Abstract
Background/Aims
Recent evidence has implicated the involvement of aquaporins (AQPs) in cellular functions that are unrelated to transepithelial water transport. Although AQPs are expressed in the gastrointestinal tract, their importance has so far been unclear. AQP3 is a water/glycerol transporter expressed at the basolateral membrane of colonic epithelial cells. The aim of this study was to investigate the involvement of AQP3 in enterocyte proliferation using mouse models of inflammatory bowel disease.
Methods
Expression and function of AQP3 in mouse colonic epithelium were established. Colitis was induced in wild‐type and AQP3 null mice by oral dextran sulphate administration or intracolonic acetic acid administration. Outcome measures included clinical disease severity, survival, pathology and cellular responses. Some mice were administered glycerol to test whether disease progression could be altered.
Results
AQP3 null mice given dextran sulphate developed severe colitis after 3 days, with colonic haemorrhage, marked epithelial cell loss and death. Wild‐type mice, which had comparable initial colonic damage as assessed by cell apoptosis, developed remarkably less severe colitis, surviving to >8 days. Cell proliferation was greatly reduced in AQP3 null mice. Oral glycerol administration significantly improved survival and reduced the severity of colitis in AQP3 null mice. Survival was also reduced in AQP3 null mice in the acetic acid model.
Conclusions
The results implicate a novel role for AQP3 in enterocyte proliferation that is probably related to its glycerol‐transporting function. AQP3 is thus a potential target for therapy of intestinal diseases associated with enterocyte destruction.
The aquaporins (AQPs) are a family of water channels expressed in many epithelial, endothelial and other cell types. They facilitate transepithelial water transport in kidney tubules for urine concentration, and in glandular, choroidal and ciliary epithelia for fluid secretion.1,2 AQPs in non‐epithelial tissues in the central nervous system and eye are also involved in the regulation of tissue hydration. Recently, analysis of transgenic mice lacking specific AQPs has revealed new cellular roles for AQPs that are unrelated to transcellular water transport.3 We found impaired angiogenesis in AQP1‐deficient mice as a consequence of reduced endothelial cell migration,4 which may be caused by slowed water movement into lamellipodia at the leading edge of migrating cells. A subset of AQPs (AQPs 3, 7 and 9), called ‘aquaglyceroporins', transport water as well as glycerol, and possibly other small solutes. Mice lacking AQP3 have dry skin and delayed epidermal healing, with reduced glycerol content in epidermis and stratum corneum,5,6 caused by impaired glycerol transport from the dermis through the normally AQP3‐expressing basal keratinocytes. Recent studies provided evidence for a new role for AQP3 in cell proliferation. Mice lacking AQP3 were found to have significantly impaired epidermal proliferation in a wound healing model,7 and impaired corneal epithelial cell proliferation after epithelial injury.8 Mice lacking AQP7 manifest age‐dependent adipocyte hypertrophy and glycerol accumulation,9 which we proposed is caused by reduced glycerol exit from the normally AQP7‐expressing adipocytes.
AQPs are expressed strongly in gastrointestinal organs including the stomach (parietal cells), liver (hepatocytes and cholangiocytes), pancreas (acinar epithelia), gallbladder (epithelium), small intestine (lacteals, enterocytes) and colon (colonocytes).10,11 Consequently, roles for AQPs in the secretion of bile and pancreatic fluid have been postulated, as well as in intestinal fluid absorption and secretion. However, phenotype analysis of AQP1, AQP4 and AQP8 knockout mice has revealed little or no consequence of AQP deletion on major gastrointestinal fluid‐transporting functions.12,13,14,15,16 The absence of overt gastrointestinal phenotypes in AQP‐deficient mice is surprising in view of the renal, central nervous system, ocular, glandular, cutaneous and other phenotypes found in these mice, particularly since the magnitude of gastrointestinal fluid transport is second only to that in kidney.
