Abstract
Objectives
To derive age and sex specific estimates of transition rates from advanced adenomas to colorectal cancer by combining data of a nationwide screening colonoscopy registry and national data on colorectal cancer (CRC) incidence.
Design
Registry based study.
Setting
National screening colonoscopy programme in Germany.
Patients
Participants of screening colonoscopy in 2003 and 2004 (n = 840 149).
Main outcome measures
Advanced adenoma prevalence, colorectal cancer incidence, annual and 10 year cumulative risk of developing CRC among carriers of advanced adenomas according to sex and age (range 55–80+ years)
Results
The age gradient is much stronger for CRC incidence than for advanced adenoma prevalence. As a result, projected annual transition rates from advanced adenomas to CRC strongly increase with age (from 2.6% in age group 55–59 years to 5.6% in age group ⩾80 years among women, and from 2.6% in age group 55–59 years to 5.1% in age group ⩾80 years among men). Projections of 10 year cumulative risk increase from 25.4% at age 55 years to 42.9% at age 80 years in women, and from 25.2% at age 55 years to 39.7% at age 80 years in men.
Conclusions
Advanced adenoma transition rates are similar in both sexes, but there is a strong age gradient for both sexes. Our estimates of transition rates in older age groups are in line with previous estimates derived from small case series in the pre‐colonoscopy era independent of age. However, our projections for younger age groups are considerably lower. These findings may have important implications for the design of CRC screening programmes.
Most colorectal cancers (CRCs) develop from adenomas, among which “advanced” adenomas are considered to be the clinically relevant precursors of CRC. The natural history of colorectal adenomas is a decisive factor for the design of CRC screening measures and their cost effectiveness. Since advanced adenomas need to be removed once they are detected, any direct observation of their natural history would be unethical. Thus, available estimates for the progression of adenomas mostly stem from radiological surveillance data or from autopsy series collected prior to the colonoscopy era.1,2,3,4,5,6,7,8,9 However, these data are rather vague as they were derived from small and potentially selective samples. For example, a major source for the estimation of adenoma transition rates has been a retrospective review of Mayo Clinic records from 226 patients with colonic polyps ⩾10 mm in diameter in whom periodic radiographical examination of the colon was performed, and in whom 21 invasive carcinomas were identified during a mean follow up of 9 years.9 Despite the undoubted usefulness of available data sources from the pre‐colonoscopy era, sample size limitations did not allow reliable estimates of adenoma transition rates according to key factors, such as age and sex. Accordingly, a common estimate of these transition rates for all ages and both sexes has generally been assumed in previous studies on effectiveness and cost effectiveness of CRC screening.10,11,12,13,14,15,16,17 Given that sensitivity analyses in these studies showed that the advanced adenoma–carcinoma transition rate represents a very influential parameter,10,11,13,18 its variation by age and sex might have a large impact on relative effectiveness and cost effectiveness of various screening schemes.
The aim of this paper was to estimate risk for developing CRC according to age and sex among carriers of advanced adenomas by combining data from a large national colonoscopy screening database and national data on CRC incidence in Germany.
Methods
Data sources
German national registry of screening colonoscopies
Prevalences of advanced adenomas (defined as presence of at least one adenoma with at least one of the following features: ⩾1 cm in size, tubulovillous or villous adenoma, high grade dysplasia; no further distinction according to number of adenomas) by 5 year age groups and sex were obtained from a nationwide registry of screening colonoscopies, which was established after implementation of colonoscopic screening for women and men aged ⩾55 years in Germany in late 2002.19 Only experienced endoscopists (internists/gastroenterologists or surgeons with pertinent certified specialisations, having conducted at least 200 colonoscopies and at least 50 polypectomies under supervision in the preceding two calendar years) are admitted to conduct screening colonoscopies. Requirements for maintenance of admission include conduction of at least 200 colonoscopies and at least 10 polypectomies per year. Histopathological examination is performed decentrally by certified pathological labs.
In the nationwide screening colonoscopy registry, basic sociodemographic variables and results of screening colonoscopy are collected anonymously for all screening colonoscopies conducted in Germany using a standardised reporting form to be completed by the gastroenterologists. In case of multiple adenomas, only the largest is recorded (categories used for classification of size of adenomas: >1 cm, ⩽1 cm; categories used for histopathological classification of adenomas: tubular, tubulovillous, villous, high grade dysplasia).
