Abstract
Background and purpose
Thrombolysis of acute ischaemic stroke is based strictly on body weight to ensure efficacy and to prevent bleeding complications. Many candidate stroke patients are unable to communicate their body weight, and there is often neither the means nor the time to weigh the patient. Instead, weight is estimated visually by the attending physician, but this is known to be inaccurate.
Methods
Based on a large general population sample of nearly 7000 subjects, we constructed approximation formulae for estimating body weight from simple anthropometric measurements (body height, and waist and hip circumference). These formulae were validated in a sample of 178 consecutive inpatients admitted to our stroke unit, and their accuracy was compared with the best visual estimation of two experienced physicians.
Results
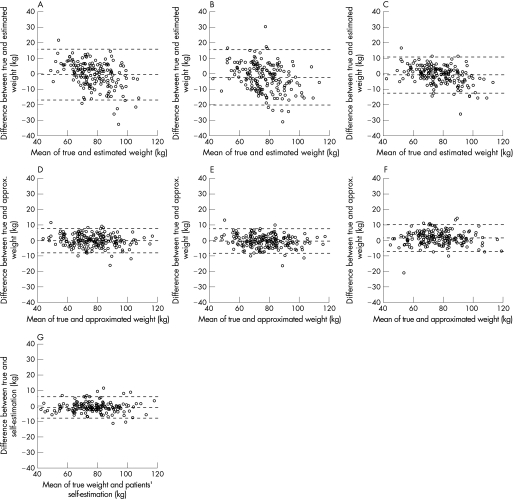
The simplest formula gave the most accurate approximation (mean absolute difference 3.1 (2.6) kg), which was considerably better than the best visual estimation (physician 1: 6.5 (5.2) kg; physician 2: 7.4 (5.7) kg). It reduced the proportion of weight approximations mismatched by >10% from 31.5% and 40.4% (physicians 1 and 2, respectively) to 6.2% (anthropometric approximation). Only the patient's own estimation was more accurate (mean absolute difference 2.7 (2.4) kg).
Conclusions
By using an approximation formula based on simple anthropometric measurements (body height, and waist and hip circumference), it is possible to obtain a quick and accurate approximation of body weight. In situations where the exact weight of unresponsive patients cannot be ascertained quickly, we recommend using this approximation method rather than visual estimation.
In acute medical care, numerous pharmacological therapies require the correct dosage and rely on knowledge of the patient's exact body weight. Many emergency patients are unable to communicate their body weight. Thrombolysis in acute stroke and the application of recombinant factor VII in spontaneous intracerebral haemorrhage are two examples where the safety and effectiveness of the treatment depends on both early application and exact dosage.1,2,3,4,5 Very often, the attending physician has neither the time nor the means of weighing the patient.6,7 Consequently, he/she has to make a visual estimation. The magnitude of this problem is illustrated by the fact that during the ECASS II trial, one of the largest trials on systemic thrombolysis in acute stroke, only a minority of patients were weighed.2
We sought an alternative approximation method for accurately assessing a patient's body weight. Such a method needs to be accurate and quick, and should preferably use simple measurement tools. One approach is the approximation of body weight based on a combination of simple anthropometric measurements. The aim of this study was to find a quick, safe and accurate anthropometric method for approximating the body weight of emergency patients.
Methods
Generation of approximation models
The dataset of the Carotid Atherosclerosis Progression Study (CAPS)8 was used to generate approximation formulae for estimating body weight. CAPS comprises a total of 6962 male and female subjects, aged 19–90 years, and resembles general population samples. During the baseline visit, the subject's body height, waist circumference and hip circumference were determined in the standing position. Body height was determined with a stadiometer, while waist circumference (umbilical level) and hip circumference (trochanter level) were established with a tape measure. The distribution of body weight was close to the normal distribution, as assessed with a QQ plot. The premises of linear regression models were assessed with residual plots, as appropriate. Three formulae were constructed with least square linear regression models, using body weight as the dependent variable: (1) a model with the parameters body height, waist and hip circumference; (2) a model with age, body height, waist and hip circumference; and (3) a model with all of the parameters of model (2) in addition to their transformations (square, cubic, reciprocal, square root, logarithmic, exponential). To optimise model (3), we used an inclusion algorithm based on a stepwise and blockwise approach, with p<0.05 and p>0.10 as the inclusion and exclusion criterion, respectively. For each model, two separate formulae were created, one for males and one for females. The resulting formulae and the respective models are listed in the appendix.
