Abstract
Background and aim
To update our 1996 review on the incidence of subarachnoid haemorrhage (SAH) and assess the relation of incidence with region, age, gender and time period.
Methods
We searched for studies on the incidence of SAH published until October 2005. The overall incidences with corresponding 95% confidence intervals were calculated. We determined the relationship between the incidence of SAH and determinants by means of univariate Poisson regression.
Results
We included 51 studies (33 new), describing 58 study populations in 21 countries, observing 45 821 896 person‐years. Incidences per 100 000 person‐years were 22.7 (95% CI 21.9 to 23.5) in Japan, 19.7 (18.1 to 21.3) in Finland, 4.2 (3.1 to 5.7) in South and Central America, and 9.1 (8.8 to 9.5) in the other regions. With age category 45–55 years as the reference, incidence ratios increased from 0.10 (0.08 to 0.14) for age groups younger than 25 years to 1.61 (1.24 to 2.07) for age groups older than 85 years. The incidence in women was 1.24 (1.09 to 1.42) times higher than in men; this gender difference started at age 55 years and increased thereafter. Between 1950 and 2005, the incidence decreased by 0.6% (1.3% decrease to 0.1% increase) per year.
Conclusions
The overall incidence of SAH is approximately 9 per 100 000 person‐years. Rates are higher in Japan and Finland and increase with age. The preponderance of women starts only in the sixth decade. The decline in incidence of SAH over the past 45 years is relatively moderate compared with that for stroke in general.
Subarachnoid haemorrhage (SAH) from a ruptured aneurysm accounts for approximately 5% of all strokes. Because it occurs at a young age and has a high case fatality, the loss of productive life years in the general population from SAH is as large as that from cerebral infarction, the most common type of stroke.1,2 Important risk factors are a familial preponderance, hypertension, smoking and alcohol abuse.3 In 1996, we performed a systematic review on the incidence of SAH between 1960 and 1994.4 In that review, the incidence of SAH had remained stable at around 8 per 100 000 person‐years over 35 years. An interesting finding was the high incidence in Finland in comparison with other European and American populations. In a small subset of studies, gender specific incidences were given, which indicated a higher incidence in women.
Since the publication of that review, many new incidence studies have been reported, including regions that were not represented in the first review. The incidence for stroke in general has declined over the past decade, and this has been attributed to a declining proportion of people who smoke and to better detection and treatment of hypertension.1 As smoking and hypertension are also risk factors for SAH, a similar decline in the incidence of SAH could be expected. We updated the previous review with new information and assessed regional differences in SAH incidence, as well as differences in incidence according to age, gender and time period.
Methods
Methods of literature search, inclusion criteria for studies and diagnostic criteria for SAH were essentially the same as in the previous overview.4 To update the review, we searched for population based studies on the incidence of SAH by performing a MEDLINE search from 1993 until October 2005. (Keywords: “stroke” or “subarachnoid haemorrhage” together with “epidemiology”, “population” or “incidence”.) In addition, we searched the reference lists of all relevant publications, searched for related articles given on MEDLINE and checked the citation list of all references found, including those from the previous version of the review. This method of cross checking was continued until no further new studies were found. The list of references thus found was compared with the personal database of references from another author (GJER) to check if references had been missed by the (retrospective) PubMed search (which was not the case). This personal database has been prospectively built by daily search of PubMed over the past 10–15 years by means of the following terms “subarachnoid hemorrhage [All Fields] OR aneurysm [All Fields] OR arteriovenous malformation [All Fields] OR perimesencephalic [All Fields] OR subarachnoid haemorrhage [All Fields] OR aneurysm*”.
Two authors (NKR and JAP) reviewed all eligible studies independently and completed a data extraction form. These forms included items regarding design of the study, study population, case finding and diagnostic criteria of SAH. The inclusion criteria were: (1) prospective design; (2) study population is representative of the population in general; (3) upper age limit for the study not below 75 years and lower age limit not above 25 years; (4) for studies about stroke in general, SAH should be considered as a separate entity; (5) results include or at least allow calculation of the overall crude incidence of SAH; (6) the majority of cases were reviewed by the study investigator; (7) case finding methods include at least involvement of all hospitals in the region, and either involvement of general practitioners or reviewing death certificates during the study period; and (8) diagnostic criteria include at least lumbar puncture or autopsy in the pre‐CT era, or in case the proportion of patients investigated with CT was lower than 90%. In the event of disagreement in the data extraction forms, the article was re‐read by another author (GJER or FHHL) and discussed until agreement was achieved. Excellent case finding was defined as involvement of all hospitals in the region as well as involvement of general practitioners and reviewing death certificates during the study period. Excellent diagnostics was defined as more than 90% of SAH patients had undergone CT.
We used incidence rates relating to the entire population, without adjustment for age or sex. Authors were contacted for missing data on crude incidence of SAH if necessary. To assess geographical differences, we compared studies by region. In addition, we extracted gender and age specific incidence rates for those studies that provided sufficient data.
Data analysis
For each of the selected studies, the overall incidence was computed if necessary. Ninety‐five per cent confidence intervals were calculated with Poisson methods. We determined the relationship of the incidence of SAH with region, age, gender and time period by means of univariate Poisson regression. Incidences by region were calculated with the subset of studies from the specific area. Relationship of incidence of SAH with age and gender was analysed using demographics of the study populations, and age and gender specific incidences of SAH were calculated with the subset of studies that provided sufficient data. Time trend was analysed using midyear of the study, taking into account regional differences. Multivariate Poisson regression was used to asses the independent contribution of age, gender and time trend to SAH incidence. To examine the influence of design of the study, we selected a subset of studies with excellent case finding and excellent diagnostic criteria for sensitivity analysis.
Results
Literature search
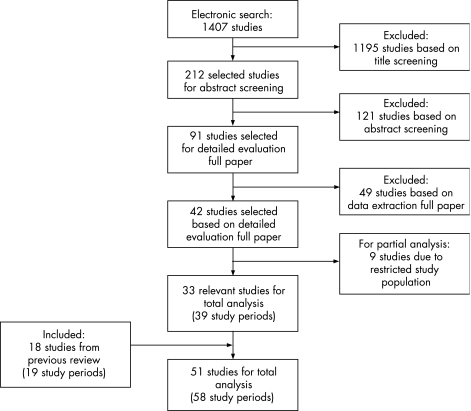
The literature search resulted in 42 new studies (fig 1).5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 Thirty‐three were relevant for overall analysis. The remaining nine studies were not included in the overall analyses because only incidences of limited age categories were provided.6,14,15,16,19,28,32,34,38 These nine studies were included only for analysis on age specific incidences. Eleven authors were contacted for missing information; in four cases the information was retrieved.14,15,39,45 Together with the 18 investigations from the previous review,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64 51 studies were used in the total analysis. As four studies provided incidences for 2, 3 or 4 periods or areas,17,27,39,55 the number of study periods and study regions that we analysed was 58, of which 39 were new. The studies covered populations in 21 countries with 45 821 896 person‐years of observation.