The forms are scanned, processed and checked for completeness and plausibility using standardised algorithms at regional data centres before transmission to the national data centre for analysis. For this analysis, 840 149 records from screening colonoscopies conducted by more than 2000 endoscopists in 2003 and 2004 were available.
Population‐based cancer registry data
National estimates of colorectal cancer incidence by age and sex are provided by the Robert Koch Institute, a Federal Institute for Disease Surveillance in Germany.20 These estimates are derived from the best available incidence data from multiple state cancer registries and nationwide available CRC mortality data. Coding of cancers in the cancer registries follows international standards for epidemiological cancer registries.21 For this analysis, we used CRC incidence data for the year 2002, the year immediately preceding introduction of screening colonoscopy, as the latter may affect observed CRC incidence by prevention of CRC or by detection of asymptomatic CRC.
Statistical analysis
For each 5 year age group i (i = 55–59, 60–64, 65–69, 70–74, 75–79, 80+ years) and each sex j (j = females, males), annual transition rates from advanced adenomas to CRC, denoted Aij, were estimated from CRC incidence Iij and advanced adenoma prevalence Pij as follows:
Aij = (Iij × Q)/Pij.
The factor Q reflects the proportion of CRCs assumed to develop through adenomas. This proportion has been assumed to equal values between 0.7 and 1.0 by various authors (regardless of age and sex).10,11,12,13,15,16,17,18 Similar to the recent study by Ladabaum and Song,17 we set Q to 0.85 in our main analysis and we varied it between 0.7 and 1.0 in additional sensitivity analyses.
Rates of CRC reported by population‐based cancer registries are in reality clinical detection rates rather than true incidence rates. It has been estimated that onset of preclinical CRC on average precedes clinical detection by 3.6 years.13 We therefore recalculated age specific rates of CRC onset by “shifting” age specific observed incidence rates to younger ages. In the main analysis, a shift by 3.6 years was made. In sensitivity analyses, this shift was varied between 2 and 5 years, according to the range of duration from preclinical CRC onset to CRC diagnosis estimated by other authors.12,15,22
Finally, cumulative risk of developing CRC within 10 years (conditional on not dying from other causes) among carriers of advanced adenomas at the beginning of each age group i—that is, at ages 55, 60, 65, 70, 75 and 80 years—was calculated as 1‐exp(−5(Aij + A(i+1)j)).23
Results
In total, records from the national screening colonoscopy database of 840 149 women and men who underwent a screening colonoscopy in Germany in 2003 or 2004 were included in the estimates of advanced adenoma prevalences (table 1). Prevalences of advanced adenomas were higher in men than in women, and they increased with age in both sexes (from age 55–59 years to ⩾80 years they increased from 3.4% to 7.3% among women, and from 6.2% to 9.5% among men, table 1). All age and sex specific prevalence estimates were based on at least 600 cases and most prevalence estimates were based on more than 2000 cases.
Table 1 Advanced adenoma prevalence, colorectal cancer incidence and estimated annual progression of advanced adenomas according to sex and age.
| Sex | Age(y) | National colonoscopy registry | National estimates of colorectal cancer occurrence | Annual transition in %‡ | ||
|---|---|---|---|---|---|---|
| Number screened | Advanced adenomas n (%) | Observed “incidence” * | Estimated onset† | |||
| Women | 55–59 | 112 399 | 3866 (3.4) | 82.1 | 104.3 | 2.6 |
| 60–64 | 160 880 | 6773 (4.2) | 113.0 | 161.3 | 3.3 | |
| 65–69 | 125 133 | 6005 (4.8) | 180.1 | 238.4 | 4.2 | |
| 70–74 | 55 582 | 3219 (5.8) | 261.0 | 326.2 | 4.8 | |
| 75–79 | 27 414 | 1785 (6.5) | 351.5 | 440.4 | 5.8 | |
| 80+ | 9492 | 691 (7.3) | 479.0 | 481.2 | 5.6 | |
| Men | 55–59 | 66 330 | 4130 (6.2) | 132.4 | 188.7 | 2.6 |
| 60–64 | 110 597 | 8312 (7.5) | 210.5 | 284.8 | 3.2 | |
| 65–69 | 97 411 | 8217 (8.4) | 313.6 | 405.7 | 4.1 | |
| 70–74 | 47 018 | 4336 (9.2) | 441.5 | 452.6 | 4.2 | |
| 75–79 | 21 220 | 2051 (9.7) | 456.9 | 512.9 | 4.5 | |
| 80+ | 6673 | 632 (9.5) | 551.4 | 564.7 | 5.1 | |
*Patients diagnosed with colorectal cancer within age group per 100 000 per year; †patients estimated to have onset of CRC within age group per 100 000 per year; ‡onset of colorectal cancers per 100 advanced adenomas.