Validation study
To validate the formulae, we prospectively included 178 consecutive inpatients admitted to the stroke unit of our institution between March and September 2006. The inclusion criterion was an admission diagnosis of “stroke” and the exclusion criteria were gravidity, lactation, amputated limb(s) and any condition that prohibited the patients from lying horizontally on their back. Age, sex, diagnosis, mobility and modified Rankin Scale were documented for every patient. Once written informed consent had been obtained from the patient or their proxy (if the patient was unable to communicate), the patient's body weight was estimated visually by two physicians independently.
Visual estimation of body weight
For every patient in the validation sample, two physicians were recruited out of a pool of five (MH, PJ, CS, SS, FT), who had at least 2 years' experience working with acute neurological patients and thrombolysis. The coordinating physician (the last author) chose the estimators according to their availability and aimed to ensure even assignment. None of the five estimators were on the stroke unit team, and if any of them knew the patient, they were excluded from making an estimation. The two physicians were asked to visually estimate, without physical contact, the body weight of the patient, who was supine on a hospital bed and wearing light clothing. Other estimations (including the patient's), actual body weight or anthropometric measurements (see below) were not disclosed to the estimators at any point during the study. To increase motivation, we prospectively donated a prize of €750 for the physician with the least mean absolute difference from the actual body weight at the end of the study and €250 for the runner‐up. To avoid conflicts of interest, none of the estimating physicians are on the author list.
Anthropometric measurements and weighing
Within 4 h of the patient's body weight being estimated, simple anthropometric measurements (body height, and waist and hip circumference) were taken in the supine position with a tape measure. We used the same anatomical landmarks as those used in the CAPS cohort (see above).
In a small series of healthy volunteers (n = 20), we determined the reproducibility of the anthropometric measurements: intraclass correlation coefficients (ICC) were 0.9978 for body height (95% CI 0.9954 to 0.9991), 0.9891 (0.9771 to 0.9953) for waist circumference and 0.9921 (0.9836 to 0.9966) for hip circumference.
All patients who were able to stand were weighed in the standing position on a standard set of calibrated scales (Seca 710; Seca, Hamburg, Germany; recently calibrated by the Hessian Bureau of Standards). Bedridden or wheelchair bound patients were weighed on a bed scales construction designed for this study. We placed large boxes under the four bedposts of a hydraulic hospital bed (S 960‐2; Voelker, Witten, Germany) and lowered the bed and the patient onto four flat digital bathroom scales (Nando 62836, Soehnle, Nassau, Germany) placed on these boxes until the bed wheels lost ground contact and the bed was supported only by the scales. The weight displayed on the four scales was documented and added together to determine the total weight of the bed and the patient. The weight of the empty bed had been ascertained beforehand and was subtracted from the total weight to establish the patient's body weight. We validated this construction in a small series of healthy volunteers (n = 20, weight range 48–107 kg). The ICC was 0.9986 (0.9959 to 0.9995) between repeated measurements, the mean (SD) absolute deviation of the bed scales from the calibrated standing scales was 0.68 (0.96) kg.
All anthropometric measurements and weighings were carried out by one person (MG) who did not disclose any of the data until the end of the study.
The study was approved by the ethics review committee of the Johann Wolfgang Goethe University, Frankfurt, Germany.