Figure 1 Flowchart of literature search on population based studies on subarachnoid haemorrhage.
Calculated incidences, case finding methods and diagnostic criteria from all of the included study periods and regions are summarised in table 1. Table 2 represents the nine studies describing study populations with limited age categories.
Table 1 Incidence, case finding methods and diagnostic criteria of subarachnoid haemorrhage in newly identified study periods and regions*.
| Study population | Region | Midyear of study | No of patient‐ years | No of SAH patients | Incidence per 100 000 person years (95% CI) | Additional case finding methods‡ | % of patients with CT | Additional diagnostic criteria†† |
|---|---|---|---|---|---|---|---|---|
| Rochester39 | USA | 1955 | 331 081 | 29 | 8.8 (5.9 to 12.6) | adhjm | 0 | AB |
| Rochester39 | USA | 1965 | 451 611 | 52 | 11.5 (8.6 to 15.1) | adhjm | 0 | AB |
| Espoo17 | Finland | 1972 | 226 200 | 42 | 18.6 (13.4 to 25.1) | ae | 0 | B |
| Rochester39 | USA | 1975 | 543 561 | 61 | 11.2 (8.6 to 14.4) | adhjm | 27¶ | ABD |
| Espoo17 | Finland | 1979 | 273 700 | 33 | 12.1 (8.3 to 16.9) | ae | 11 | B |
| Copenhagen46 | Denmark | 1984 | 295 470 | 49 | 16.6 (12.3 to 21.9) | ak | 47 | ABE |
| Izumo city†27 | Japan | 1985 | 807 490 | 170 | 21.1 (18.0 to 24.5) | a | 99§ | ABC |
| Rochester39 | USA | 1985 | 617 554 | 43 | 7.0 (5.0 to 9.4) | adhjm | 85¶ | ABD |
| Finland17 | Finland | 1990 | 269 608 | 39 | 14.5 (10.3 to 19.8) | aeh | 60 | B |
| Izumo city†26 | Japan | 1990 | 496 074 | 123 | 24.8 (20.6 to 29.6) | ai | 100§ | BE |
| Asturias37 | Spain | 1991 | 417 033 | 28 | 6.7 (4.5 to 9.7) | b | 70 | ‡‡ |
| Ahmadi29 | Kuwait | 1992 | 291 199 | 4 | 1.4 (0.4 to 35.2) | ab | 100 | A |
| Novosibirsk35 | Russia | 1992 | 158 234 | 14 | 8.9 (4.8 to 14.8) | abehjm | 0** | BC |
| Auckland†31 | New Zealand | 1992 | 1 890 738 | 166 | 8.8 (7.5 to 10.2) | ae | 82 | ABC |
| Belluno22 | Italy | 1992 | 211 389 | 12 | 5.7 (2.9 to 9.9) | abefjk | 90 | AB |
| Sweden north42 | Sweden | 1993 | 8 212 800 | 984 | 12.0 (11.2 to 12.8) | abkh | 87 | ABC |
| L'Aquila21 | Italy | 1994 | 297 838 | 24 | 8.0 (5.2 to 12.0) | abcefj | 89¶ | AB |
| Shimokita†24 | Japan | 1994 | 899 910 | 198 | 22.0 (19.0 to 25.3) | ai | 100§ | AC |
| Izumo City25 | Japan | 1994 | 509 124 | 123 | 24.2 (20.1 to 28.8) | ai | 98 | ABC |
| Malmo45 | Sweden | 1995 | 2 674 144 | 197 | 7.4 (6.4 to 8.5) | abde | 89 | AB |
| Izumo city†27 | Japan | 1995 | 763 686 | 188 | 24.7 (21.2 to 28.4) | ai | 98 | ABC |
| Perth7 | Australia | 1995 | 134 000 | 4 | 3.0 (0.8 to 7.6) | abdfj | >78 | BC |
| Sweden south40 | Sweden | 1996 | 1 140 000 | 106 | 9.3 (7.6 to 11.2) | ai | 100¶ | ABC |
| Melbourne5 | Australia | 1996 | 133 816 | 12 | 9.0 (4.6 to 15.7) | bdg | 91¶ | AB |
| London11 | UK | 1996 | 938 132 | 74 | 7.9 (6.2 to 9.9) | abefj | 88¶ | AB |
| Vibo Valentia20 | Italy | 1996 | 179 186 | 12 | 6.7 (3.5 to 11.7) | abdejk | 96¶ | B |
| Dijon43 | France | 1996 | 429 264 | 12 | 2.8 (1.4 to 4.9) | abdeh | 96 | ‡‡ |
| Valle d' Aosta44 | Italy | 1997 | 118 723 | 14 | 11.8 (6.4 to 19.8) | abdej | 97¶ | AB |
| Erlangen10 | Germany | 1997 | 202 900 | 12 | 5.9 (3.1 to 10.3) | abdejk | 96 | D |
| Kumamoto†23 | Japan | 1998 | 9 300 000 | 2115 | 22.7 (21.8 to 23.7) | bij | 100§ | AC |
| Martinique30 | Caribbean | 1998 | 360 000 | 20 | 5.6 (3.4 to 8.6) | abeijk | 93 | A |
| Scotland36 | UK | 1999 | 212 704 | 23 | 10.8 (6.9 to 16.2) | abhfl | 91 | B |
| Portugal north33 | Portugal | 1999 | 246 224 | 23 | 9.3 (5.9 to 14.0) | abdefhikm | 97 | B |
| Orebro41 | Sweden | 1999 | 123 503 | 11 | 8.9 (4.4 to 15.9) | abdefkm | 84 | AB |
| Tartu13 | Estonia | 2000 | 101 122 | 8 | 7.9 (3.4 to 15.6) | abei | 92 | B |
| Iqueque9 | Chile | 2001 | 396 712 | 15 | 3.8 (2.1 to 6.2) | abdefgh | 91 | AB |
| Tbilisi18 | Georgia | 2002 | 140 926 | 23 | 16.3 (10.3 to 24.5) | aehijl | 78§ | A |
| Barbados8 | Caribbean | 2002 | 239 068 | 7 | 2.9 (1.2 to 6.0) | abcdefh | 96 | B |
| Oxford12 | UK | 2003 | 181 084 | 16 | 8.8 (5.1 to 14.3) | abefk | 98¶ | AB |
SAH, subarachnoid haemorrhage.
