Abstract
Aim
The clinical and epidemiological characteristics of chronic inflammatory demyelinating polyneuropathy (CIDP) in an Italian population were assessed.
Subjects and methods
All subjects with a diagnosis of demyelinating neuropathy after 1990 in Piemonte and Valle d'Aosta (4 334 225 inhabitants) were considered. The diagnosis of CIDP was based on the research criteria of the American Academy of Neurology. 165 of 294 patients met the diagnostic criteria.
Results
The crude prevalence rate was 3.58/100 000 population (95% CI 3.02 to 4.20). At the prevalence day, 76 (49.0%) cases had definite, 67 (43.2%) probable and 12 (7.7%) possible CIDP; disability was mild in 105 (67.7%) cases, moderate in 32 (20.6%) and severe in 18 (11.6%). The course was remitting–relapsing in 40 cases (25.8%), chronic progressive in 96 (61.9%) and monophasic in 19 (12.3%). Considering the 95 patients whose disorder presented in the period 1995–2001, the mean annual crude incidence rate was 0.36/100 000 population (95% CI 0.29 to 0.44), with a male to female ratio of 2.3:1. 14 cases were affected by diabetes mellitus. In multivariate analysis, factors related to severe disability at the prevalence day were: age>60 years; failure of immunomodulating therapies at the time of diagnosis; worse disability at nadir; and chronic course.
Conclusion
Incidence and prevalence rates of CIDP in Italy were higher than those observed in most previous studies. At the prevalence day, more than 80% of cases had a mild or moderate disability, indicating either a good response to immunomodulating therapy or a tendency of CIDP to have a mild course in most cases.
Chronic inflammatory demyelinating polyneuropathy (CIDP) was identified as a separate disease entity, different from Guillain–Barré syndrome (GBS), in 1975.1 In contrast with GBS, CIDP is a chronic, demyelinating, mainly distal polyneuropathy, with clinical progression over 2 months, and marked responsiveness to steroids and immunomodulating therapies.2 According to the Ad Hoc Subcommittee of the American Academy of Neurology (AHSAAN),3 the diagnosis of CIDP is based on the presence of explicit clinical features, associated with a demyelinating pattern at EMG, and may be confirmed by nerve biopsy. The pathogenesis of CIDP is unknown, but immunomediated inflammatory mechanisms have been postulated, with the involvement of macrophages, T lymphocytes, cytokines, oxygen reactive species and proteases.4,5,6,7,8,9 The epidemiological characteristics of CIDP are still unclear as only very few studies have been published.10,11,12,13
The aim of the study was to assess the clinical and epidemiological characteristics of CIDP in a large population, using a register methodology, and to compare the findings with those of an epidemiological study on GBS performed in the same area and with a similar case finding methodology.14
Methods
Study area
The study was conducted in the Piemonte and Valle d'Aosta regions in northern Italy. On the prevalence day, 31 December 2001, the total population was 4 334 225.
Case finding
Several concurrent sources were used for identifying patients affected by chronic demyelinating neuropathies: (a) archives of the 28 neurology departments of Piemonte and Valle d'Aosta from 1 January 1990; these departments cover all neurology departments in the area; (b) Regional Centralised Archive, using the following International Classification of Diseases, 9th revision, codes: 357.0 (acute infective polyneuritis), 357.8 (other polyneuritis, including chronic inflammatory demyelinating polyneuritis) and 357.9 (other, not specified, polyneuritis); and (c) archives of the neurophysiology laboratories of the two regions.
For all cases, a standardised record form was used, including first and family name, demographic data, information related to the disorder (symptom onset, antecedent events, exposure to toxic substances, type of course, comorbidities, treatments) and examinations performed, with particular attention to neurophysiological assessment, CSF examination and sural nerve biopsy. We considered as antecedent events those potentially related to the disorder and which occurred no more than 1 month before the onset of symptoms, as registered in the clinical charts of the patients. Clinical status at nadir and at the prevalence day was defined using the Modified Rankin Scale (MRS)15: 0, asymptomatic; 1, non‐disabling symptoms that do not interfere with daily activities; 2, slight disability (unable to carry out all activities, but still able to look after themselves); 3, moderate disability (requiring assistance with some activities but still able to walk without assistance); 4, moderate severe disability (unable to walk without assistance); and 5, severe disability (totally dependent, requiring constant care). Electrodiagnostically, demyelination was defined according to AHSAAN criteria3 (presence of any three of the following four criteria: partial conduction block in at least one motor nerve; reduced conduction velocity in at least two motor nerves; prolonged distal latency in at least two motor nerves; absence of F waves or prolonged F wave latency in at least two motor nerves).