Statistical analyses
ICCs for the single rater were calculated with two way mixed effects models. To display the accuracy of the different approximation methods, we used Bland and Altman plots.9 All statistical calculations were made using SPSS (SPSS Inc., Cary, USA).
Results
The validation sample comprised 178 patients with a mean age of 67.3 (15.6) years; 90 (50.6%) were male. The final diagnosis was transient ischaemic attack in 37 patients (20.8%), ischaemic stroke in 111 patients (62.4%) and haemorrhagic stroke in 13 patients (7.3%). Seventeen patients (9.6%) suffered “stroke mimics” linked to other neurological diseases. Mean body weight was 77.2 (14.5) kg. Median modified Rankin Scale was 2 (range 0–5). Thrombolysis had been applied to 11 patients (6.2%). A total of 138 patients (77.5%) were able to stand and were weighed on the stand‐up scales; the remainder were weighed using the bed scales construction (see methods). One hundred and fifty‐one patients (84.8%) were able to give their own estimation of their body weight. In four patients, only one physician estimated their body weight because of organisational difficulties. The mean time required to make the anthropometric measurements was 99.5 (36.7) s (range 50–190).
Accuracy
The accuracy of the physicians' estimations, anthropometric approximations and the patients' own estimations are shown in table 1 and fig 1. The patient's own estimation was the most accurate and the physician's estimation the least accurate approximation. The second best approximation was given by model 1. Other measures of accuracy were calculated to enable comparison with other studies (see discussion). When the approximated body weight was used for thrombolysis in acute stroke, the resulting recombinant tissue plasminogen activator (rt‐PA) dose was compared with the correct dose. The rt‐PA dosage mismatch is shown in table 2. Following the rt‐PA criterion (with an upper dose limit of 90 mg), model 2 gave the best approximation, but model 1 was almost as accurate. Interestingly, for the purposes of determining the required rt‐PA dose, the approximation models were more accurate than the patient's own estimation.
Table 1 Properties and accuracy of the estimating physicians.
| Physician | Sex | Length of service as physician (y) | No of estimations | Absolute of difference between estimation and actual weight (mean (SD)) | Proportion of patients with >10% mismatch of weight approximation (%) |
|---|---|---|---|---|---|
| MH | M | 2.5 | 75 | 7.49 (5.60) | 42.7 |
| PJ | M | 4.0 | 68 | 5.67 (3.94) | 26.5 |
| CS | F | 4.5 | 68 | 6.31 (5.52) | 27.9 |
| SS | M | 8.0 | 72 | 8.70 (6.55) | 50.0 |
| FT | M | 7.5 | 69 | 6.49 (5.14) | 33.3 |
Figure 1 Bland and Altman plots for comparison of different approximations with actual body weight. (A) Estimation physician 1. (B) Estimation physician 2. (C) Best estimation of two physicians. (D) Anthropometric approximation, linear formula with three parameters and gender (model 1). (E) Anthropometric approximation, linear formula with four parameters and gender (model 2). (F) Anthropometric approximation, formula with four parameters and gender, with inclusion of transformed parameters (model 3). (G) Patients' self‐estimation.
Table 2 Accuracy of different approximations.