The epithelium lining the intestine maintains its architecture by a balance between the continuous processes of epithelial cell generation from clonal stem cells17 at the base of intestinal crypts, and death of cells near the luminal surface. Recent evidence has suggested dysregulation of these processes in inflammatory bowel diseases (IBDs) such as ulcerative colitis, and has emphasised the importance of cell proliferation in disease progression.18,19 There is evidence for a crucial role for Toll‐like receptor (TLR) signalling and commensal bacteria in the initiation and transduction of the inflammatory and tissue repair responses.20,21 TLR‐4 null mice and related MyD88 null mice show significantly more severe disease progression in murine models of colitis as a result of impaired epithelial cell proliferation.20
Here, we present evidence for a new AQP function in the gastrointestinal tract. We found remarkably greater colonic pathology and mortality in mice lacking AQP3 than in wild‐type mice in experimental models of colitis, with impaired epithelial cell proliferation at the base of colonic crypts in the null mice. The motivations for this study included the strong expression of AQP3 in colonic epithelial cells that undergo rapid turnover,17 and, as mentioned above, the impairment of epidermal and corneal cell proliferation in AQP3 deficiency.7,8 We used the dextran sulphate and intracolonic acetic acid models of colitis21,22,23,24 to induce epithelial damage and restitution in the colon. The primary model used was the dextran sulphate model, a well‐described oral model of colitis. In order to verify the findings using a direct damage, non‐oral model, we also used intracolonic instillation of acetic acid.25,26 Enterocyte turnover is strongly stimulated in these models, which we predicted could expose defects in enterocyte proliferation and/or migration in AQP3 deficiency. Consequences of the findings here include the potential involvement of AQP3 in epithelial cell proliferation in a variety of intestinal inflammatory diseases, and the possibility of new therapies based on pharmacological modulation of AQP3 expression.
Materials and methods
Mice
AQP3 null mice in a CD1 genetic background were generated by targeted gene disruption as described.25 Six‐ to ten‐week‐old male weight‐matched wild‐type and AQP3 null mice were used. For some control studies, AQP1 null mice in a CD1 genetic background were used.28 The mice were maintained in air‐filtered cages and fed normal mouse chow in the UCSF Animal Care facility. All procedures were approved by the UCSF Committee on Animal Research.
Dextran sulphate model of colitis
Dextran sulphate sodium (molecular weight 36 000–50 000 Da; MP Biomedicals, Solon, OH, USA) was given ad libitum in the drinking water. Wild‐type mice were given 6% dextran sulphate to induce acute colitis after 4 days. Since AQP3 null mice have increased daily water intake (see the Results section), in order to achieve the same dextran sulphate load24 AQP3 null mice were given 2% dextran sulphate. Colitis induction in AQP1 mice was carried out as for AQP3 mice. Fluid consumption, body weight, stool consistency and colonic bleeding were monitored, and a disease activity index (DAI) was calculated as described.29,30
Acetic acid model of colitis
Prior to acetic acid administration, mice were deprived of food for 24 h (but given 5% sucrose in water). Mice were then anaesthetised using 125 mg/kg 2,2,2‐tribromoethanol (Avertin; Sigma‐Aldrich, St Louis, MO, USA). As modified from published protocols,25,26 a PE‐50 catheter was inserted 3–4 cm into the lumen of the colon via the anus for infusion of 0.1 ml of 3% acetic acid in saline. After 1 min, the acetic acid was washed out with three lavages of saline, 0.3 ml each. Mice were assessed daily for body weight and survival for 10 days. Colon histology was obtained in some mice on day 3.
Histology, immunocytochemistry and immunoblot analysis
Mice were killed and colons excised, fixed in 10% neutral buffered formalin, and embedded in paraffin. For immunohistochemistry, tissue sections (5 μm) were cut, deparaffinised, and stained with a rabbit polyclonal AQP3 antibody (Chemicon, Temecula, CA, USA) and Texas Red mouse anti‐rabbit IgG (Vector Labs, Burlingame, CA, USA). Wide‐field fluorescence images were taken using a Leica DM4000B epifluorescence microscope and Spot RT CCD camera; confocal images were taken on a Leitz microscope with a Nipkow wheel confocal attachment. Immunoblot analysis was done on homogenates of full‐thickness colon. For histology, tissue sections (5 μm) were stained with H&E. Colon length was measured after excision. Crypt length and density were measured from at least two sections per mouse colon. Colonic epithelial damage was scored as follows: 0 = intact crypt; 1 = mild crypt loss (10–33%); 2 = moderate crypt loss (33–66%); 3 = severe crypt loss (66–100%); 4 = complete loss of crypt and surface epithelium intact. Infiltration with inflammatory cells was also scored (0 = normal, 1 = mild, 2 = modest, 3 = severe, 4 = very severe).
Glycerol transport
To measure transepithelial glycerol permeability, closed colonic loops were created as described.16,31 Loops were filled with phosphate‐buffered saline containing 10 mM glycerol and 250 mM raffinose (to maintain loop volume over the course of the experiment). Based on preliminary experiments showing an ∼2 h half‐time for glycerol disappearance from loops, fluid was sampled using a syringe from loops at 0 and 2 h for colorimetric assay of glycerol concentration (R‐Biopharm AG, Germany).
