In humans and some other animals, bilirubin is excreted as the end product of haem originating from haemoglobin and other haemoproteins, and it has been the cause of jaundice and kernicterus.1,2 The conversion of haem to bilirubin is a two‐step process.1 First, haem is converted to biliverdin by haem oxygenase. Then biliverdin is converted to bilirubin by biliverdin reductase. Interestingly, many animals take biliverdin as the end product of haem, without converting it further to bilirubin.2 Biliverdin is water soluble and readily excreted through bile or urine.
Why the energy‐consuming conversion in some animals of the innocuous biliverdin to the water‐insoluble, potentially toxic bilirubin, which needs more resources for transportation and excretion, takes place has been a great puzzle.1,2 Discovery of the antioxidant property and other properties of bilirubin has suggested that the change of biliverdin to bilirubin might be an advance in evolution.1 However, this notion is challenged by the fact that biliverdin‐ or bilirubin‐predominant species exist in animals at different stages of evolution, from fishes to mammals.2 The discrepancy in bile pigments exists even in closely related species. For instance, nutria is a rodent like rats and mice, but the bile of nutria is predominantly biliverdin, in contrast to the predominant bilirubin in rats and mice.2 Change of biliverdin to bilirubin needs only one enzyme—biliverdin reductase.1 This enzyme exists in the oldest bacteria like cyanobacteria,1 far before eukaryotic cells appeared on earth. So what has caused the bilirubin or biliverdin predominance in animals? Here I suggest that the inactivation of digestive proteases is the evolutionary driving force for bilirubin or biliverdin predominance.
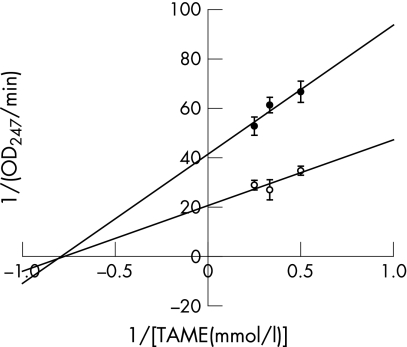
Table 1 shows that trypsin and chymotrypsin are significantly inhibited by free bilirubin, but not conjugated bilirubin or biliverdin. The nature of this inhibition and its physiological relevance were further explored for trypsin. Figure 1 shows a Lineweaver–Burk plot of the inhibition of trypsin by free bilirubin. It indicates that the inhibition is non‐competitive, suggesting that free bilirubin can inactivate the enzyme.
Table 1 Effect of free bilirubin, biliverdin and conjugated bilirubin on trypsin and chymotrypsin activity.
| Control (μmol/l) | Free bilirubin (μmol/l) | Biliverdin (μmol/l) | Conjugated bilirubin (μmol/l) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | 5 | 10 | 10 | 10 | |
| Trypsin | |||||||
| Increase of OD per min | 0.0367 (0.0023) | 0.0255 (0.0013)† | 0.0197 (0.0018)† | 0.0142 (0.0033)† | 0.0102 (0.0006)† | 0.0459 (0.0025)† | 0.0461 (0.0007)† |
| Test/control | 1.00 | 0.70 | 0.54 | 0.39 | 0.28 | 1.25 | 1.26 |
| Chymotrypsin | |||||||
| Increase of OD per min | 0.0218 (0.0030) | 0.0166 (0.0025)* | 0.0121 (0.0008)† | 0.0061 (0.0017)† | 0.0002 (0.0006)† | 0.0271 (0.0023)* | 0.0237 (0.0016) |
| Test/control | 1.00 | 0.76 | 0.56 | 0.28 | 0.01 | 1.24 | 1.09 |
Data are expressed as mean (SD) of quadruplicate measurements.
*p<0.05; †p<0.001 versus control.
Trypsin and chymotrypsin activities were measured using Nα‐p‐tosyl‐l‐arginine methyl ester (TAME) or N‐benzoyl‐l‐tyrosine ethyl ester (BTEE) as the substrate, respectively, by a method modified from that described by Hummel.9 Free bilirubin (3 μl 50 X; Sigma, St Louis, Missouri, USA), biliverdin (Frontier Scientific, Logan, Utah, USA) or conjugated bilirubin (Frontier Scientific) was added to a 96‐well ultraviolet‐transparent microplate. . The final volume of the reaction solution was 150 μl, giving the final concentrations indicated in table 1. For trypsin assay, 72 μl buffer (0.046 M Tris/HCl, 0.0115 M CaCl2, pH 8.1) containing 150 ng trypsin was added to the microplate by multiple pipette, followed by 75 μl of buffer containing 150 nmol TAME to start the reaction. The plate was then read at 247 nm on a microplate reader. For chymotrypsin assay, BTEE was dissolved in dimethylsulphoxide (DMSO) at 4.054 mg/ml, and then added with nine volumes of water to obtain a concentration of 1.07 mmol/l. DMSO was used instead of methanol (which was used in the original paper) because methanol greatly reduces the solubility of free bilirubin. After adding 77 μl buffer (0.080 M Tris/HCl, 0.1 M CaCl2, pH 7.8) containing 150 ng chymotrypsin, the reaction was started by adding 70 μl BTEE solution. Then the plate was read at 256 nm on a microplate reader. Data were collected at 30‐second intervals for 5 minutes and expressed as the increase in optical density (OD) per minute in the linear range (the first 2–3 minutes).