*Studies listed in ascending order of midyear of data collection and are additional to those in the previous review.
†Studies based primarily on SAH, in contrast with general stroke studies.
‡Case finding methods. For inclusion, involvement of all hospitals in the region necessary and at least a or b. a = death certificates; b = general practitioners; c = rehabilitation; d = nursing homes; e = regular search; f = review radiology requests; g = media attention (campaign/newspaper); h = outpatient clinics, health centres; i = sudden deaths, very early death; j = emergency, ambulance, on call medical services; k = ICD‐codes; l = door‐to‐door, home visit, social services, phone calls; m = autopsy reports.
§Studies providing the proportion of CT use in SAH patients exclusively, in contrast with % of CT in patients with stroke in general.
¶Studies not providing the exact proportion of patients with CT exclusively, but only the proportion of patients investigated with CT, autopsy or MRI.
**CT was available after 1992, and before 1992, all patients were diagnosed with lumbar puncture or autopsy.
††Additional diagnostic criteria, besides CT. For inclusion, at least A or B was necessary in pre‐CT era or when CT percentage was below 90%. A = Lumbar puncture; B = autopsy; C = angiography; D = MRI; E = surgery.
‡‡Proportion of patients investigated with CT or diagnostic criteria unknown, but inclusion after discussion among authors of this review.
Table 2 Incidence, case finding methods and diagnostic criteria of subarachnoid haemorrhage in newly identified studies describing study populations with limited age categories*.
| Study population | Region | Midyear of study | No of patient years | No of SAH patients | Incidence per 100 000 person years (95% CI) | Additional case finding methods‡ | % of patients with CT | Additional diagnostic criteria†† | Restriction of study population: age (y) |
|---|---|---|---|---|---|---|---|---|---|
| Oyabe28 | Japan | 1984 | 492 885 | 124 | 25.2 (20.7 to 29.6) | abj | ‡‡ | ‡‡ | >25 |
| Novosibirsk34 | Russia | 1987 | 971 751 | 64 | 6.6 (5.0 to 8.2) | aehjm | ** | AB | 25–74 |
| Turku15 | Finland | 1987 | 1 249 992 | 278 | 22.2 (19.6 to 24.9) | abeam | ‡‡ | AB | >25 |
| FINMONICA†16 | Finland | 1988 | 3 863 088 | 956 | 24.7 (23.2 to 26.3) | aeh | 84§ | ABC | 25–74 |
| FINSTROKE14 | Finland | 1993 | 1 933 660 | 360 | 18.6 (16.7 to 20.5) | aehk | 86 | AB | 25–74 |
| Arcadia19 | Greece | 1994 | 161 548 | 14 | 8.7 (4.1 to 13.2) | abeh | 82 | AB | >20 |
| Manhattan38 | USA | 1994 | 428 775 | 39 | 9.1 (6.2 to 12.0) | befikl | 99¶ | AC | >20 |
| Innhered32 | Norway | 1995 | 69 295 | 13 | 18.8 (8.6 to 29.0) | abdegk | 88 | BD | >15 |
| ACROSS†6 | Australia, N Zealand | 1997 | 4 916 154 | 400 | 8.1 (7.3 to 8.9) | aefk | 90§ | ABC | >15 |
SAH, subarachnoid haemorrhage.
*Studies listed in ascending order of midyear of data collection and are additional to those in the previous review.
†Studies based primarily on SAH, in contrast with general stroke studies.
‡Case finding methods. For inclusion, involvement of all hospitals in the region necessary and at least a or b. a = death certificates; b = general practitioners; c = rehabilitation; d = nursing homes; e = regular search; f = review radiology requests; g = media attention (campaign/newspaper); h = outpatient clinics, health centres; i = sudden deaths, very early death; j = emergency, ambulance, on call medical services; k = ICD‐codes; l = door‐to‐door, home visit, social services, phone calls; m = autopsy reports.
§Studies providing the proportion of CT use in SAH patients exclusively, in contrast with % of CT in patients with stroke in general.
¶Studies not providing the exact proportion of patients with CT exclusively, but only the proportion of patients investigated with CT, autopsy or MRI.
**CT was available after 1992, and before 1992, all patients were diagnosed with lumbar puncture or autopsy.
††Additional diagnostic criteria, besides CT. For inclusion, at least A or B was necessary in pre‐CT era or when CT percentage was below 90%. A = Lumbar puncture; B = autopsy; C = angiography; D = MRI; E = surgery.
‡‡Proportion of patients investigated with CT or diagnostic criteria unknown, but inclusion after discussion among authors of this review.
Region
There was wide variation in SAH incidence, ranging from 2 to 25 per 100 000 person‐years, with most regional incidences between 7 and 13 per 100 000 person‐years. We defined all countries other than Japan, Finland and South or Central America as the reference group. Overall incidences were 9.1 (95% CI 8.8 to 9.5) per 100 000 person‐years in the reference group (42 studies); 22.7 (95% CI 21.9 to 23.5) in Japan (seven studies); 19.7 (95% CI 18.1 to 21.3) in Finland (six studies); and 4.2 (95% CI 3.1 to 5.7) in South and Central America (three studies) (fig 2). The incidence in Japan was 2.5 (95% CI 2.4 to 2.6) times higher than that of the reference region and in Finland 2.2 (95% CI 2.0 to 2.4) times higher, whereas the incidence in South and Central America was 2.2 (95% CI 1.6 to 2.9) times lower.