Bromodeoxyuridine and apoptosis measurements
For measurement of cell proliferation, mice were injected intraperitoneally with 1 mg of bromodeoxyuridine (BrdU; Sigma, St Louis, MO, USA) at 3 days after initiation of dextran sulphate administration. Intestines were excised 2 h post‐injection, fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections (5 μm) were cut, deparaffinised, stained with rat anti‐BrdU antibody (Chemicon, Temecula, CA, USA) and peroxidase/diaminobenzidine (DAB; Vector Labs), and counterstained with haematoxylin. Apoptosis staining was done on fixed paraffin sections at 2 days using a DNA fragmentation assay kit (APO‐BrdU‐IHC, Biovision Inc., Mountain View, CA, USA). The extent of apoptosis was quantified from the staining intensity by image analysis (Image J, Wayne Rasband, NIH) of 3–5 areas per section (two sections per mouse).
Glycerol replacement experiments
In some studies, wild‐type and AQP3 null mice were supplemented with oral glycerol (2% (w/v) in drinking water, Fluka Chemie GmBH, Germany). Dextran sulphate was administered as described above, and assessment of DAI and histology were done.
Data analysis
All values are expressed as mean ± SEM for data on different mice. Differences between groups were analysed using one‐way analysis of variance (ANOVA) with Tukey–Kramer post hoc test or Student t test where appropriate.
Results
AQP3 expression and function in murine colon
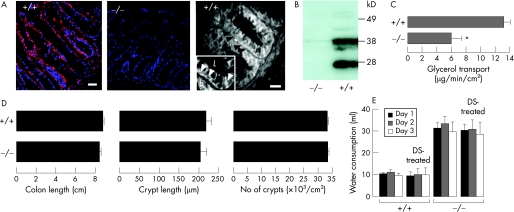
Previous studies have shown AQP3 expression in human and rat colon.32,33 Immunostaining of mouse colon showed AQP3 localisation in the basolateral membrane of colonic epithelial cells, mainly at the base of colonic crypts (fig 1A). Immunoblot analysis confirmed the antibody specificity, with AQP3 expression seen in colon homogenates from wild‐type but not AQP3 null mice (fig 1B). To confirm functional AQP3 expression in colon, glycerol transport from lumen to blood was measured in closed colonic loops. Figure 1C shows an ∼2‐fold reduced rate of glycerol disappearance from colonic loops of AQP3 null mice, indicating reduced transepithelial glycerol permeability. The actual fold difference in enterocyte basolateral membrane glycerol permeability may be much greater because of unstirred layer effects and serial permeability barriers. Histology of the colonic mucosa in wild‐type and AQP3 null mice were indistinguishable, without significant differences in crypt length or density (fig 1D).
Figure 1 Expression and function of aquaporin 3 (AQP3) in mouse colon. (A) AQP3 immunofluorescence in the proximal colon of a wild‐type mouse (wide‐field left, confocal right) and an AQP3 null mouse (middle). Nuclei stained blue with 4′,6‐diamidino‐2‐phenylindole (DAPI). Arrows indicate basolateral membrane staining. Scale bars = 20 μm. The inset shows a higher magnification view (b, basolateral; l, lumen). (B) Immunoblot of whole colon homogenate. (C) Transepithelial glycerol transport measured in closed colonic loops in vivo (three loops per mouse) (SEM, n = 4 mice. p<0.05). (D) Colon length (left), crypt length (middle) and crypt density (right) (SEM, n = 5 mice). (E) Daily water consumption (SEM, n = 16 mice). DS, dextran sulphate.
Reduced survival with severe colonic pathology in dextran sulphate‐treated AQP3 null mice
Based on the postulated roles of AQP3 in epidermal and corneal cell proliferation, we tested whether AQP3 has a role in colonic epithelial cell regeneration/restitution after inflammatory damage. A well‐established model of acute colitis produced by oral dextran sulphate was used to induce colonic inflammation and epithelial cell damage.22 Pilot studies on wild‐type mice established the dextran sulphate load required in our mouse strain to produce colitis reliably within 7 days. Previous studies have shown that AQP3 null mice are polyuric because of reduced water permeability in the kidney collecting duct,27 with consequently greater daily fluid consumption than wild‐type mice.34 In order to match dextran sulphate load in the wild‐type and AQP3 null experimental groups, the amount of dextran sulphate was normalised by daily water intake (fig 1E).