Diagnosis confirmation and classification
The diagnosis of CIDP was based on the diagnostic criteria of AHSAAN.3 The clinical records of each potential case were examined by the Scientific Committee (see appendix) to verify if they met the criteria. Axonal neuropathies, as well as toxic, metabolic (with the exception of diabetes mellitus), hereditary, vasculitic and paraneoplastic neuropathies were excluded. Moreover, HIV associated neuropathies were also excluded, as well as those with paraproteinaemias detected by immunoelectrophoresis of serum proteins. For all patients less than 20 years of age, Charcot–Marie–Tooth type 1 and X linked dominant Charcot–Marie–Tooth were excluded by genetic analysis.
According to AHSAAN criteria, cases were classified as definite CIDP (fulfilling clinical, CSF, electrophysiological and biopsy criteria), probable CIDP (fulfilling clinical, CSF and all four electrophysiological criteria, but lacking nerve biopsy) and possible CIDP (fulfilling clinical, CSF and at least three of the four electrophysiological criteria, but lacking nerve biopsy). Therefore, patients who, even in presence of a formal diagnosis of CIDP, lacked enough clinical, neurophysiological, CSF and nerve biopsy information to reach at least the possible CIDP level, and those cases who did not have a demyelinating neuropathy, were excluded.
Classification of CIDP clinical course
Clinical course was classified as follows12: monophasic CIDP, when clinical criteria for CIDP where met and the disorder remained stable or improved for at least 6 months, without further relapses during follow‐up; relapsing–remitting CIDP, when at least two episodes of rapid worsening, demonstrable symptomatically or through clinical examination and with or without treatment, lasting more than 7 days, were observed and following a period of stability or improvement of at least 4 weeks; chronic progressive CIDP, when the disorder worsened steadily, showing no improvement with or without treatment up to the time of observation.
Statistical methods
We calculated 95% confidence intervals (CI) assuming a Poisson distribution.16 Age and gender standardisation of incidence and prevalence rates was performed using the direct method on the Italian 2001 census population. The Student's t test was used to compare means. Other comparisons were performed with Fisher's exact test. All test were two tailed. A p value <0.05 was considered significant. Factors related to long term prognosis were analysed using a forward stepwise multivariate logistic regression, considering as the outcome measure MRS and analysing all possible interactions between putative prognostic factors. Data were processed using the SAS statistical package (v 8.2).
Results
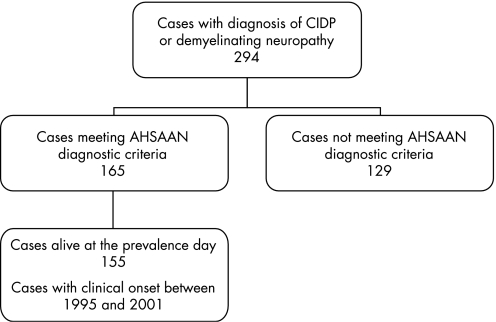
We identified a total of 294 patients with a diagnosis of CIDP or idiopathic demyelinating neuropathy. Of these, 165 (53 women and 112 men) met the AHSAAN diagnostic criteria. Of these patients, 155 (50 women and 105 men) were alive at the prevalence day. A flowchart of the diagnostic process is shown in fig 1. Reasons for exclusion of the other 129 patients were: absence of enough information to reach a diagnosis of possible CIDP (n = 86); presence of paraproteinaemia (n = 29); presence of malignancies (n = 12); and HIV associated neuropathy (n = 2). Mean age at onset was 59.6 (SD 14.8) years (range 4–93), with no significant differences between the sexes (men 58.8 (SD 15.2), women 61.8 (SD 14) years). Mean duration of follow‐up was 7.3 years (SD 4.3; median 6.5; range 2–18).
Figure 1 Flowchart of case finding. See text for details on cases who did not meet the diagnostic criteria. AHSAAN, Ad Hoc Subcommittee of the American Academy of Neurology; CIDP, chronic inflammatory demyelinating polyneuropathy.