Figure 2 Incidence of subarachnoid haemorrhage by region. Incidences per 100 000 person‐years, with corresponding 95% CI. All countries other than Japan, Finland and South and Central America were pooled in a reference group. Overall incidences were 9.1 (95% CI 8.8 to 9.5) in the reference group (42 studies); 22.7 (95% CI 21.9 to 23.5) in Japan (seven studies); 19.7 (95% CI 18.1 to 21.3) in Finland (six studies); and 4.2 (95% CI 3.1 to 5.7) in South and Central America (three studies). Age specific incidences by region reveal the same trend (see text)
Age
The mean age of the study population was mentioned in 37 studies, and univariate Poisson regression analysis was performed for this subset of studies. In populations with a mean age of 35 years, calculated incidence was 8.6 (95% CI 8.0 to 9.2), and for every year of increase in mean age, the incidence was 1.06 times higher (95% CI 1.05 to 1.07).
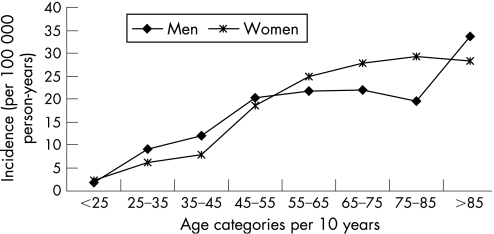
Twenty studies, including the nine studies with only age specified subsets of the population, reported separately on incidences per age group.5,6,8,9,10,16,18,19,20,22,27,36,45,49,50,52,54,57,60,64 The overall incidence of these 20 studies was 13.9 (95% CI 13.3 to 14.5) per 100 000 person‐years. In this subset, incidence increased with age: taking age 45–55 years as the reference category, incidence ratios increased from 0.10 (95% CI 0.08 to 0.14) for age <25 years, to 1.61 (95% CI 1.24 to 2.07) for ⩾85 years (table 3).
Table 3 Incidence of subarachnoid haemorrhage per age category in 20 studies.
| Age (y) | Incidence per 100 000 person‐years (95% CI) | Incidence ratio (95% CI) | Ratio women/men (95% CI) |
|---|---|---|---|
| <25 | 2.0 (1.6 to 2.6) | 0.10 (0.08 to 0.14) | 1.36 (0.82 to 2.27) |
| 25–35 | 7.7 (6.8 to 8.8) | 0.40 (0.34 to 0.46) | 0.67 (0.51 to 0.88) |
| 35–45 | 10.5 (9.0 to 11.3) | 0.52 (0.44 to 0.60) | 0.65 (0.51 to 0.82) |
| 45–55 | 19.5 (17.8 to 21.4) | Reference | 0.91 (0.76 to 1.09) |
| 55–65 | 24.8 (22.7 to 27.2) | 1.27 (1.12 to 1.45) | 1.15 (0.95 to 1.38) |
| 65–75 | 25.4 (23.1 to 28.0) | 1.30 (1.14 to 1.49) | 1.26 (1.04 to 1.54) |
| 75–85 | 26.2 (22.5 to 30.4) | 1.34 (1.13 to 1.60) | 1.50 (1.07 to 2.10) |
| >85 | 31.3 (24.6–39.8) | 1.61 (1.24 to 2.07) | 0.84 (0.49 to 1.44) |
For Japan, incidences per age decade were given in two studies. Based on these two studies, increase in age specific incidence seemed to be steeper in Japan than in other regions, ranging from 0.56 (95% CI 0.18 to 1.75) per 100 000 person years for age <25 years to 7.96 (95% CI 5.33 to 11.88) for ⩾85 years. For Finland, no age specific incidence per age decade was available for analysis, and for South and Central America, numbers were too small to provide reliable estimates.
Age adjusted incidences per 100 000 person‐years in Japan varied from 21 (95% CI 18 to 24) to 23 (95% CI 19 to 28),23,24,25,26,27 and in Finland from 14 (95% CI 10 to 19) to 30 (95% CI 22 to 40).17,58 From studies in South and Central America, age adjusted incidence was given in only one study (4; 95% CI 2 to 6 per 100 000 person‐years), which was also adjusted for sex.9
Gender
Gender distribution was provided in 37 studies. Univariate Poisson regression analysis showed that for each additional per cent of women, the incidence became 1.07 times higher (95% CI 1.04 to 1.10).
Eighteen studies reported incidences for men and women separately.5,9,10,17,18,20,22,27,29,35,44,45,47,49,50,52,54,57 The overall incidence in this subset of studies was 10.5 (95% CI 9.9 to 11.2) per 100 000 person‐years; the incidence for men was 9.2 (95% CI 8.4 to 10.2) and for women 11.5 (95% CI 10.6 to 12.6). Thus the incidence in women was 1.24 (95% CI 1.09 to 1.42) times higher than in men. Separate women–men ratios per region were 1.26 (95% CI 1.03 to 1.52) for the reference region, 1.16 (95% CI 0.95 to 1.42) for Japan, 1.58 (95% CI 1.08 to 2.30) for Finland and 0.89 (95% CI 0.32 to 2.47) for South and Central America.
Age and gender
In the 37 studies that reported mean age of the study population and gender distribution, mean age and proportion of women were analysed by multivariate analysis. After adjustment for age, incidence increased by a factor of 1.03 (95% CI 0.99 to 1.06) for each additional percentage point of women in the study population. After adjustment for gender, incidence increased by a factor of 1.06 (95% CI 1.05 to 1.07) for each additional year.
Incidences were reported separately for women and men by age category in 16 studies.5,6,9,10,16,18,19,20,22,27,45,49,50,52,54,57 In this subset of studies, the women–men ratio ranged from 0.65 (95% CI 0.51 to 0.82) to 1.50 (95% CI 1.07 to 2.10). In the age group 25–45 years, incidence was significantly higher in men than in women, but in the age group 55–85 years, incidence was significantly higher in women than in men (table 3, fig 3).
Figure 3 Incidence of subarachnoid haemorrhage by age and gender.
Time trend
Midyear of the study was analysed by univariate and multivariate analysis for evaluation of a time trend. Because studies in Japan and Finland were confined to more years, analyses on time trend were performed for the reference region only. During the observation period, incidence decreased by a factor of 0.994 (95% CI 0.987 to 1.001) per year in the reference region after adjustment for gender and age. When all 42 studies were analysed from the reference region without adjustment for age and gender, the rate ratio was 1.001 (0.997 to 1.004) for year‐to‐year annual change, thus showing no decrease in incidence. In the subset of studies that reported on study periods after 1990 and that provided exact proportions of patients investigated by CT, the rate ratio for use of CT on reported incidence was 0.997 (95% CI 0.993 to 1.002) in the reference region (n = 14), 0.963 (95% CI 0.908 to 1.022) in Japan (n = 4) and 0.963 (0.821 to 1.13) in South and Central America (n = 3). Thus after 1990 there was no obvious relation between the use of CT and reported incidence of SAH. To exclude the influence of percentage of CT use, separate analyses were performed including only studies after 1990. For this subset of 24 studies after 1990 from the reference region, results were essentially the same after adjustment for age and gender (table 4).