| Absolute difference between approximation and actual weight | Mean (SD) (kg) | Mean (SD) (%) | Proportion of patients with >10% mismatch of weight approximation (%) | Proportion of patients with ⩾5 mg hypothetical rt‐PA dosage failure (%) |
|---|---|---|---|---|
| Estimation physician 1 | 6.49 (5.25) | 8.6 (6.9) | 32.0 | 48.3 |
| Estimation physician 2 | 7.43 (5.73) | 9.7 (7.7) | 40.7 | 53.9 |
| Best estimation of two physicians | 4.54 (3.88) | 6.0 (5.1) | 15.2 | 28.1 |
| Anthropometric approximation, linear formula with 3 parameters plus gender (model 1) | 3.11 (2.60) | 4.2 (3.7) | 6.2 | 14.6 |
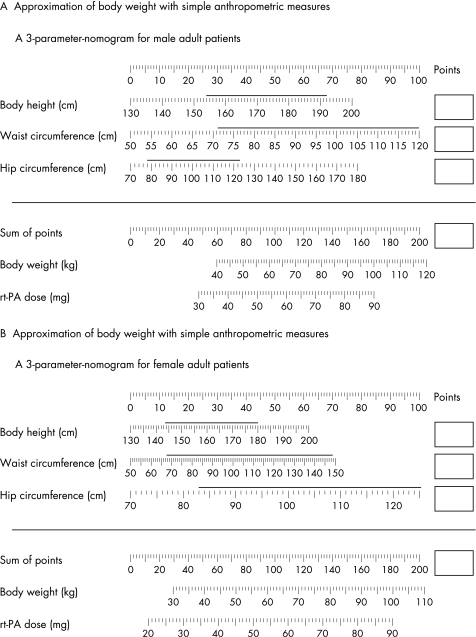
| Anthropometric approximation, linear formula with 3 parameters plus gender (model 1), calculated with the nomogram (fig 2) | 3.11 (2.69) | 4.2 (3.6) | 7.3 | 14.6 |
| Anthropometric approximation, linear formula with 4 parameters plus gender (model 2) | 3.19 (2.57) | 4.3 (3.8) | 6.7 | 14.0 |
| Anthropometric approximation, formula with 4 parameters plus gender with inclusion of transformed parameters (model 3) | 3.73 (3.08) | 5.0 (4.8) | 7.3 | 20.8 |
| Patient's own estimation | 2.65 (2.37) | 3.4 (3.0) | 3.4 | 23.6 |
rt‐PA, recombinant tissue plasminogen activator.
Calculation tools
As the best and simplest of the models (model 1, see appendix) is still too complex to calculate manually, we developed two tools for obtaining quick and precise calculations. We programmed a JavaScript calculator, which is available online (http://www.kgu.de/znn/neurologie/bw/), and designed a nomogram (see fig 2). Strictly speaking, the formulae are only validated for the range of input variables given by the distribution of the validation sample (see appendix). The JavaScript calculator automatically displays a warning when the validated range is exceeded, and in the nomogram, the validated range is marked with a horizontal line over the scale. The nomogram does not cover the entire validated range of waist and hip circumference, as it was optimised for maximal accuracy in the range of body weights relevant for systemic thrombolysis, where the dosage is limited at a body weight of 100 kg. As calculation using a nomogram introduces a new source of inaccuracy, we validated the nomogram calculations. In a subsample of 30 patients, the ICC between raters was 0.9198 (0.8386 to 0.9610). The accuracy of the nomogram calculations is also shown in table 2. The mean time for calculation using the nomogram was 56.7 (15.3) s (range 37–113).
Figure 2 Nomograms for approximation of body weight and recombinant tissue plasminogen activator (rt‐PA) dose for acute stroke. (A) Nomogram for male patients. (B) Nonogram for female patients. How to use: identify the patient's body height, and waist and hip circumference on the respective scales; read the number of points given from the topmost scale and note in the box to the right for all three parameters; the sum of the points is calculated and identified in the topmost scale of the lower part of the nomogram; the corresponding body weight and rt‐PA dose can be read on the scales below. The validated range of input variables is marked with a horizontal line over the scale.
Discussion
A considerable number of emergency drugs have a low therapeutic range and require knowledge of the patient's exact body weight in order for the correct dosage to be established.3,10,11 Thrombolysis is a standard therapy that is increasingly applied in acute ischaemic stroke, and is based strictly on exact body weight. Overdosage may lead to toxicity (eg, an increased rate of bleeding complications, such as intracranial haemorrhage10) and underdosage may result in loss of efficacy.