Prevalence rate
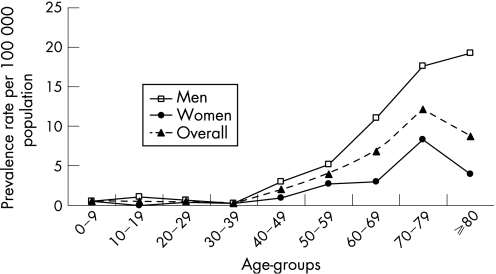
The crude prevalence rate of CIDP in Piemonte and Valle d'Aosta was 3.58/100 000 population (95% CI 3.02 to 4.20) and the standardised prevalence rate was 3.41/100 000 population (95% CI 2.92 to 3.98). Gender specific prevalence rates were 5.02/100 000 (95% CI 4.13 to 6.10) for men and 2.23/100 000 (95% CI 1.65 to 2.94) for women (p<0.001) (table 1). Age specific prevalence rates increased up to a peak in the 70–79 decade in women, whereas in men, prevalence rates progressively increased up to the 80+ age group (fig 2).
Table 1 Crude prevalence rates (per 100 000 population) by age group and gender.
| Age group (y) | Women | Men | Total | ||
|---|---|---|---|---|---|
| n | Rates (95% CI) | n | Rates (95% CI) | Rates (95% CI) | |
| 0–19 | 1 | 0.30 (0.01–1.67) | 4 | 0.82 (0.27–1.91) | 0.57 (0.22–1.24) |
| 20–39 | 2 | 0.34 (0.04–1.23) | 3 | 0.54 (0.15–1.38) | 0.43 (0.16–0.94) |
| 40–59 | 11 | 1.89 (0.94–3.38) | 24 | 4.20 (2.93–5.80) | 2.99 (2.19–3.98) |
| 60–79 | 30 | 5.58 (3.77–7.98) | 61 | 14.04 (11.31–17.27) | 9.45 (7.88–11.25) |
| 80⩾ | 6 | 4.03 (1.48–8.79) | 13 | 19.24 (11.58–30,01) | 8.78 (5.68–13.00) |
| Overall | 50 | 2.23 | 105 | 5.02 | 3.58 |
Figure 2 Crude prevalence rates (per 100 000 population) of chronic inflammatory demyelinating polyneuropathy in Piemonte and Valle d'Aosta by age and gender.
Incidence rate
Considering the 95 patients whose disorder presented in the 1995–2001 period, the mean annual crude incidence rate was 0.36/100 000 population (95% CI 0.29 to 0.44) and was higher among men (0.51 (95% CI 0.39 to 0.65)) than among women (0.22 (95% CI 0.15 to 0.31)) (p<0.0001). The mean annual standardised incidence rate was 0.34/100 000 population (95% CI 0.28 to 0.42).
Clinical characteristics
At the prevalence day, 76 cases (49.0%) had definite CIDP, 67 probable CIDP (43.2%) and 12 possible CIDP (7.7%). In 40 cases (25.8%), the course was remitting–relapsing, in 96 (61.9%) chronic progressive and in 19 (12.3%) monophasic (table 2). There was no significant relationship between the type of course and age, gender or presence of preceding events, although remitting–relapsing cases had an age of onset slightly earlier than other types (data not shown). Only nine (5.8%) subjects had signs of involvement of the cranial nerves. Twenty (12.9%) patients had involvement of the autonomic nervous system; the most common dysfunctions were orthostatic hypotension, sphincter disturbances and cardiac arrhythmias. Only 15 (9.7%) cases referred antecedent events within 1 month before the onset of CIDP (eight pharyngolaryngitis, seven influenza). Fourteen cases (9.0%) were also affected by diabetes mellitus.
Table 2 Frequency of types of clinical course of chronic inflammatory demyelinating polyneuropathy.
| Study area | Remitting– relapsing (%) | Monophasic (%) | Chronic (%) |
|---|---|---|---|
| New South Wales11 | 51 | – | 49* |
| SE England12† | 43 | 13 | 37 |
| Vest‐Agder, Norway13 | 33 | 6 | 61 |
| Piemonte, Italy | 26 | 12 | 62 |
*Includes all non‐remitting–relapsing forms.
†7% of cases were classified as relapsing Guillain–Barré syndrome.
At nadir, 77 patients (49.6%) had an MRS score of 1 or 2 (mild disability), 65 (41.9%) a score of 3 (moderate disability) and 12 (8.5%) a score of 4 or 5 (severe disability). At the prevalence day, 105 cases (67.7%) had a mild disability, 32 (20.6%) a moderate disability and 18 (11.6%) a severe disability. In multivariate analysis, factors related to severe disability at the prevalence day were: age>60 years (p = 0.0003); failure of immunomodulating therapies at the time of diagnosis (ie, none or only 1 point improvement in MRS) (p = 0.002); disability at nadir (p = 0.008); and a chronic course (p = 0.02).