Figure 1 Lineweaver‐Burk plot of the inhibition of trypsin by free bilirubin. Trypsin activity was measured using different concentrations of TAME as the substrate with (•) or without (○) 2.5 μM bilirubin. TAME, Nα‐p‐tosyl‐l‐arginine methyl ester.
The effect of free bilirubin on protein digestion by trypsin was further investigated using chymotrypsinogen as the substrate. Bilirubin (10 μmol/l) showed 61% inhibition for the proteolytic activation of α‐chymotrypsinogen A to chymotrypsin in a system containing 1 μg/ml trypsin and 1 μg/ml chymotrypsinogen for 30 min. Digestive proteases are inhibited by free bilirubin but not conjugated bilirubin. As bilirubin is secreted from the bile to the lumen mainly in the conjugated form,2 the digestion of dietary proteins in the upper small intestine would proceed smoothly. Deconjugation of bilirubin by β‐glucuronidase from the mucosal cells3 would form a protective layer on the surface of the gut. A more dramatic deconjugation of bilirubin by the high amounts of β‐glucuronidase from gut bacteria3 would further cause a prompt and effective inactivation of these digestive proteases in the lower intestine. Here we can see the wonderful design of nature that turns a waste byproduct into a precious treasure. The amount of digestive proteases secreted by the pancreas largely depends on the amount of protein in the diet.4 This would provide an explanation for the observation that bilirubin‐predominant species tend to be carnivores or omnivores, while biliverdin‐predominant species tend to be herbivores.2 Large amounts of bilirubin exist in the bile of cats, dogs, opossums, armadillos, alligators, African clawed toads, bullfrogs, mudpuppies, sharks (spiny dogfish), small skates, trout, goosefish, and perch, while biliverdin is the main bile pigment of rabbits, nutrias (rodents that eat water plants), sloths (leaf eaters), birds and tilapia (fish that eat algae).2 Although cattle and sheep are herbivores and their bile consists mainly of bilirubin, the activity of their hepatic biliverdin reductase was just 4–5% of that of the rats,5 and their bile indeed contains certain amounts of biliverdin,2 suggesting that they may be in an intermediary state of transition.
Studies have also shown that bile pigments change from bilirubin to biliverdin in fishes after starvation.6 This further suggests bilirubin is only produced as necessary, which depends in some way on the feeding activities. On the other hand, the energy‐consuming conversion of biliverdin to bilirubin suggests that bilirubin may have an important role for the body. An impairment in inactivation of digestive proteases by deconjugated bilirubin may have a causative role in diseases such as inflammatory bowel disease.7 It would also provide a possible explanation as to why chronic blockage of bile flow in diseases such as primary sclerosing cholangitis is often accompanied by gut damage like inflammatory bowel disease.8 These areas require further investigation.
References
- 1.McDonagh A F. Turning green to gold. Nat Struct Biol 20018198–200. [DOI] [PubMed] [Google Scholar]
- 2.Cornelius C E. Comparative bile pigment metabolism in vertebrates. In: Ostrow JD, ed. Bile pigments and jaundice: molecular, metabolic, and medical aspects. New York: Dekker, 1986601–647.
- 3.Rod T O, Midtvedt T. Origin of intestinal beta‐glucuronidase in germfree, monocontaminated and conventional rats. Acta Pathol Microbiol Scand [B] 197785271–276. [DOI] [PubMed] [Google Scholar]
- 4.Howard F, Yudkin J. Effect of dietary change upon the amylase and trypsin activities of the rat pancreas. Br J Nutr 196317281–294. [DOI] [PubMed] [Google Scholar]
- 5.George J W, Nulk K, Weiss A.et al Biliverdin reductase‐activity in cattle, sheep, rabbits and rats. Int J Biochem 198921477–481. [DOI] [PubMed] [Google Scholar]
- 6.Fang L S. Study on the heme catabolism of fish. Comparative Biochemistry and Physiology B‐Biochemistry & Molecular Biology 198788667–673. [Google Scholar]
- 7.Qin X F. Impaired inactivation of digestive proteases by deconjugated bilirubin: the possible mechanism for inflammatory bowel disease. Med Hypoth 200259159–163. [DOI] [PubMed] [Google Scholar]
- 8.Qin X. Primary sclerosing cholangitis and inflammatory bowel disease: where is the link? Am J Gastroenterol 20071021332–1333. [DOI] [PubMed] [Google Scholar]
- 9.Hummel B C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol 1959371393–1399. [PubMed] [Google Scholar]