In emergency stroke care, there is often neither the time nor the means to weigh the patient. To weigh a bedridden patient, either he/she must be lifted onto a special weigh bed, or a weighbridge can be used provided the empty bed or trolley is weighed beforehand or afterwards. These procedures take up time that is valuable in acute stroke. In many accident and emergency departments, and even in intensive care units, there is no access to bed scales for weighing supine patients.6,7 During the ECASS II trial, exact body weights were determined in only a minority of cases.2 In many situations, especially with unresponsive patients, visual estimation is the unofficial standard to determine body weight.
The inaccuracy of visual estimation of body weight in adults has been illustrated in several publications.6,7,11,12,13,14,15 Martin and colleagues13 retrospectively compared the body weight of 133 patients after cardiac arrest with visual estimations of paramedics. These estimations differed from actual weight by more than 10% in 26% of cases. Coe and colleagues12 compared visual estimations of experienced physicians with actual weight of 38 patients lying covered on trolleys. The exact number of outliers is not given in the text of the publication, but we can read from the figures that the number of estimations with more than a 10% error ranged from 29% to 45% for the four estimating physicians. Fernandes and colleagues15 weighed 177 adult patients in an emergency department, had their weight estimated visually by nurses and physicians and asked the patients for their own estimation. The nurses' and physicians' estimations both had an error of >10% in 34% of cases, the patients' own estimations differed by >10% in only 3% of cases. Leary and colleagues7 asked experienced ICU physicians and nurses to estimate the body weight of 30 healthy volunteers in the supine position. They too did not give the number of outliers, but we can read from the scatterplots that the proportion of estimations with a more than 10% error ranged from 23% to 40% for four estimators. Sanchez14 included 255 adult emergency patients in their study, where a physician, nurse and the patients themselves were asked to estimate body weight. The error rate of >10% is not given in the report, but the mean absolute error was 11.5% for the physician, 11.1% for the nurse and 2.8% for the patient. Cubison and Gilbert6 retrospectively compared the estimated weight (accident and emergency department) with the exact weight (weighbridge) of 32 patients with severe burns (31 adults, one child). Again, the error rates are not given in the text, but the individual weights can be reconstructed from a figure. Assuming the display in the figure is accurate, 43% of the estimated weights documented in the accident and emergency department deviated from the actual weight by more than 10%. Menon and Kelly11 had the largest sample, comprising 1137 patients, who gave their own estimations, were estimated visually in the standing position by nurses and physicians and weighed on a stand‐up scale. The nurses' estimations were >10% inaccurate in 22% of cases and the physicians' in 41%. The patients' own estimations deviated from the actual weight by more than 10% in 9% of cases.
The accuracy of our physicians (>10% deviation in 32.0–40.7% in individual patients, 26.5–50.0% for individual physicians) was in the same range as that of the professionals in the literature reviewed above (22–45%). The patients' own estimations (>10% deviation in 3.4%) were within the range specified in the three corresponding publications (2.8–9%).11,14,15 Hence the accuracy of the visual estimation of body weight in stroke patients was equally as poor as in other samples.
The idea of anthropometric approximation of body weight is not new. The approach is common in paediatric emergency medicine where body height is used mostly as the only anthropometric variable.16,17,18,19 Such a simple form of approximation has the advantage that the approximated body weight (or drug doses) can be printed on a tape measure, which saves time in calculation. In adults, formulae based on body height alone give a crude approximation, even when stratified for obesity status.20 Chumlea and colleagues21 developed approximation formulae using arm circumference, calf circumference, subscapular skinfold thickness and knee height. In a clinical validation sample, the mean (SD) signed differences between predicted and measured weight were −4.3 (3.9) kg for men and 5.1 (8.3) kg for females. Our best formula (model 1) resulted in mean signed differences of 0.7 (3.9) kg for males and −0.5 (4.2) kg for females. Atiea and colleagues22 used a sample of 211 elderly patients to develop optimal formulae for approximating body weight with anthropometric measurements. They used 19 candidate variables (skinfold thickness at 10 body sites, circumference at six areas, length of extremities) to find the best predictive models with only two parameters for both sexes. They found the best models included arm circumference and chest girth for males and thigh circumference and waist skinfold thickness for females. These results were then validated in a second cohort. The accuracy of these models was given as percentages of deviation: there was a deviation of more than 5 kg in 37.5% of female patients but in only 5% of male patients. For our formulae (model 1), the percentage deviations were 21.1% and 20.4%, respectively.