The age gradient was considerably stronger for observed CRC “incidence”. For women, a more than fivefold increase between ages 55–59 and 80+ years was observed. For men, higher incidence rates at all ages and a more than fourfold increase from the youngest to the oldest age group was observed. Within each age group, estimated CRC onset was somewhat higher than observed incidence, given that a substantial proportion of cases diagnosed in each age group were assumed to have had their onset in the preceding younger age group and given the strong increase of incidence with age. The age gradient of estimated rates of CRC onset was somewhat lower than the age gradient of observed incidence rates, but it was still considerably stronger than the age gradient of advanced adenoma prevalence (table 1).
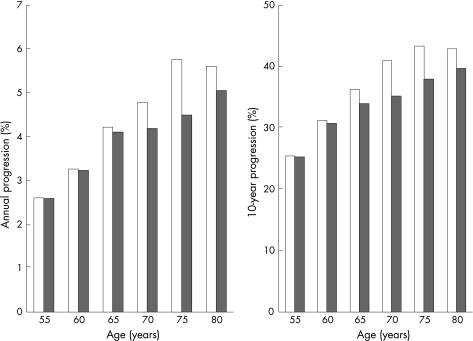
Combining the advanced adenoma prevalences and the estimated CRC onset rates as outlined in the Methods section, and assuming that 85% of CRCs arise from advanced adenomas, yielded estimates of annual transition rates from 2.6% in the youngest age group to 5.6% in age group 80+ among women, and from 2.6% in the youngest age group to 5.1% in the oldest age group among men (table 1 and fig 1, left graph). Estimates of 10 year cumulative risk increased from 25.4% at age 55 years to 42.9% at age 80 years in women, and from 25.2% at age 55 to 39.7% at age 80 years among men (table 2 and fig 1, right graph). These estimates varied by ±3.8–3.9 percentage points at age 55 years and by ±5.1–5.9 percentage points at age 80 years when the proportion of CRCs arising from adenomas was varied between 70% and 100% in sensitivity analyses. Variation of the time from CRC onset to CRC diagnosis between 2 and 5 years led to relatively minor variation in the estimated 10 year cumulative risk (table 2).
Figure 1 Projected annual progression (left graph) and 10 year progression (right graph) of advanced adenomas to colorectal cancer according to sex and age in Germany. Women = left columns, men = right columns.
Table 2 Estimated 10 year cumulative risk of colorectal cancer among women and men with advanced adenomas at various ages.
| Sex | Age (y) | 10 year cumulative risk (%) | ||||
|---|---|---|---|---|---|---|
| Main analysis Q = 0.85 D = 3.6y | One‐way sensitivity analyses | |||||
| Q = 0.70 | Q = 1.00 | D = 2y | D = 5y | |||
| Women | 55 | 25.4 | 21.5 | 29.2 | 22.9 | 27.3 |
| 60 | 31.2 | 26.5 | 35.6 | 28.1 | 33.5 | |
| 65 | 36.2 | 31.0 | 41.1 | 33.4 | 38.3 | |
| 70 | 41.0 | 35.2 | 46.2 | 38.1 | 43.0 | |
| 75 | 43.3 | 37.4 | 48.7 | 41.8 | 44.5 | |
| 80 | 42.9 | 37.0 | 48.3 | 42.7 | 43.0 | |
| Men | 55 | 25.2 | 21.3 | 29.0 | 22.5 | 27.2 |
| 60 | 30.7 | 26.1 | 35.0 | 27.9 | 32.7 | |
| 65 | 33.9 | 28.9 | 38.6 | 32.4 | 35.1 | |
| 70 | 35.2 | 30.0 | 40.0 | 34.3 | 35.8 | |
| 75 | 38.0 | 32.5 | 43.0 | 36.9 | 38.7 | |
| 80 | 39.7 | 34.0 | 44.8 | 38.9 | 40.2 | |
The proportion of colorectal cancers (CRC) arising from polypoid adenomas Q and the duration from CRC onset to CRC detection D are set to 0.85 and 3.6 years, respectively, in the main analyses, and they are varied between 0.7 and 1.0 and between 2 and 5 years, respectively, in the sensitivity analyses.