Discussion
Assessment of the epidemiological characteristics of CIDP has various methodological problems: (1) as CIDP is a relatively rare disorder, it is necessary to evaluate large populations to obtain an adequate number of patients; (2) there is no diagnostic gold standard, and existing diagnostic criteria are relatively complex, not widely accepted and have never formally been validated; (3) the clinical boundaries of the disorder are not clear as several comorbidities are described whose pathogenetic significance is unclear (ie, diabetes mellitus, monoclonal gammopathy of undetermined significance, active chronic hepatitis, etc). These aspects may account for the large differences found in the published studies, concerning the prevalence rate and also the clinical characteristics of the disorder. Moreover, even though all available epidemiological studies have used AHSAAN diagnostic criteria, application of these criteria showed wide differences; for example, in one study patients who did not meet the EMG criteria for demyelinating neuropathy were included if they had a “positive opinion of an expert neurologist or neurophysiologist”.12
The prevalence rates reported in the various studies are compared in table 3. We could not standardise the prevalence rates to a common reference population as all studies but one did not report in detail the age and gender distributions of the study population. The prevalence rate in Piemonte and Valle d'Aosta was higher than those found by most studies,10,11,12 but lower than that reported in the study from Northern Norway.13 The mean annual incidence rate found in Piemonte and Valle d'Aosta was more than twofold that reported in New South Wales11 (0.15 vs 0.36/100 000 population). Interestingly, the incidence rate of CIDP in Piemonte and Valle d'Aosta was about a quarter of that found in the same area for GBS in the 2 year period 1995–1996 (1.36/100 000 population/year).14
Table 3 Crude prevalence rates for chronic inflammatory demyelinating polyneuropathy: comparison of the literature.
| Study area (ref) | Population | Prevalence rate (M+W) (95% CI) | Prevalence rate (M) (95% CI) | Prevalence rate (W) (95% CI) | Mean age at onset (y) |
|---|---|---|---|---|---|
| Japan | 614 725 | 0.8 (0.3–1.9) | 1.4 (0.4–3.6) | 0.3 (0.01–1.7) | NS |
| New South Wales, Australia | 5 995 544 | 1.9 (1.5–2.2) | 2.2 (1.7–2.8) | 1.6 (1.2–2.1) | 53.5 |
| SE England | 3 717 638 | 1.2 (0.9–1.7) | NR | NR | 54.4 |
| Vest‐Agder, Norway | 155 464 | 7.7 (3.2–12.2) | 14.7 (7.3–26.4) | 5.0 (1.4–12.8) | 48 |
| Piemonte, Italy | 4 334 225 | 3.6 (3.0–4.2) | 5.0 (4.1–6.1) | 2.2 (1.7–2.9) | 59.6 |
M, men; NR, not reported; W, women.
Prevalence rates are per 100 000 population.
In our series, the prevalence rate increased in the 70–79 year age group in women, similar to the Australian study,11 whereas a progressive increase up to the 80+ year age group was observed among men. The higher frequency of CIDP in the elderly has no satisfactory explanation.
In all epidemiological studies,10,11,12,13 and in clinical series,17,18 CIDP was found to be more frequent among men, similar to that observed in other dysimmune neuropathies, such as GBS and motor neuropathy with multiple conduction blocks.19 The cause of this finding remains unclear, in particular when considering the higher incidence of immune related disorders of the central nervous system in women, such as multiple sclerosis.
