Prolongation of QT may occur during seizures but this association was not seen in patients who subsequently succumbed to SUDEP.12
Centrally mediated respiratory failure has also been proposed as a mechanism for SUDEP.13 Pulmonary oedema was found in the majority of SUDEP patients in a population study of 44 cases in Denver.14 In a sheep model of SUDEP, apnoea was associated with pulmonary oedema in animals that died of SUDEP but not in those that survived.15 In a series of 335 SUDEP cases, 15 deaths were witnessed. They too suggested that the mechanism of death is likely to be central apnoea. However, there were no EEG data available in these cases.
Bird et al described a case report that demonstrated flattening of the EEG, first on the right hemisphere and then bilaterally. Pulse artefacts continued for a further 120 s, suggesting that the primary cause of death was not cardiac.3 Lee reported a similar case in 1998.4 This case too showed sudden electrical silence following a seizure. A case of near SUDEP showed postictal central apnoea followed by cardiac arrest (successfully resuscitated).16 They suggested centrally mediated apnoea as a cause of SUDEP. However, the exact timing of apnoea to EEG changes that followed the flattening was not stated in the paper.
The patient described in this paper died during a seizure and the ambulatory EEG showed a sudden change in electrical activity to a flat line. Sudden cessation of all electrical activity of the brain during a seizure is a unique phenomenon. Abrupt flattening of the EEG does not occur in cerebral ischaemia or hypotension. Electrophysiological sequelae of ischaemia and hypotension is well described in textbooks of electroencephalography: “A sequence of electrical events can clearly be demonstrated in complete cerebral ischaemia due to cardiac arrest, excessive hypotension or mechanical interruption of cerebral blood flow. During the first 3–6 s after the arrest of circulation, no clinical or electrical EEG changes can be observed. When the arrest lasts about 3–13 s, slow waves of increasing amplitude and decreasing frequency appear. If the arrest of circulation is prolonged the attenuation of activity and flattening of the EEG occurs”.17
It is of interest that all cases of SUDEP captured on EEG (Bird and colleagues3 and Lee4) were associated with sudden flattening of the EEG prior to death. We propose that one of the causes of SUDEP is sudden irreversible “cerebral electrical shutdown” (CES) associated with a seizure. In other words, the death occurs secondary to primary brain failure. Cardiorespiratory failure that leads to death, therefore, is mediated centrally by CES.
It is possible that primary brain failure is due to seizure related neuronal injury. A recent comprehensive study using HSP‐70 and c‐JUN immunohistochemistry showed markers of neuronal injury in patients with SUDEP but not in controls.18
Timely intervention (attempts at resuscitation) at early stages may reverse the consequence of CES, as in the case of the report of So and colleagues.16
Abbreviations
CES - cerebral electrical shutdown
SUDEP - sudden death in epilepsy
Footnotes
Competing interests: None.
References
- 1.Nashef L, Brown S. Epilepsy and sudden death. Lancet 19963481324–1325. [DOI] [PubMed] [Google Scholar]
- 2.Nashef L, Sander J W A S. Sudden unexpected deaths in epilepsy—where are we now ? Seizure 19965225–238. [DOI] [PubMed] [Google Scholar]
- 3.Bird J M, Dembny K A T, Sandeman D.et al Sudden unexplained death in epilepsy: an intracranially monitored case. Epilepsia 199738S52–S56.9092961 [Google Scholar]
- 4.Lee M A. EEG video recording of sudden unexpected death in epilepsy (SUDEP). Epilepsia 199839(Suppl 6)123 [Google Scholar]
- 5.Blumhardt L D, Smith P E M, Owen L. Electrographic accompaniments of temporal lobe epileptic seizures. Lancet 198611051–1056. [DOI] [PubMed] [Google Scholar]
- 6.Nei M, Ho R T, Abou‐Khalil B W.et al EEG and ECG in sudden unexpected death in epilepsy. Epilepsia 200445338–345. [DOI] [PubMed] [Google Scholar]
- 7.Nashef L, Walker F, Allen P.et al Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 199660297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iani C, Colicchoi G, Attanasio A.et al Cardiogenic syncope in temporal lobe epileptic seizures. J Neurol Neurosurg Psychiatry 199763259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott C, Fish D, Butterworth R. Cardiac systole in association with partial seizures. Proceedings of the British Society for Clinical Neurophysiology, London, UK, 5 October 1999
- 10.Ballardie F W, Murphy R P, Davis J. Epilepsy: a presentation of the Romano–Ward Syndrome. BMJ 1983287896–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacia S V, Devinsky O, Luciano D J.et al The prolonged QT syndrome presenting as epilepsy: a report of two cases and literature review. Neurology 1994441408–1410. [DOI] [PubMed] [Google Scholar]
- 12.Tavernor S J, Brown S W, Tavernor R M E.et al Electrocardiograph QT lengthening associated with epileptiform EEG discharges—a role in sudden unexplained death in epilepsy ? Seizure 1996579–83. [DOI] [PubMed] [Google Scholar]
- 13.Terrence C F, Rao G R, Perper J A. Neurogenic pulmonary oedema in unexpected, unexplained death of epileptic patients. Ann Neurol 19819458–464. [DOI] [PubMed] [Google Scholar]
- 14.Earnest M P, Thomas G E, Eden R A.et al The sudden unexplained death syndrome in epilepsy: demographic, clinical and post mortem features. Epilepsia 199233310–316. [DOI] [PubMed] [Google Scholar]
- 15.Johnston S C, Horn J K, Valente J.et al The role of hypoventilation in a sheep model of epileptic sudden death. Ann Neurol 199537531–537. [DOI] [PubMed] [Google Scholar]
- 16.So E L, Sam M C, Lagerlund T L. Post control apnoea as a cause of SUDEP: Evidence from NEAR‐SUDEP Incident. Epilepsia 2000411494–1497. [DOI] [PubMed] [Google Scholar]
- 17.Neidemeyer E, Da Silva F L.Electroencephalograpy, basic principles, clincial applications and relateid fields. 2nd Edn. Baltimore: Urban and Schanarzenberg, 1987;385
- 18.Thom M, Seetah S, Sirodiya S.et al Sudden and unexpected death in epilepsy (SUDEP): evidence of acute neuronal injury using HSP‐70 and c‐JUN immunohistochemistry. Neuropathol Appl Neurobiol 200329123–143. [DOI] [PubMed] [Google Scholar]