Discussion
In this paper, we combined data from a unique nationwide colonoscopy registry and from population‐based cancer registries from Germany to project age and sex specific risk of CRC development among carriers of advanced colorectal adenomas. Any direct estimation of this parameter from cohorts of adenoma carriers would be unethical, as advanced adenomas have to be removed once they are detected, and pertinent data from the pre‐colonoscopy era are very sparse. Our analysis shows that the risk of advanced adenoma–CRC transition is comparable among women and men, but strongly increases with age.
Our projections of advanced adenoma progression to CRC in the older age groups are in line with previous estimates based on much smaller samples, which have been used for all age groups in previous analyses of cost effectiveness of CRC screening. For example, the cost effectiveness analyses of colorectal cancer screening by Frazier et al11 and O'Leary et al16 assumed a base‐case value of 5% for the annual transition rate from high‐risk polyps to localised cancer regardless of age, citing the 1987 report of Stryker et al from the Mayo clinic case series as pertinent reference.9 The same estimate was used, again for all age groups and both sexes, in the recent cost‐effectiveness analysis by Ladabaum and Song.17 Furthermore, polyp dwell time, which is closely related to the adenoma–CRC transition rate, has been found to be an important influential parameter in pertinent sensitivity analyses in a number of cost effectiveness studies.10,13,16,18 Therefore, the finding of a strongly age‐dependent transition rate may be of high relevance for the optimal design of CRC screening programmes.
In our analysis, we implicitly made the assumption that all CRCs arising from adenomas were arising from advanced adenomas—that is, advanced adenomas represent a necessary interim stage in the adenoma–CRC sequence. A similar assumption (eg large adenomas or high risk polyps being necessary intermediate stages in the development of CRC from small adenomas or from low risk polyps, respectively) has been implicitly or explicitly made by other authors, and it appears plausible on biological grounds.11,16,17
In the interpretation of our results, specific strengths and limitations of this study require careful consideration. Strengths include the large population‐based data sources, especially a unique nationwide registry with more than 840 000 records from screening colonoscopies. The latter allowed estimating age and sex‐specific prevalences of advanced adenomas in an unselected screening population with very small random error. Unfortunately, no data for age group 50–54 years could be provided, as colonoscopy screening in Germany is offered from 55 years on only. Given the lack of information on adenoma location in the national colonoscopy database, it was not possible to further assess potential differences in transition rates of proximal and distal advanced adenomas. The latter might be of interest when (cost‐) effectiveness of screening colonoscopy and screening sigmoidoscopy are compared. Furthermore, although high levels of qualification and experience are a prerequisite for conducting screening colonoscopies and for certification of pathology labs in Germany, and all screening colonoscopies have to be documented on a standardised form, the degree of standardisation of information given on colonoscopy reports is likely to be less perfect in such a nationwide database than it could be achieved in a trial conducted by a few specialised centres with centralised review of all adenomas. However, our results might be more relevant for judging the risk that advanced adenomas detected in routine practice will progress to cancer. Furthermore, potentially less than perfect classification of adenomas provided on colonoscopy reports would be expected to apply to all ages. The resulting potential misclassification would therefore be expected to be “non‐differential” with respect to age, which, according to epidemiological theory, implies that differences in age‐specific transition rates would tend to be underestimated rather than overestimated.24 Potentially less than perfect classification of histopathological findings would thus not explain the major differences in estimates of CRC transition rates between age groups.