Table 4 Annual time trend of incidence of subarachnoid haemorrhage in the reference region*.
| No of studies | Incidence ratio† (95% CI) | Incidence ratio† (95% CI) adjusted for gender and age | |
|---|---|---|---|
| Reference region | 42 | 1.001 (0.997 to 1.004) | 0.994 (0.987 to 1.001) |
| Reference region after 1990 | 24 | 0.973 (0.961 to 0.985) | 0.994 (0.967 to 1.022) |
*All countries other than Japan, Finland and South and Central America.
†Incidence ratio represents coefficient for year‐to‐year annual change.
Sensitivity analysis
The criterion for excellent case finding was met by 33 studies (20 new, 13 from the previous review) and the criterion for excellent diagnostics by seven studies (five new, two from the previous review). If we combine both “excellent” case finding methods and “excellent” diagnostic criteria, none of the studies fulfilled these criteria. Therefore, we were unable to perform a sensitivity analysis with excellent studies.
Discussion
We found that wide variation exists in the incidence of SAH. The overall incidence of SAH was approximately 9 per 100 000 person‐years but varied significantly by region, with doubled rates in Japan and Finland and far lower rates in South and Central America. The incidence was higher in women and increased with age. The gender distribution varied with age. At young ages, incidence was higher in men, while after the age of 55 years, the incidence was higher in women. The incidence of SAH has probably decreased slightly over the past 45 years.
Several factors may contribute to the higher incidence in Finland and Japan, but the extent of their contributions remains speculative. In Japan and Finland, a higher risk of rupture of intracranial aneurysms is described.65 Genetic factors may also play an important role in both Japan and Finland.
The relatively older age in Japan may be another explanation. Global statistics report the Japanese as being the oldest population in the world, with a median age of 43 years in 2005.66 However, this older age cannot entirely explain the high incidence, because age specific incidences were also higher in Japan than in the reference population. Another explanation may be better case finding, but case finding in the studies from Japan was not more exhaustive than in other regions. Five of the seven studies did not describe regular contacts with general practitioners, and none mentioned contacting rehabilitation facilities or nursing homes as a case finding method. However, the majority of studies from Japan examined instances of sudden death more extensively than studies from other regions. Most studies from Japan used in addition to autopsy, neuroimaging of patients who had died suddenly or during transportation to the hospital. Probably more patients dying early after SAH were detected by scrutinising all of these events, which increased the incidence of SAH compared with studies in which such instances of sudden death were not examined in this way. However, sudden death accounts for only 12% of all SAH patients67; more extensive examination of patients dying early may contribute to, but cannot entirely explain, the higher incidence in Japan. The proportion of patients in whom the diagnosis of SAH was confirmed by CT scanning was almost 100% in Japan. However, a large proportion of patients investigated by means of CT does not lead to a higher incidence. In our previous review, we found a higher percentage of CT use to be associated with a lower incidence of SAH and in recent studies we found no relation between the proportion of patients investigated by means of CT and the reported incidence. The greater use of neuroimaging in Japan is therefore unlikely to be an explanation for the high incidence rates reported in Japan.
Age adjusted incidences were also higher in Finland than in the reference region. In Finnish studies, the proportions of patients in whom the diagnosis of SAH was confirmed by CT were low (varying between 0% and 60%). If we apply the rate ratio for proportion investigated by CT on incidence found in the previous version of the review, and if we assume a hypothetical 100% proportion of patients investigated by CT, the incidence of SAH would be 10.6 (95% CI 8.9 to 12.5) in Finland, which is still higher than the incidence in the reference region. Thus the low proportions of CT in Finnish studies do not entirely explain the higher incidences found. Case finding methods in Finnish studies were not more exhaustive compared with other studies, thereby not increasing the incidence found. Other explanations for the high incidence in Finland include high prevalence of smoking and hypertension,68 and heavy episodic alcohol abuse.69
The low incidences in South and Central America can perhaps be explained in part by the relatively young mean age of people in these regions. Reported mean ages in the study populations varied between 25 and 35 years, whereas for the reference population this mean age was 37 years. However, the age adjusted incidence given in one study was also lower than in the reference region.9 Thus other factors are likely to be involved in the lower incidence in this region. No differences in case finding methods were noted, but access to hospitals in these regions may be less than in other regions. Another explanation might be racial differences, although in some studies the incidence of SAH in black populations was higher in comparison with white populations.70
In summary, none of these explanations can completely explain the regional differences, and other factors are likely to be involved.
The higher incidence of SAH in women was found in the previous version of our review but the age dependent gender difference is a new finding. While previous literature describes a peak incidence in the sixth decade,71 some recent studies found a continuous increase with age, or an age dependent gender difference.6 The current review confirms these observations from some individual studies. The reasons for the overall higher incidence in women are not clear, but hormonal factors (including use of hormone replacement therapy) are a possible explanation.72,73 Our finding that the preponderance of women starts only after the sixth decade further supports this suggestion.
Although several studies have reported a statistically significant decline in stroke of approximately 2% per year over the past two decades,12,74,75 it is still uncertain if the reduction in cardiovascular risk factors has also translated into a reduction in the incidence of SAH. Our study found a decrease in incidence of 0.6% per year, which is modest compared with the decline in stroke in general. In our analysis, the influences of region, age, gender and improved diagnostic criteria by CT were taken into account. In our previous review, we found that the apparent decline in the incidence of SAH until 1990 was entirely explained by the increasing proportion of patients investigated by CT.4 In this update, we found that in studies performed after 1990, the proportion of patients investigated by means of CT was no longer significantly related to incidence in any region. The most likely explanation is that after 1990, almost all hospitalised patients were investigated using CT. Thus the contrast between studies with small proportions investigated by CT (with over reporting of SAH)76 and studies with large proportions investigated by CT has disappeared. The time trend found in our study is therefore not explained by percentages of CT use for confirmation of diagnosis of SAH. The small magnitude of the decline in incidence of SAH may in part be explained by the stronger influence of genetic factors in SAH than in stroke in general.77 However, genetic factors explain only 10% of SAH, and most cases are attributed to smoking, hypertension and excessive use of alcohol.77 Perhaps the reduction in risk factors is more effective in older people (where most stokes in general occur) than in younger people (who are most at risk of SAH), but we have no data to support this hypothesis.