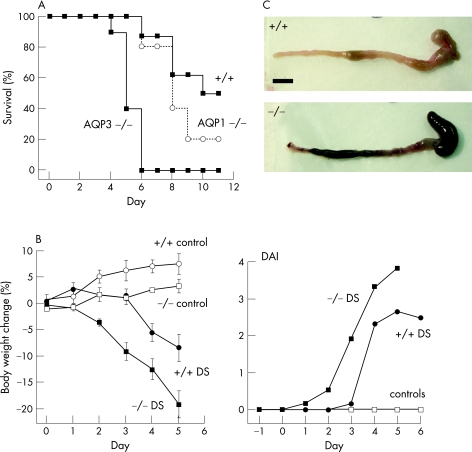
Figure 2A shows remarkably reduced survival of AQP3 null mice after dextran sulphate‐induced colitis, with no mice surviving to 6 days. Figure 2B summarises the progression of body weight and DAI following dextran sulphate administration, revealing significantly more severe colitis in AQP3 null than wild‐type mice, with an earlier appearance of clinical signs of colitis. At 3 days, the colons of AQP3 null mice were grossly haemorrhagic with decreased colon length (fig 2C).
Figure 2 Aquaporin 3 (AQP3) deficiency worsens the severity of dextran sulphate (DS)‐induced colitis. (A) Survival after administration of DS. AQP1 −/−, aquaporin 1 null. (B) Left: body weight (SEM, 4 mice for control groups, 8 mice for DS‐treated groups). Right: disease activity index (DAI) after DS treatment (4 mice for control groups, 8 mice for DS‐treated groups). (C) Diseased bowel.
Control experiments were done to rule out the possibility that systemic factors related to polyuria in AQP3 null mice could account for their worse survival. Dextran sulphate experiments were done on mice lacking AQP1, which have similar polyuria to AQP3 null mice.28 AQP1 is not expressed in colonic epithelium. Figure 2A shows that the survival of AQP1 null mice was similar to that of wild‐type mice, and notably better than that of AQP3 null mice, suggesting that the greater disease progression in AQP3 null mice results from greater colonic pathology.
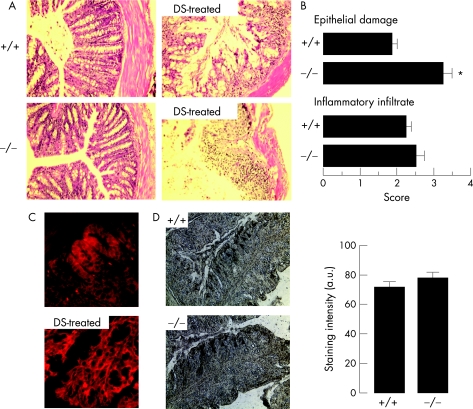
Sections of colon from wild‐type and AQP3 null mice were compared to assess structural and epithelial damage after dextran sulphate administration. Figure 3A shows considerably more epithelial damage in AQP3 null mice than in wild‐type mice at 5 days, with near complete loss of the epithelium. Because dextran sulphate‐induced colonic epithelial damage can be patchy, histological scoring of many sections was done (fig 3B), showing significantly increased mucosal damage in AQP3 null mice. In contrast, markers of inflammation, such as infiltration of leucocytes and neutrophils into the mucosa, were similar in wild‐type and AQP3 null mice (fig 3B). Interestingly, by immunostaining, colonic AQP3 expression was elevated in wild‐type mice at 3 days after dextran sulphate administration compared with untreated wild‐type mice (fig 3C), providing further evidence for involvement of AQP3 in the colonic cellular response to injury. Figure 3D shows similar numbers of apoptotic epithelial cells in wild‐type and AQP3 null mice at 2 days after dextran sulphate administration, suggesting similar cellular insult.
Figure 3 Analysis of colonic structural responses to dextran sulphate (DS)‐induced colitis. (A) H&E‐stained sections of colon from control and DS‐treated mice at 5 days. (B) Histological scores of epithelial damage and inflammatory cell infiltrate (5 mice per group, p<0.005). (C) Increased AQP3 immunostaining in wild‐type mice at 3 days vs before DS treatment (representative of sections from four wild‐type and AQP3 null mice). (D) Micrographs (left) showing apoptosis 2 days after DS treatment. Arrows indicate apoptotic cells. Right: staining intensity (SEM, n = 3 mice).