A possible concern could be that adenoma prevalences among screening participants might not be representative for adenoma prevalences among the general population. On the one hand, prevalences could be somewhat lower among screening participants, assuming that they are more health conscious than non‐participants. However, in contrast to other cancers, no major variation of CRC risk by factors related to health consciousness, such as indicators of education or socioeconomic status, has been found. However, adenoma prevalences among screening participants might even be somewhat higher than in the general population, assuming that people at higher risk of CRC, such as people with a family history of CRC, might be more likely to undergo screening. Overall, the estimates of advanced adenoma prevalences from the German national screening registry were in line with25,26,27 or somewhat lower than28 previous, less detailed, estimates from much smaller colonoscopy studies conducted in the USA. Although selection patterns could have led to some underestimation or overestimation of advanced adenoma prevalences overall, it is unlikely that they could account for the strong age gradient in advanced adenoma progression estimates found in our study.
National estimates of CRC incidence in Germany referred to the year 2002—the year immediately preceding introduction of screening colonoscopy to avoid “contamination” by detection of asymptomatic CRC by screening colonoscopy. As described in the Methods section, CRC onset rates were derived from CRC incidence rates in our calculations. Therefore, major changes in CRC incidence in the years preceding 2003 and 2004 could, in theory, have influenced our results. However, incidence rates of colorectal cancer have been very stable in Germany since the middle of the 1990s,20 and it appears particularly unlikely that the strong age gradient, which is a universal finding in CRC incidence,29 should have markedly changed in recent years.
Like other studies, we had to rely on external sources for estimates of the proportion of CRC arising from adenomas, but uncertainty with respect to this parameter was taken care of in sensitivity analyses. The same applies to estimates of the duration from CRC onset to CRC detection. The main finding of our study, the strong age dependency of adenoma–CRC transition rates, was not affected by variation of overall levels of these parameters. However, we cannot rule out that potential variation of these parameters between age groups might affect the projections of age‐specific CRC transition rates to some extent.
Despite its limitations, our analysis provides information on age and sex‐specific advanced adenoma transition rates, which could be helpful in the design of endoscopy based screening programmes and their optimisation regarding effectiveness and cost effectiveness. Our finding that advanced adenoma transition rates are strongly age‐dependent could have important clinical implications, possibly including a higher age at first screening or differential endoscopy intervals according to age. However, additional risk factors, such as family history of CRC, also have to be taken into account. To further differentiate estimates of the natural history of colorectal adenomas—for example, by various types and by location—large studies with more detailed information about colonoscopic findings are needed.
Abbreviations
CRC - colorectal cancer
Footnotes
Competing interests: None.
References
- 1.Muto T, Bussey H J, Morson B C. The evolution of cancer of the colon and rectum. Cancer 1975362251–2270. [DOI] [PubMed] [Google Scholar]
- 2.Kozuka S, Nogaki M, Ozeki T.et al Premalignancy of the mucosal polyp in the large intestine: II. Estimation of the periods required for malignant transformation of mucosal polyps. Dis Colon Rectum 197518494–500. [DOI] [PubMed] [Google Scholar]
- 3.Eide T J, Stalsberg H. Polyps of the large intestine in Northern Norway. Cancer 1978422839–2848. [DOI] [PubMed] [Google Scholar]
- 4.Vatn M H, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer 198249819–825. [DOI] [PubMed] [Google Scholar]
- 5.Williams A R, Balasooriya B A W, Day D W. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 198223835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morson B C. The evolution of colorectal carcinoma. Clin Radiol 198435425–431. [DOI] [PubMed] [Google Scholar]
- 7.Wegener M, Borsch G, Schmidt G. Colorectal adenomas. Distribution, incidence of malignant transformation, and rate of recurrence. Dis Colon Rectum 198629383–387. [DOI] [PubMed] [Google Scholar]
- 8.Eide T J. Risk of colorectal cancer in adenoma‐bearing individuals within a defined population. Int J Cancer 198638173–176. [DOI] [PubMed] [Google Scholar]
- 9.Stryker S J, Wolff B G, Culp C E.et al Natural history of untreated polyps. Gastroenterology 1987931009–1013. [DOI] [PubMed] [Google Scholar]
- 10.Wagner J, Tunis S, Brown M.et al Cost‐effectiveness of colorectal cancer screening in average‐risk adults. In: Young G, Rozen P, Levin B, eds. Prevention and early detection of colorectal cancer. London, UK: Saunders, 1996321–356.