It seems contradictory that the incidence of SAH decreased over time, although the overall incidence in our update was higher than the incidence found in our previous review. However, by updating the review, we included five new studies in the reference region published after 1993 presenting data from before 1990. These five studies had a combined incidence of 10.4 per 100 000 person‐years, which is higher than the overall incidence from the studies that had been included in the previous version of the review. The net result is that the incidence of all studies (including the newly found ones) for the observation period from the previous review (1972–1990) has increased compared with that in the previous review. This effect in part explains the paradox of higher incidence in the current review despite declining incidence over time. Furthermore, we found the decrease in incidence only after adjustment for gender and age. Thus the increased incidence in the updated version of the review may be explained in part by inclusion of study populations with higher ages in the more recent years.
The number of population based studies (51) and number of person‐years (45 821 896) included in this review was large and therefore overall estimates are precise. Subgroup analyses according to region, age, gender and time trend were based on smaller numbers of studies and person‐years. Nevertheless, even for these analyses, CI values were narrow. This current review also included data from additional parts of the world compared with the previous version; only African, South Asian and Chinese populations were not represented.
Our study shows that the incidence of SAH has declined over the past decades, although to a lesser extent than that of stroke in general. Moreover, incidence continues to increase until older age, is higher in women than in men only after the fifth decade and varies considerably per region. Further studies should address the reasons for the relative moderate decline in incidence of SAH, the higher incidence in women only after the fifth decade and the regional differences in SAH incidence. The answers to these questions will probably provide further clues to the aetiology of SAH.
Abbreviations
SAH - subarachnoid haemorrhage
Footnotes
Competing interests: None.
References
- 1.Feigin V L, Lawes C M, Bennett D A.et al Stroke epidemiology: a review of population‐based studies of incidence, prevalence, and case‐fatality in the late 20th century. Lancet Neurol 2003243–53. [DOI] [PubMed] [Google Scholar]
- 2.Johnston S C, Selvin S, Gress D R. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998501413–1418. [DOI] [PubMed] [Google Scholar]
- 3.Feigin V L, Rinkel G J E, Lawes C M.et al Risk factors for subarachnoid hemorrhage. An updated systematic review of epidemiological studies. Stroke 2005362773–2780. [DOI] [PubMed] [Google Scholar]
- 4.Linn F H H, Rinkel G J E, Algra A.et al Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta‐analysis. Stroke 199627625–629. [DOI] [PubMed] [Google Scholar]
- 5.Thrift A G, Dewey H M, Macdonell R A.et al Incidence of the major stroke subtypes: initial findings from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2001321732–1738. [DOI] [PubMed] [Google Scholar]
- 6.ACROSS study Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand. Stroke 2000311843–1850. [DOI] [PubMed] [Google Scholar]
- 7.Jamrozik K D, Broadhurst R J, Lai N.et al Trends in the incidence, severity, and short‐term outcome of stroke in Perth, Western Australia. Stroke 1999302105–2111. [DOI] [PubMed] [Google Scholar]
- 8.Corbin D O, Poddar V, Hennis A.et al Incidence and case fatality rates of first‐ever stroke in a black Caribbean population: the Barbados Register of Strokes. Stroke 2004351254–1258. [DOI] [PubMed] [Google Scholar]
- 9.Lavados P M, Sacks C, Prina L.et al Incidence, 30‐day case‐fatality rate, and prognosis of stroke in Iquique, Chile: a 2‐year community‐based prospective study (PISCIS project). Lancet 20053652206–2215. [DOI] [PubMed] [Google Scholar]
- 10.Kolominsky‐Rabas P L, Sarti C, Heuschmann P U.et al A prospective community‐based study of stroke in Germany—the Erlangen Stroke Project (ESPro): incidence and case fatality at 1, 3, and 12 months. Stroke 1998292501–2506. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe C D, Rudd A G, Howard R.et al Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry 200272211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell P M, Coull A J, Giles M F.et al Change in stroke incidence, mortality, case‐fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 20043631925–1933. [DOI] [PubMed] [Google Scholar]
- 13.Vibo R, Korv J, Haldre S.et al First‐year results of the third stroke registry in Tartu, Estonia. Cerebrovasc Dis 200418227–231. [DOI] [PubMed] [Google Scholar]
- 14.Sivenius J, Tuomilehto J, Immonen‐Raiha P.et al Continuous 15‐year decrease in incidence and mortality of stroke in Finland. The FINSTROKE Study. Stroke 200435420–425. [DOI] [PubMed] [Google Scholar]
- 15.Immonen‐Raiha P, Sarti C, Tuomilehto J.et al Eleven‐year trends of stroke in Turku, Finland. Neuroepidemiology 200322196–203. [DOI] [PubMed] [Google Scholar]
- 16.Jakovljevic D, Sivenius J, Sarti C.et al Socioeconomic inequalities in the incidence, mortality and prognosis of subarachnoid hemorrhage: the FINMONICA Stroke Register. Cerebrovasc Dis 2001127–13. [DOI] [PubMed] [Google Scholar]
- 17.Numminen H, Kotila M, Waltimo O.et al Declining incidence and mortality rates of stroke in Finland from 1972 to 1991. Results of three population‐based stroke registers. Stroke 1996271487–1491. [DOI] [PubMed] [Google Scholar]
- 18.Tsiskaridze A, Djibuti M, van Melle G.et al Stroke incidence and 30‐day case‐fatality in a suburb of Tbilisi. Results of the first prospective population‐based study in Georgia. Stroke 2004352523–2528. [DOI] [PubMed] [Google Scholar]