Chronic forms were the most common in our series and in the Norwegian study whereas remitting–relapsing forms were more common in the Australian and English studies.11,12,13 In general, in referral series, remitting–relapsing cases are more common. These differences may reflect either the diverse duration of follow‐up or a possible shift of patients from one type of course to another. Moreover, it is likely that in clinical referral series, there is selection bias of patients more responsive to therapy (ie, patients with a remitting–relapsing course). However, in all epidemiological series, monophasic CIDP accounted for approximately 10% of cases. We did not find any significant difference in age of onset according to the type of course; therefore, we cannot confirm the younger age of onset of CIDP with a relapsing course reported in clinical series.20
The frequency of autonomic dysfunction in our series was 12.9%, and higher among patients with more serious CIDP: 7/11 patients (64%) with a score of 4 or 5 on the Rankin Scale had signs of autonomic dysfunction. Autonomic involvement was less frequent in CIPD than in GBS in the same area (22.5%).14
At the prevalence day, the majority of cases had only a mild disability, indicating that most cases had a positive response to therapy or a mild disease course; the rate of patients with severe disability in our study (8.5%) was similar to that reported in a study on 38 cases followed‐up for 5 years in Japan (13%).21 In the present series, a higher residual disability was related to an older age at onset, a reduced response to therapy at the time of diagnosis, a higher disability score at nadir and a chronic course of the disease. Because of the retrospective design of the study, we could not compare the various therapies performed in our population of patients (intravenous immune globulin, plasma exchange or corticosteroids). In other studies, a worse course of CIDP was related to a chronic onset (progression over more than 8 weeks)22 and to asymmetrical symptoms and demyelination in the intermediate nerve segments.21
In conclusion, the use of a population based register allowed us to evaluate the prevalence and incidence of CIDP in a defined area. CIDP in Piemonte and Valle d'Aosta showed an incidence rate that was about a quarter of that observed for GBS in the same area. However, both disorders had a higher frequency among men and older subjects. The response to therapy was generally good, and residual disability was mild in most cases. The response to immune therapy at the time of diagnosis was, together with age, the major prognostic factor.
Abbreviations
AHSAAN - Ad Hoc Subcommittee of the American Academy of Neurology
CIDP - chronic inflammatory demyelinating polyneuropathy
GBS - Guillain–Barré syndrome
MRS - Modified Rankin Scale
Appendix
Piemonte and Valle d'Aosta Register for CIDP (PARCIDP).
Coordinating centre: 1st Division of Neurology, Department of Neuroscience, University of Torino, Italy.
Project coordinator: A Chiò, MD.
Study monitors: F Plano, MD, A Calvo, MD, N Di Vito, MD, P Ghiglione, MD.
Scientific Committee: E Bottacchi, MD, A Chiò, MD, D Cocito, MD, MT Giordana, MD, M Leone, MD, G Mora, MD.
Collaborating centers: A Chiò, MD, AA Terreni, MD, R Mutani, MD, D Cocito, MD, B Bergamasco, MD (Department of Neuroscience, Section of Neurology, University of Torino, and ASO San Giovanni Battista, Torino); A Bertolotto, MD, R Sciolla, MD, MT Giordana, MD (Department of Neuroscience, Section of Neurology, University of Torino, and Azienda Ospedaliera San Luigi Gonzaga, Orbassano); M Leone, MD, P Gaviani, MD, F Monaco, MD (Department of Neurology, “Amedeo Avogadro” University, Novara); M De Mattei, MD, E Morgando, MD (Department of Neurology, ASO San Giovanni Battista, Torino); L Sosso, MD, M Gionco, MD (Department of Neurology, Ospedale Mauriziano, Torino); U Morino, MD, M Nobili, MD (Department of Neurology, Ospedale Martini, Torino); L Appendino, MD (Department of Neurology, Ospedale Maria Vittoria, Torino); R Cavallo (Department of Neurology, Ospedale S Giovanni Bosco, Torino); E Oddenino, MD (Department of Neurology, Ospedale Gradenigo, Torino); G Vaula, MD, G Ferrari, MD (Department of Neurology, Ivrea); M Favero, MD, C Doriguzzi Bozzo, MD (Department of Neurology, Pinerolo); P Santamaria, MD (Department of Neurology, Vercelli); U Massazza, MD, E Bollani, MD (Department of Neurology, Biella); A Villani, MD, R Conti, MD (Department of Neurology, Domodossola); G Mora, MD, C Balzarini, MD (Department of Neurological Rehabilitation, Fondazione Salvatore Maugeri, Clinica del Lavoro e della Riabilitazione, IRCCS, Scientific Institute of Veruno); M Palermo, MD (Department of Neurology, Alessandria); F Vergnano, MD (Department of Neurology, Casale Monferrato); S Cordera, MD, C Buffa, MD (Department of Neurology, Novi Ligure); MT Penza, MD (Department of Neurology, Tortona); F Fassio, MD (Department of Neurology, Asti); P Meineri, MD (Department of Neurology, Azienda Ospedaliera Santa Croce e Carle, Cuneo); A Cognazzo, MD, A Dutto, MD, A Cucatto, MD (Department of Neurology, Savigliano); W Troni, MD (Department of Neurology, Alba); G Corso, MD, E Bottacchi, MD (Department of Neurology, Aosta).