Reduced survival in acetic acid‐treated AQP3 null mice
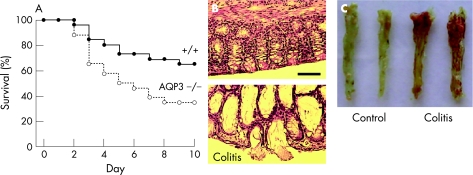
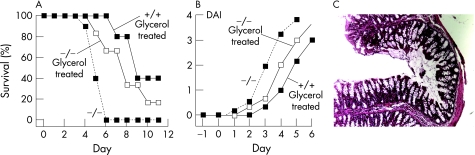
An intracolonic infusion acetic acid model was developed as an alternative model of colitis that does not require oral administration. After testing a series of models, including intracolonic trinitrobenzenesulphonic acid (TNBS), we adapted the well‐described acetic acid model used previously to produce acute colitis in mice.25,26 Following pilot studies to determine acetic acid dose and volume, administration details and washout procedures, conditions were established in which survival of wild‐type mice was 30–40% at 10 days after treatment (fig 4A). Under identical treatment conditions there was significantly reduced survival of AQP3 null mice at 3–10 days after treatment. Acetic acid treatment produced marked epithelial and goblet cell loss, with distortion of crypt architecture and marked infiltration of inflammatory cells in the lamina propria (fig 4B). Prominent mucosal congestion and haemorrhage was seen at the macroscopic level (fig 4C). These results support the conclusion that AQP3 deficiency is associated with a greater severity of colitis.
Figure 4 Reduced survival of aquaporin 3 (AQP3) null mice in an acetic acid model of colitis. Acetic acid (3%) applied by intracolonic administration as described in the Materials and methods section. (A) Mouse survival after acetic acid treatment (26 mice per group, p = 0.02, log‐rank test). (B) H&E‐stained sections of ascending colon from a wild‐type control (top) and an acetic acid‐treated (bottom) mouse at 3 days. Scale bar = 100 μm. (C) Colonic mucosa of control and acetic acid‐treated mice.
Impaired enterocyte proliferation in AQP3 deficiency
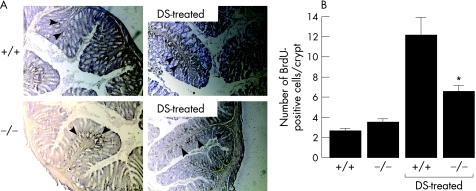
Proliferation of epithelial cells after mucosal damage is an important determinant of disease progression in colitis,35 with studies showing marked enterocyte proliferation in dextran sulphate‐induced colitis.36 BrdU pulse–chase experiments were done to compare colonic epithelial cell proliferation in wild‐type and AQP3 null mice in the dextan sulphate model. BrdU‐labelled sections of colonic mucosa (fig 5A) showed the expected increase in cell proliferation in wild‐type mice at 3 days after dextran sulphate treatment, with 12±2 BrdU‐labelled cells per crypt (fig 5B). Cell proliferation in AQP3 null mice was also increased, though significantly less than in wild‐type mice, with 6.6±0.6 BrdU‐labelled cells per crypt (fig 5B)
Figure 5 Enterocyte hypoproliferation in colitis in aquaporin 3 (AQP3) null mice. Bromodeoxyuridine (BrdU) pulse–chase experiments were carried out as described in the Materials and methods section at 3 days after dextran sulphate (DS) administration. Sections are representative of four or more obtained per mouse (n = 5 mice). (A) BrdU‐stained cells in control and DS‐treated colons. (B) Number of BrdU‐positive cells per crypt (SEM, n = 5 mice).
Oral glycerol administration reduces the severity of colitis in AQP3 null mice
To test whether AQP3‐facilitated glycerol transport may be involved in enterocyte proliferation and responsible for the colitis phenotype difference in wild‐type vs AQP3 null mice, we administered glycerol to dextran sulphate‐treated mice to determine whether the course of the colitis could be modified. A similar glycerol replacement strategy was used to demonstrate that the glycerol‐transporting function of AQP3 was responsible for the difference in skin phenotype in wild‐type vs AQP3 null mice.6,7 Figure 6A and B shows significantly greater survival and reduced severity of colitis in the glycerol‐treated AQP3 null mice. Histological examination at 5 days (fig 6C) showed evidence of reduced crypt/epithelial damage compared with that seen in untreated AQP3 null mice (fig 4A).