- 19.Vemmos K N, Bots M L, Tsibouris P K.et al Stroke incidence and case fatality in southern Greece. The Arcadia Stroke Registry. Stroke 199930363–370. [DOI] [PubMed] [Google Scholar]
- 20.Di Carlo A, Inzitari D, Galati F.et al A prospective community‐based study of stroke in Southern Italy: The Vibo Valentia Incidence of Stroke Study (VISS). Methodology, incidence and case fatality at 28 days, 3 and 12 months. Cerebrovasc Dis 200316410–417. [DOI] [PubMed] [Google Scholar]
- 21.Carolei A, Marini C, Di Napoli M.et al High stroke incidence in the prospective community‐based L'Aquila registry (1994–1998). First year's results. Stroke 1997282500–2506. [DOI] [PubMed] [Google Scholar]
- 22.Lauria G, Gentile M, Fassetta G.et al Incidence and prognosis of stroke in the Belluno province, Italy. First‐year results of a community‐based study. Stroke 1995261787–1793. [DOI] [PubMed] [Google Scholar]
- 23.Hamada J, Morioka M, Yano S.et al Incidence and early prognosis of aneurysmal subarachnoid hemorrhage in Kumamoto Prefecture, Japan. Neurosurgery 20045431–37. [DOI] [PubMed] [Google Scholar]
- 24.Ohkuma H, Fujita S, Suzuki S. Incidence of aneurysmal subarachnoid hemorrhage in shimokita, Japan, from 1989 to 1998. Stroke 200233195–199. [DOI] [PubMed] [Google Scholar]
- 25.Inagawa T, Takechi A, Yahara K.et al Primary intracerebral and aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Part 1: Incidence and seasonal and diurnal variations. J Neurosurg 200093958–966. [DOI] [PubMed] [Google Scholar]
- 26.Inagawa T, Tokuda Y, Ohbayashi N.et al Study of aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Stroke 199526761–766. [DOI] [PubMed] [Google Scholar]
- 27.Inagawa T. Trends in incidence and case fatality rates of aneurysmal subarachnoid hemorrhage in Izum City, Japan, between 1980–1989 and 1990–1998. Stroke 2001321499–1507. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa Y, Nakagawa H, Naruse Y.et al Trends in stroke incidence and acute case fatality in a Japanese rural area : the Oyabe study. Stroke 2000311583–1587. [DOI] [PubMed] [Google Scholar]
- 29.Abdul‐Ghaffar N U, el Sonbaty M R, el‐Din Abdul‐Baky M S.et al Stroke in Kuwait: a three‐year prospective study. Neuroepidemiology 19971640–47. [DOI] [PubMed] [Google Scholar]
- 30.Smadja D, Cabre P, May F.et al ERMANCIA: Epidemiology of Stroke in Martinique, French West Indies: Part I: methodology, incidence, and 30‐day case fatality rate. Stroke 2001322741–2747. [DOI] [PubMed] [Google Scholar]
- 31.Truelsen T, Bonita R, Duncan J.et al Changes in subarachnoid hemorrhage mortality, incidence, and case fatality in New Zealand between 1981–1983 and 1991–1993. Stroke 1998292298–2303. [DOI] [PubMed] [Google Scholar]
- 32.Ellekjaer H, Holmen J, Indredavik B.et al Epidemiology of stroke in Innherred, Norway, 1994 to 1996. Incidence and 30‐day case‐fatality rate. Stroke 1997282180–2184. [DOI] [PubMed] [Google Scholar]
- 33.Correia M, Silva M R, Matos I.et al Prospective community‐based study of stroke in Northern Portugal: incidence and case fatality in rural and urban populations. Stroke 2004352048–2053. [DOI] [PubMed] [Google Scholar]
- 34.Feigin V L, Nikitin Y P, Bots M L.et al A population‐based study of the associations of stroke occurrence with weather parameters in Siberia, Russia (1982–92). Eur J Neurol 20007171–178. [DOI] [PubMed] [Google Scholar]
- 35.Feigin V L, Wiebers D O, Nikitin Y P.et al Stroke epidemiology in Novosibirsk, Russia: a population based study. Mayo Clin Proc 199570847–852. [DOI] [PubMed] [Google Scholar]
- 36.Syme P D, Byrne A W, Chen R.et al Community‐based stroke incidence in a Scottish population: the Scottish Borders Stroke Study. Stroke 2005361837–1843. [DOI] [PubMed] [Google Scholar]
- 37.Caicoya M, Rodriguez T, Lasheras C.et al Stroke incidence in Asturias, 1990–1991. Rev Neurol 199624806–811. [PubMed] [Google Scholar]
- 38.Sacco R L, Boden‐Albala B, Gan R.et al Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 1998147259–268. [DOI] [PubMed] [Google Scholar]
- 39.Brown R D, Whisnant J P, Sicks J R D.et al Stroke incidence, prevalence and survival. Secular trends in Rochester, Minnestota, through 1989. Stroke 199627373–380. [PubMed] [Google Scholar]
- 40.Nilsson O G, Lindgren A, Ståhl N.et al Incidence of intracerebral and subarachnoid haemorrhage in southern Sweden. J Neurol Neurosurg Psychiatry 200069601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appelros P, Nydevik I, Seiger A.et al High incidence rates of stroke in Orebro, Sweden: Further support for regional incidence differences within Scandinavia. Cerebrovasc Dis 200214161–168. [DOI] [PubMed] [Google Scholar]
- 42.Stegmayr B, Eriksson M, Asplund K. Declining mortality from subarachnoid hemorrhage. Changes in incidence and case fatality from 1985 through 2000. Stroke 2004352059–2063. [DOI] [PubMed] [Google Scholar]
- 43.Wolfe C D, Giroud M, Kolominsky‐Rabas P.et al Variations in stroke incidence and survival in 3 areas of Europe. European Registries of Stroke (EROS) Collaboration. Stroke 2000312074–2079. [DOI] [PubMed] [Google Scholar]
- 44.D'Alessandro G, Bottacchi E, Di Giovanni M.et al Temporal trends of stroke in Valle d'Aosta, Italy. Incidence and 30‐day fatality rates. Neurol Sci 20002113–18. [DOI] [PubMed] [Google Scholar]
- 45.Khan F A, Engstrom G, Jerntorp I.et al Seasonal patterns of incidence and case fatality of stroke in Malmo, Sweden: the STROMA study. Neuroepidemiology 20052426–31. [DOI] [PubMed] [Google Scholar]
- 46.Truelsen T, Gronbaek M, Schnohr P.et al Stroke case fatality in Denmark from 1977 to 1992: the Copenhagen City Heart Study. Neuroepidemiology 20022122–27. [DOI] [PubMed] [Google Scholar]