- 11.Frazier A L, Colditz G A, Fuchs C S.et al Cost‐effectiveness of screening for colorectal cancer in the general population. JAMA 20002841954–1961. [DOI] [PubMed] [Google Scholar]
- 12.Ness R M, Holmes A M, Klein R.et al Cost‐utility of one‐time colonoscopic screening for colorectal cancer at various ages. Am J Gastroenterol 2000951800–1811. [DOI] [PubMed] [Google Scholar]
- 13.Loeve F, Brown M L, Boer R.et al Endoscopic colorectal cancer screening: a cost‐saving analysis. J Natl Cancer Inst 200092557–563. [DOI] [PubMed] [Google Scholar]
- 14.McMahon P M, Bosch J L, Gleason S.et al Cost‐effectiveness of colorectal cancer screening. Radiology 200121944–50. [DOI] [PubMed] [Google Scholar]
- 15.Vijan S, Hwang E W, Hofer T P.et al Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med 2001111593–601. [DOI] [PubMed] [Google Scholar]
- 16.O'Leary B A, Olynk J K, Neville A M.et al Cost‐effectiveness of colorectal cancer screening: comparison of community‐based flexible sigmoidoscopy with fecal occult blood testing and colonoscopy. J Gastroenterol Hepatol 20041938–47. [DOI] [PubMed] [Google Scholar]
- 17.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology 20051291151–1162. [DOI] [PubMed] [Google Scholar]
- 18.Pignone M, Saha S, Hoerger T.et al Cost‐effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 200213796–104. [DOI] [PubMed] [Google Scholar]
- 19.Knöpnadel J, Altenhofen L, Lichtner F.et al Früherkennung des Darmkrebses und möglicher Vorstufen. Wissenschaftliche Begleitung zur Einführung der Früherkennungskoloskopie in Deutschland im Auftrag der Spitzenverbände der Gesetzlichen Krankenkassen und der Ärztlichen Bundesvereinigung. Auswertungen der Dokumentationen zur Früherkennungskoloskopie Berichtszeitraum 2003 (1. Quartal 2003 – 4. Quartal 2003). (Wissenschaftliche Reihe des Zentralinstitutes, Band 59). Köln: Deutscher Ärzte‐Verlag 2005
- 20.Gesellschaft epidemiologischer Krebsregister in Deutschland e V. Krebs in Deutschland. Häufigkeiten und Trends, 5. Ausgabe. Saarbrücken 2006
- 21.Tyczynski J E, Demaret E, Parkin D M. eds. Standards and guidelines for cancer registration in Europe. The ENCR recommendations, vol. 1. IARC Technical Publication No. 40. Lyon: International Agency for Research on Cancer 2003
- 22.Koretz R L. Malignant polyps: Are they sheep in wolves' clothing? Ann Intern Med 199311863–68. [DOI] [PubMed] [Google Scholar]
- 23.Day N. Cumulative rate and cumulative risk. In: Muir C, Waterhouse J, Mack T, et al eds. Cancer Incidence in Five Continents, Vol V. IARC Scientific Publication No. 88. Lyon (France): International Agency for Research on Cancer, 1987; Chapter 10.
- 24.Rothman K J, Greenland S. Modern epidemiology, 2nd edition. Philadelphia: Lippincott‐Raven 1998
- 25.Schoenfeld P, Cash B, Flood A, for the CONCeRN Study Investigators et al Colonoscopic screening of average‐risk women for colorectal neoplasia. N Engl J Med 20053522061–2068. [DOI] [PubMed] [Google Scholar]
- 26.Strul H, Kariv R, Leshno M.et al The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average‐risk individuals aged 40–80 years. Am J Gastroenterol 2006101255–262. [DOI] [PubMed] [Google Scholar]
- 27.Imperiale T F, Wagner D R, Lin C Y.et al Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med 2000343169–174. [DOI] [PubMed] [Google Scholar]
- 28. Lieberman DA, Weiss DG, for the Veterans Affairs Cooperative Study Group 380. One‐time screening for colorectal cancer with combined fecal occult‐blood testing and examination of the distal colon. N Engl J Med 2001345555–560. [DOI] [PubMed] [Google Scholar]
- 29.Parkin D M, Bray F, Ferlay J.et al Global cancer statistics, 2002. CA Cancer J Clin 20055574–108. [DOI] [PubMed] [Google Scholar]