Figure 6 Oral glycerol administration reduces the severity of dextran sulphate (DS)‐induced colitis in aquaporin 3 (AQP3) null mice. (A) Survival curve for wild‐type and AQP3 null mice after DS administration with oral glycerol treatment (6 mice per group). For comparison, survival is shown for AQP3 null/DS‐treated mice without glycerol (dashed line). (B) Disease activity index (DAI) after glycerol treatment (SEM, n = 6 mice). (C) H&E‐stained sections of colon from a glycerol‐treated AQP3 null mouse at 5 days after DS administration.
Discussion
These studies revealed an interesting gastrointestinal phenotype in AQP3 null mice with potential clinical relevance and broad implications to tissues outside of the colon. In a dextran sulphate model of colitis, mice lacking AQP3 had remarkably more severe colonic pathology and reduced survival than control mice. Wild‐type mice showed basal crypt cell hyperproliferation in response to colonic epithelial cell injury, whereas the AQP3 null mice had reduced epithelial cell proliferation, probably accounting for their more rapid worsening of clinical signs and more severe colonic pathology. Differences in outcome and cell proliferation in the AQP3 null mice could not be accounted for by differences in initial epithelial cell damage, as both groups of mice had the same dextran sulphate exposure and showed a comparable apoptotic cell response. Reduced survival of AQP3 null mice was also demonstrated in an acetic acid model of colitis, which involved intracolonic infusion rather than oral administration. Our results provide the first evidence for involvement of AQP3 in intestinal cell proliferation, which adds to a growing list of aquaporin functions that are unrelated to their classical role in transcellular water transport.3
Previous phenotype studies of mice lacking aquaglyceroporins provide clues about possible mechanisms for AQP3 involvement in cell proliferation. In skin, AQP3 deficiency reduces stratum corneum hydration and elasticity, and delays epidermal wound healing and restoration of barrier function after stratum corneum removal.5,6 Mechanistic analysis indicated reduced glycerol content in the epidermis and stratum corneum in AQP3 null mice as a consequence of reduced glycerol transport through basal epidermal cells. Reduced glycerol content impairs skin hydration because of its humectant (water‐retaining) properties, and delays healing because of its precursor role in epidermal biosynthesis. Topical or oral glycerol supplementation corrected the skin abnormalities in AQP3 null mice.6 More direct evidence for AQP3 involvement in epidermal cell proliferation was found recently,7 where AQP3 deficiency was associated with impaired cellular ATP generation and p38 mitogen‐activated protein (MAP) kinase signalling, and absent tumorigenesis (unpublished results). Whether a similar mechanism exists in colon was not determined here, since, unlike epidermis, suitable primary enterocyte cell culture models do not exist. Existing transformed colonic cell lines such as T84 and HT29 are not suitable for proliferation studies, as they are derived from colon carcinomas.
Deficiency of AQP7, another aquaglyceroporin that transports both water and glycerol, produced progressive adipocyte hypertrophy with intracellular accumulation of glycerol and triglycerides.9 Mechanistic analysis suggested that reduced adipocyte permeability in AQP7 deficiency results in progressive glycerol accumulation and a consequent shift in adipocyte metabolism favouring triglyceride biosynthesis. Taken together with the data here showing partial correction of the AQP3 defect by glycerol administration, these studies suggest an important physiological role for aquaglyceroporin‐mediated glycerol transport in cellular metabolism and biosynthesis.
Based on the previous work, the impaired cell proliferation in AQP3 deficiency reported here might be related to reduced AQP3‐facilitated glycerol transport and consequent altered cell metabolism. AQP involvement in cell proliferation may thus be a general paradigm in tissues with rapid cell turnover. In addition to, or as an alternative to altered cell metabolism, AQP3 involvement in cell proliferation may be via interactions with other associated proteins. Recent studies have begun to link aquaglyceroporins with activation of peroxisome proliferator‐activated receptors (PPARs) and also to regulation of proteins involved with glycerol metabolism.35 Activation of PPARs has also been shown to be involved in colitis, with evidence for reduced disease progression with PPAR agonist treatment.36,37 As described in the Introduction, recent studies have highlighted the central role of cell proliferation in response to a wide range of intestinal stimuli, including TLR activation and parasitic infection.18,20,38
Though the primary defect producing severe colitis in AQP3 deficiency appears to be impaired crypt cell proliferation, there may also be impairment of cell migration as a consequence of reduced colonocyte water permeability. Re‐epithelialisation of the colon involves proliferation of basal crypt epithelial cells, as well as epithelial cell migration from the bottom of the crypts towards the colonic surface. Evidence from vascular endothelial and AQP‐transfected cells has suggested a role for AQP‐mediated water transport in cell migration, which could have a number of physiologically important consequences such as in endothelial cell migration in tumour angiogenesis.4 Recent studies have shown impaired corneal re‐epithelialisation in response to injury in AQP3‐deficient mice, with impairment of both cell migration and proliferation.8 Thus, impairment of AQP3‐facilitated migration of new colonocytes might also contribute to the severe colitis in AQP3 deficiency, though we believe that this is less likely in the colon than in cornea because AQP3 is a substantially less efficient water transporter than the water‐selective AQPs,39 and colonocytes also express at least two water‐selective AQPs, AQP4 and AQP8.14,16,40 Previous studies have demonstrated AQP4‐dependent transepithelial water permeation in colon, though little effect of AQP4 or AQP8 deletion on vital colon functions was found, including faecal dehydration, fluid absorption and fluid secretion.14,16
In summary, our results support a novel function of AQP3 in enterocyte proliferation, and provide the first evidence for a major gastrointestinal phenotype associated with AQP deficiency. Further work is needed to establish the precise metabolic link between AQP3‐facilitated enterocyte glycerol transport and proliferation, and to investigate the possibility of modulation of AQP3 expression in therapy for diseases, such as Crohn's disease, that are associated with enterocyte hyper‐ or hypoproliferation.