- 47.Bamford J M, Sandercock P A G, Dennis M S.et al A prospective study of acute cerebrovascular disease in the community: the Oxfordshire community stroke project 1981–1986. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 19905316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longstreth W T, Jr, Nelson L M, Koepsell T D.et al Clinical course of spontaneous subarachnoid hemorrhage: a population‐based study in King County, Washington. Neurology 199343712–718. [DOI] [PubMed] [Google Scholar]
- 49.Jerntrop P, Berglund G. Stroke registry in Malmö, Sweden. Stroke 199223357–361. [DOI] [PubMed] [Google Scholar]
- 50.Anderson C S, Jamrozik K D, Burvill P W.et al Determining the incidence of different subtypes of stroke: results from the Perth community stroke study 1989–1990. Med J Aust 199315885–89. [DOI] [PubMed] [Google Scholar]
- 51.Bonita R, Thomson S. Subarachnoid hemorrhage: epidemiology, diagnosis, management and outcome. Stroke 198516591–594. [DOI] [PubMed] [Google Scholar]
- 52.Herman B, Leyten A C, van Luijk J H.et al Epidemiology of stroke in Tilburg, the Netherlands. The population‐based stroke incidence register: 2. Incidence, initial clinical picture and medical care, and three‐week case fatality. Stroke 198213629–634. [DOI] [PubMed] [Google Scholar]
- 53.Ricci S, Celani M G, La Rosa F.et al A community‐based study of incidence, risk factors and outcome of transient ischaemic attacks in Umbria, Italy: the SEPIVAC study. J Neurol 199123887–90. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka H, Ueda Y, Date C. Incidence of stroke in Shibata, Japan: 1976–1978. Stroke 198112460–466. [DOI] [PubMed] [Google Scholar]
- 55.Terent A. Increasing incidence of stroke among Swedish women. Stroke 198819598–603. [DOI] [PubMed] [Google Scholar]
- 56.Sarti C, Tuomilehto J, Salomaa V.et al Epidemiology of subarachnoid hemorrhage in Finland from 1983 to 1985. Stroke 199122848–853. [DOI] [PubMed] [Google Scholar]
- 57.Giroud M, Milan C, Beuriat P.et al Incidence and survival rates during a two‐year period of intracerebral and subarachnoid haemorrhages, cortical infarcts, lacunes and transient ischaemic attacks. The Stroke Registry of Dijon: 1985–1989. Int J Epidemiol 199120892–899. [DOI] [PubMed] [Google Scholar]
- 58.Sivenius J, Heinonen O P, Pyörälä K.et al The incidence of stroke in the Kuopio area of east Finland. Stroke 198516188–192. [DOI] [PubMed] [Google Scholar]
- 59.Aho K, Fogelhom R. Incidence and early prognosis of stroke in Espoo‐Kauniainen area; Finland in 1972. Stroke 1974558–61. [PubMed] [Google Scholar]
- 60.Hansen B S, Marquardsen J. Incidence of stroke in Frederiksberg, Denmark. Stroke 19778663–665. [DOI] [PubMed] [Google Scholar]
- 61.Gross C R, Kase C S, Mohr J P.et al Stroke in South Alabama: incidence and diagnostic features—a population based study. Stroke 198415249–255. [DOI] [PubMed] [Google Scholar]
- 62.Norrving B, Löwenheim P. Epidemiology of stroke in Lund‐Orup, Sweden, 1983–85. Incidence of first stroke and age‐related changes in subtypes. Acta Neurol Scand 198878708–713. [DOI] [PubMed] [Google Scholar]
- 63.Epstein L, Rishpon S, Bental E.et al Incidence, mortality, and case‐fatality rate of stroke in northern Israel. Stroke 198920725–729. [DOI] [PubMed] [Google Scholar]
- 64.Jorgensen H S, Plesner A M, Hubbe P.et al Marked increase of stroke incidence in men between 1972 and 1990 in Frederiksberg, Denmark. Stroke 1992231701–1704. [DOI] [PubMed] [Google Scholar]
- 65.Wermer M J, van der Schaaf I C, Algra A.et al Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: a meta‐analysis. Stroke 2007381404–1410. [DOI] [PubMed] [Google Scholar]
- 66. United Nations Populations Division, DESA. World Population Prospects: The 2006 Revision population ageing. http://www.un.org/esa/Population/publications/wpp2006/wpp2006_ageing.pdf (accessed 4 october 2007)
- 67.Huang J, van Gelder J M. The probability of sudden death from rupture of intracranial aneurysms: a meta‐analysis. Neurosurgery 2002511101–1105. [DOI] [PubMed] [Google Scholar]
- 68.Stegmayr B, Asplund K, Kuulasmaa K.et al Stroke incidence and mortality correlated to stroke risk factors in the WHO MONICA Project. An ecological study of 18 populations. Stroke 1997281367–1374. [DOI] [PubMed] [Google Scholar]
- 69.Makela P, Fonager K, Hibell B.et al Episodic heavy drinking in four Nordic countries: a comparative survey. Addiction 2001961575–1588. [DOI] [PubMed] [Google Scholar]
- 70.Kissela B, Schneider A, Kleindorfer D.et al Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 200435426–431. [DOI] [PubMed] [Google Scholar]
- 71.van Gijn J, Rinkel G J E. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001124249–278. [DOI] [PubMed] [Google Scholar]
- 72.Longstreth W T, Nelson L M, Koepsell T D.et al Subarachnoid hemorrhage and hormonal factors in women. A population‐based case‐control study. Ann Intern Med 1994121168–173. [DOI] [PubMed] [Google Scholar]
- 73.Mhurchu C N, Anderson C S, Jamrozik K D, ACROSS study et al Hormonal factors and risk of aneurysmal subarachnoid hemorrhage. An international population‐based, case‐control study. Stroke 200132606–612. [DOI] [PubMed] [Google Scholar]
- 74.Vibo R, Korv J, Roose M. The Third Stroke Registry in Tartu, Estonia: decline of stroke incidence and 28‐day case‐fatality rate since 1991. Stroke 2005362544–2548. [DOI] [PubMed] [Google Scholar]
- 75.Pajunen P, Paakkonen R, Hamalainen H.et al Trends in fatal and nonfatal strokes among persons aged 35 to > or = 85 years during 1991–2002 in Finland. Stroke 200536244–248. [DOI] [PubMed] [Google Scholar]
- 76.van Gijn J, van Dongen K J. Computed tomography in the diagnosis of subarachnoid haemorrhage and ruptured aneurysm. Clin Neurol Neurosurg 19808211–24. [DOI] [PubMed] [Google Scholar]
- 77.Ruigrok Y M, Buskens E, Rinkel G J E. Attributable risk of common and rare determinants of subarachnoid hemorrhage. Stroke 2001321173–1175. [DOI] [PubMed] [Google Scholar]