Previous approaches to anthropometric approximations have several disadvantages in terms of their use in emergency situations: Chumlea's21 formulae require two different callipers to accurately measure skinfold thickness and knee height. Atiea's22 method uses only a tape measure for men, but also needs a skinfold calliper for women. These approximations were not developed and optimised for use in emergency situations, but rather for a geriatric setting. Our method relies on tape measurements throughout and gives more accurate approximations. With the calculation tools we offer, measurements and calculations take a mean time of 2.5 (maximum 5) min. Using our internet calculator may make the calculation time even shorter.
Limitations
One limitation of our study was that in the construction sample (CAPS), all anthropometric measurements were taken in standing probands, while in the validation study they were assessed in supine subjects. It has already been shown that when measured in the supine position, body height is greater than when measured in the standing position.23 However, despite this source of measurement error, the approximation was still proven to be highly accurate.
Another potential drawback is the fact that, for practical reasons, we did not use a certified weighbridge to weigh bedridden patients, but instead constructed our own. Our validation data showed, however, that this construction sufficiently met the quality criteria. Moreover, the clinical validation of our formulae was based on a cohort where the majority (77.5%) were able to use the certified stand‐up scales.
Conclusion
Visual estimation of body weight in acute stroke patients is prone to relevant error. If it is possible without great loss of time, the patient should always be weighed. The patients' own estimation is the second best approximation, if available. Before visual estimation is attempted, we recommend using our approximation method, which can be done with a tape measure in a very short time.
Appendix
Table A1 provides a description of the models on which the best approximation formulae were based. Table A2 gives the validated range of input variables.
Table A1 Description of the models on which the best approximation formulae are based.
| Model | Explanatory variable | Coefficient (B) | 95% CI | R2 |
|---|---|---|---|---|
| 1 (males) | Constant | −137.432 | [−141.497; −133.366] | 0.846 |
| Body height (cm) | 0.60035 | [0.57856; 0.62213] | ||
| Waist circumference (cm) | 0.785 | [0.765; 0.805] | ||
| Hip circumference (cm) | 0.392 | [0.367; 0.418] | ||
| 1 (females) | Constant | −110.924 | [−115.565; −106.283] | 0.815 |
| Body height (cm) | 0.4053 | [0.37851; 0.43208] | ||
| Waist circumference (cm) | 0.325 | [0.309; 0.342] | ||
| Hip circumference (cm) | 0.836 | [0.810; 0.863] |
Complete information on the other models can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental.
Table A2 Validated range of input variables.
| Male patients | Female patients | |
|---|---|---|
| Body height (cm) | 154–192 | 144–180 |
| Waist circumference (cm) | 71–128 | 68–148 |
| Hip circumference (cm) | 78–123 | 83–132 |
Complete information on the models, others than those in the appendix, can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental.
Copyright © 2007 BMJ Publishing Group Ltd
Supplementary Material
Acknowledgements
We thank the patients included in this study for their patience and cooperation. We thank Marek Humpich, Carola Seifried, Patrick Jung, Frank Trostdorf and Sebastian von Stuckrad‐Barre for their willing participation and their best visual estimations.
Abbreviations
CAPS - Carotid Atherosclerosis Progression Study
ICC - intraclass correlation coefficients
rt‐PA - recombinant tissue plasminogen activator
Footnotes
Competing interests: None.
Complete information on the models, others than those in the appendix, can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental.