Acknowledgements
We thank Dr Mariko Hara‐Chikuma for assistance with glycerol permeability and replacement experiments, and Liman Qian for mouse breeding. Supported by grants DK35124, HL59198, EY13574, EB00415, HL73856 and HL73856 from the National Institutes of Health, and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation.
Abbreviations
AQP - aquaporin
BrdU - bromodeoxyuridine
DAI - disease activity index
IBD - inflammatory bowel disease
PPAR - peroxisome proliferator‐activated receptor
TLR - Toll‐like receptor
Footnotes
Competing interests: None.
References
- 1.Verkman A S. Novel roles of aquaporins revealed by phenotype analysis of knockout mice. Rev Physiol Biochem Pharmacol 200515531–55. [DOI] [PubMed] [Google Scholar]
- 2.King L S, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 20045687–698. [DOI] [PubMed] [Google Scholar]
- 3.Verkman A S. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci 20051183225–3232. [DOI] [PubMed] [Google Scholar]
- 4.Saadoun S, Papadopoulos M C, Hara‐Chikuma M.et al Impairment of angiogenesis and cell migration by targeted aquaporin‐1 gene disruption. Nature 2005434786–792. [DOI] [PubMed] [Google Scholar]
- 5.Hara M, Ma T, Verkman A S. Selectively reduced glycerol in skin of aquaporin‐3‐deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J Biol Chem 200227746616–46621. [DOI] [PubMed] [Google Scholar]
- 6.Hara M, Verkman A S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin‐3‐deficient mice. Proc Natl Acad Sci USA 20031007360–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara‐Chikuma M, Verkman A S. Aquaporin‐3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med 2007; in press [DOI] [PubMed]
- 8.Levin M H, Verkman A S. Aquaporin‐3 dependent cell migration and proliferation during corneal re‐epithelialization. Invest Opthamol Vis Sci 2006474365–4372. [DOI] [PubMed] [Google Scholar]
- 9.Hara‐Chikuma M, Sohara E, Rai T.et al Progressive adipocyte hypertrophy in aquaporin‐7‐deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem 200528015493–15496. [DOI] [PubMed] [Google Scholar]
- 10.Thiagarajah J R, Verkman A S. Water transport in the gastrointestinal tract. In: Johnson LR, Barrett K, Ghishan F, Merchant J, Said H, Wood J, eds. Physiology of the gastrointestinal tract. Volume 4, New York: Academic Press, 20061827–1845.