Footnotes
Competing interests: None.
References
- 1.Dyck P J, Lais A C, Ohta M.et al Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc 197550621–637. [PubMed] [Google Scholar]
- 2.Köller H, Kieseier B C, Jander S.et al Chronic inflammatory demyelinating polyneuropathy. N Engl J Med 20053521343–1356. [DOI] [PubMed] [Google Scholar]
- 3.Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology 199141617–618. [PubMed] [Google Scholar]
- 4.Prineas J W, Mc Leod J R. Chronic relapsing polyneuritis. J Neurol Sci 197627427–458. [DOI] [PubMed] [Google Scholar]
- 5.Dyck P J, Prineas J, Pollard J. Chronic inflammatory demyelinating polyradiculopathy. In: Dyck PJ, Thomas PK, Griffin JW, et al eds. Peripheral neuropathy. Philadelphia: WB Saunders, 19931498–1517.
- 6.Matsummuro K, Izumo S, Umehara F.et al Chronic inflammatory demyelinating polyneuropathy: Histological and immunopathological studies in biopsied sural nerves. J Neurol Sci 1994127170–178. [DOI] [PubMed] [Google Scholar]
- 7.Hartung H ‐ P, Stoll G, Toyka K V. Immune reactions in the peripheral nervous system. In: Dyck PJ, Thomas PK, Griffin JW, et al eds. Peripheral neuropathy. 3rd Edn. Philadelphia: Saunders, 1995418–444.
- 8.Misawa S, Kuwabara S, Mori M.et al Serum levels of tumor necrosis factor‐alpha in chronic inflammatory demyelinating polyneuropathy. Neurology 200156666–669. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki T, Oka N, Akiguchi I.et al Up‐regulation of cyclooxygenase‐2 in inflammatory demyelinating neuropathy. Acta Neuropathol 2001101154–158. [DOI] [PubMed] [Google Scholar]
- 10.Kusumi M, Nakashima K, Nakayama H.et al Epidemiology of inflammatory neurological and inflammatory neuromuscular diseases in Tottori Prefecture, Japan. Psychiatry Clin Neurosci 199549169–174. [DOI] [PubMed] [Google Scholar]
- 11.McLeod J C, Pollard D, Macaskill P.et al Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol 199946910–913. [PubMed] [Google Scholar]
- 12.Lunn M P T, Manji H, Choudhary P P.et al Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in South‐East England. J Neurol Neurosurg Psychiatry 199966677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mygland A, Monstad P. Chronic polyneuropathies in Vest‐Agder, Norway. Eur J Neurol 20018157–165. [DOI] [PubMed] [Google Scholar]
- 14.Chiò A, Cocito D, Leone M.et al Guillain–Barrè syndrome: a prospective, population‐based incidence and outcome survey. Neurology 2003601146–1150. [DOI] [PubMed] [Google Scholar]
- 15.van Swieten J C, Koudstall P J, Visser M C.et al Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988191263–1264. [DOI] [PubMed] [Google Scholar]
- 16.Schoenberg B S. Calculating confidence intervals for rates and ratios. Neuroepidemiology 19832257–265. [Google Scholar]
- 17.Rotta F T, Sussman A, Bradley W G.et al The spectrum of chronic inflammatory demyelinating polyneuropathy. J Neurol Sci 2000173129–139. [DOI] [PubMed] [Google Scholar]
- 18.Hattori N, Misu K, Ichimura M.et al Age of onset influences clinical features of chronic inflammatory demyelinating polyneuropathy. J Neurol Sci 200118457–63. [DOI] [PubMed] [Google Scholar]
- 19.Hughes R A C, Rees J H. Clinical and epidemiological features of Guillain–Barré Syndrome. J Infect Dis 1997176(Suppl 2)S92–S98. [DOI] [PubMed] [Google Scholar]
- 20.McCombe P A, Pollard J D, McLeod J G. Chronic inflammatory demyelinating polyradiculoneuropathy. A clinical and electrophysiological study of 92 cases. Brain 19871101617–1630. [DOI] [PubMed] [Google Scholar]
- 21.Kuwubara S, Misawas S, Mori M.et al Long term prognosis of chronic inflammatory demyelinating polyneuropathy: a five year follow‐up of 38 cases. J Neurol Neurosurg Psychiatry 20067766–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mygland A, Monstad P, Vedeler C. Onset and course of chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 200531589–593. [DOI] [PubMed] [Google Scholar]