References
- 1.Hacke W, Kaste M, Fieschi C.et al for the ECASS study group. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 19952741017–1025. [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Fieschi C.et al Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet 19983521245–1251. [DOI] [PubMed] [Google Scholar]
- 3.Mayer S A, Brun N C, Begtrup K.et al Recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med 2005352777–785. [DOI] [PubMed] [Google Scholar]
- 4.Saver J L. Time is brain—quantified. Stroke 200637263–266. [DOI] [PubMed] [Google Scholar]
- 5.Davis S M, Broderick J, Hennerici M.et al Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006661175–1181. [DOI] [PubMed] [Google Scholar]
- 6.Cubison T C, Gilbert P M. So much for percentage, but what about the weight? Emerg Med J 200522643–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leary T S, Milner Q J, Niblett D J. The accuracy of the estimation of body weight and height in the intensive care unit. Eur J Anaesthesiol 200017698–703. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz M W, von Kegler S, Steinmetz H.et al Carotid intima‐media thickening indicates a higher vascular risk across a wide age range: Prospective data from the Carotid Atherosclerosis Progression study (CAPS). Stroke 20063787–92. [DOI] [PubMed] [Google Scholar]
- 9.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 10.Gibson C M, Marble S J. Issues in the assessment of the safety and efficacy of tenecteplase (tnk‐tpa). Clin Cardiol 200124577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon S, Kelly A M. How accurate is weight estimation in the emergency department? Emerg Med Australas 200517113–116. [DOI] [PubMed] [Google Scholar]
- 12.Coe T R, Halkes M, Houghton K.et al The accuracy of visual estimation of weight and height in pre‐operative supine patients. Anaesthesia 199954582–586. [DOI] [PubMed] [Google Scholar]
- 13.Martin D R, Soria D M, Brown C G.et al Agreement between paramedic‐estimated weights and subsequent hospital measurements in adults with out‐of‐hospital cardiac arrest. Prehospital Disaster Med 1994954–56. [PubMed] [Google Scholar]
- 14.Sanchez L D I J S N. Weight estimation by emergency department personnel. Acad Emerg Med 200411546 [Google Scholar]
- 15.Fernandes C M, Clark S, Price A.et al How accurately do we estimate patients' weight in emergency departments? Can Fam Physician 1999452373–2376. [PMC free article] [PubMed] [Google Scholar]
- 16.Garland J S, Kishaba R G, Nelson D B.et al A rapid and accurate method of estimating body weight. Am J Emerg Med 19864390–393. [DOI] [PubMed] [Google Scholar]
- 17.Hughes G, Spoudeas H, Kovar I Z.et al Tape measure to aid prescription in paediatric resuscitation. Arch Emerg Med 1990721–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubitz D S, Seidel J S, Chameides L.et al A rapid method for estimating weight and resuscitation drug dosages from length in the pediatric age group. Ann Emerg Med 198817576–581. [DOI] [PubMed] [Google Scholar]
- 19.Molyneux E, Brogan R, Mitchell G.et al Children's weights: Guess or measure by tape? Lancet 19993541616. [DOI] [PubMed] [Google Scholar]
- 20.Lee S W, Park G H, Lee S Y.et al Who class‐specific equations using height for predicting body weight: Crude indicator for dry weight in haemodialysis patients. Nephrology (Carlton) 200510446–452. [DOI] [PubMed] [Google Scholar]
- 21.Chumlea W C, Guo S, Roche A F.et al Prediction of body weight for the nonambulatory elderly from anthropometry. J Am Diet Assoc 198888564–568. [PubMed] [Google Scholar]
- 22.Atiea J A, Haboubi N Y, Hudson P R.et al Body weight estimation of elderly patients by nomogram. J Am Geriatr Soc 199442763–765. [DOI] [PubMed] [Google Scholar]
- 23.Gray D S, Crider J B, Kelley C.et al Accuracy of recumbent height measurement. JPEN J Parenter Enteral Nutr 19859712–715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.