- 11.Masyuk A I, Marinelli R A, LaRusso N F. Water transport by epithelia of the digestive tract. Gastroenterology 2002122545–562. [DOI] [PubMed] [Google Scholar]
- 12.Ma T, Jayaraman S, Wang K S.et al Defective dietary fat processing in transgenic mice lacking aquaporin‐1 water channels. Am J Physiol 2001280C126–C134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mennone A, Verkman A S, Boyer J L. Unimpaired osmotic water permeability and fluid secretion in bile duct epithelia of AQP1 null mice. Am J Physiol 2002283G739–G746. [DOI] [PubMed] [Google Scholar]
- 14.Wang K S, Ma T, Filiz F.et al Colon water transport in transgenic mice lacking aquaporin‐4 water channels. Am J Physiol 2000279G463–G470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K S, Komar A R, Ma T.et al Gastric acid secretion in aquaporin‐4 knockout mice. Am J Physiol 2000279G448–G453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Song Y, Zhao D, Verkman A S. Phenotype analysis of aquaporin‐8 null mice. Am J Physiol Cell Physiol . 2005;288C1161–C1170. [DOI] [PubMed]
- 17.Potten C S. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc B: Biol Sci 1998353821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pull S L, Doherty J M, Mills J C.et al Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA 200510299–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dohi T, Ejima C, Kato R.et al Therapeutic potential of follistatin for colonic inflammation in mice. Gastroenterology 2005128411–423. [DOI] [PubMed] [Google Scholar]
- 20.Rakoff‐Nahoum S, Paglino J, Eslami‐Varzaneh F.et al Recognition of commensal microflora by toll‐like receptors is required for intestinal homeostasis. Cell 2004118229–241. [DOI] [PubMed] [Google Scholar]
- 21.Katakura K, Lee J, Rachmilewitz D.et al Toll‐like receptor 9‐induced type I IFN protects mice from experimental colitis. J Clin Invest 2005115695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okayasu I, Hatakeyama S, Yamada M.et al A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 199098694–702. [DOI] [PubMed] [Google Scholar]
- 23.Vowinkel T, Kalogeris T J, Mori M.et al Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig Dis Sci 200449556–564. [DOI] [PubMed] [Google Scholar]
- 24.Vowinkel T, Mori M, Krieglstein C F.et al Apolipoprotein A‐IV inhibits experimental colitis. J Clin Invest 2004114260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh H, Beck P L, Inoue N.et al A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest 19991041539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh H, Tomita M, Uchino H.et al cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid‐induced colitis. Biochem J 1996318939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma T, Song Y, Yang B.et al Nephrogenic diabetes insipidus in mice lacking aquaporin‐3 water channels. Proc Natl Acad Sci USA 2000974386–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma T, Yang B, Gillespie A.et al Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin‐1 water channels. J Biol Chem 19982734296–4299. [DOI] [PubMed] [Google Scholar]
- 29.Rachmilewitz D, Karmeli F, Takabayashi K.et al Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 20021221428–1441. [DOI] [PubMed] [Google Scholar]
- 30.Rachmilewitz D, Katakura K, Karmeli F.et al Toll‐like receptor 9 signaling mediates the anti‐inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004126520–528. [DOI] [PubMed] [Google Scholar]
- 31.Thiagarajah J R, Broadbent T, Hsieh E.et al Prevention of toxin‐induced intestinal ion and fluid secretion by a small‐molecule CFTR inhibitor. Gastroenterology 2004126511–519. [DOI] [PubMed] [Google Scholar]
- 32.Silberstein C, Kierbel A, Amodeo G.et al Functional characterization and localization of AQP3 in the human colon. Braz J Med Biol Res 1999321303–1313. [DOI] [PubMed] [Google Scholar]
- 33.Ishibashi K, Sasaki S, Fushimi K.et al Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci USA 1994916269–6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S W, Gresz V, Rojek A.et al Decreased expression of AQP2 and AQP4 water channels and Na,K‐ATPase in kidney collecting duct in AQP3 null mice. Biol Cell 200597775–778. [DOI] [PubMed] [Google Scholar]
- 35.Dignass A U. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis 2001768–77. [DOI] [PubMed] [Google Scholar]
- 36.Vetuschi A, Latella G, Sferra R.et al Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium‐induced colitis in rats. Dig Dis Sci 2002471447–1457. [DOI] [PubMed] [Google Scholar]
- 37.Patsouris D, Mandard S, Voshol P J.et al PPARalpha governs glycerol metabolism. J Clin Invest 200411494–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassaganya‐Riera J, Reynolds K, Martino‐Catt S.et al Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 2004127777–791. [DOI] [PubMed] [Google Scholar]
- 39.Lytle C, Tod T J, Vo K T.et al The peroxisome proliferator‐activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis 200511231–243. [DOI] [PubMed] [Google Scholar]
- 40.Cliffe L J, Humphreys N E, Lane T E.et al Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 20053081463–1465. [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Verkman A S. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope‐tagged constructs in Xenopus oocytes. J Biol Chem 199727216140–16146. [DOI] [PubMed] [Google Scholar]
- 42.Koyama Y, Yamamoto T, Tani T.et al Expression and localization of aquaporins in rat gastrointestinal tract. Am J Physiol 1999276C621–C627. [DOI] [PubMed] [Google Scholar]