Endoscopic resection (ER) of early neoplastic lesions has become increasingly important in recent years, both as a diagnostic tool for the staging of oesophageal carcinomas and as a method of carrying out definitive treatment when the cancer meets certain criteria in which the risk of lymph‐node metastasis is negligible. Early diagnosis, especially of neoplastic lesions arising in Barrett's oesophagus, has become more frequent as a result of improved endoscopic technology, surveillance programmes, and increasing experience and awareness on the part of endoscopists. For many years, surgery was considered to be the treatment of choice, even in patients with high‐grade intraepithelial neoplasia (HGIN) or mucosal carcinoma, but it is associated with a 30‐day mortality of between 3 and 10% and with significant morbidity in 40–50% of cases.1,2 In low‐volume centres or with less experienced surgeons, the mortality rate with radical oesophageal resection can rise to more than 20%.3,4
These alarming data are the reason why local treatment methods such as photodynamic therapy (PDT), argon plasma coagulation (APC), electrocoagulation and ER have been introduced and investigated in several studies on early oesophageal neoplasia. In contrast to ablative treatment methods such as PDT, ER allows histological assessment of the resected specimen in order to assess the depth of infiltration of the tumour and freedom from neoplasia at the lateral and (more importantly) basal margins, imitating the surgical situation.5 These significant advantages of ER are the main reason why ER should be preferred to ablative treatment methods, even PDT, whenever possible, especially bearing in mind the low accuracy of endoscopic ultrasound (EUS) regarding local tumour staging.6,7,8,9,10,11 Arguments in favour of ER are listed in table 1.
Table 1 Points in favour of endoscopic resection of early oesophageal carcinoma.
| Surgery1,2,3,4,20 | Endoscopic resection16,17,18,19 |
|---|---|
| Morbidity 18–48% | Low morbidity (1–3%) and mortality (0%) |
| Mortality 2–20% | Low risk of lymph‐node metastasis in low‐risk mucosal carcinomas (0% in Barrett's cancer; 0–10% in squamous cell cancer) |
| Reduced quality of life | Organ preservation, quality of life not compromised |
Techniques of ER
‘Endoscopic resection' is the general term for all of the different resection techniques used to treat neoplastic and uncertain lesions in the gastrointestinal tract (table 2). The aim of ER must always be complete resection of the mucosal and submucosal layer down to the lamina muscularis propria. The widely used term ‘endoscopic mucosal resection' (EMR) suggests that only the mucosal layer is resected using this technique, sparing the submucosal layer. We therefore believe that the misleading term EMR should no longer be used and should be replaced by ER.
Table 2 Methods of endoscopic resection.
| ER without suction device |
| Single‐snare resection without submucosal injection |
| Single‐snare resection with submucosal injection |
| ER with suction device |
| Cap technique with submucosal injection |
| Ligation technique without submucosal injection |
| Endoscopic submucosal dissection |
ER, Endoscopic resection.
ER without a suction device
The simplest variant of ER is snare resection without previous submucosal injection. In this technique, a diathermy snare is advanced through the working channel of the endoscope and positioned above the target lesion. The lesion is caught by tightening the loop and is slowly resected using electric cutting current. This technique is usually only used in polypoid oesophageal lesions, because placement of the snare is difficult in flat oesophageal neoplasms as a result of the tangential position of the endoscope. At best, only small specimens can be obtained with ER using this technique.12
In flat lesions, submucosal injection of a diluted saline–epinephrine solution is usually carried out before resection. The injected fluid can lift the lesion and can produce a submucosal safety cushion to prevent perforation and bleeding. The main advantages of this method are that no special equipment is necessary and that it is fast and easily used. The major disadvantages are again that only small specimens can be obtained with the method, and also that only small lesions can be resected in one piece.13 For these reasons, several new resection techniques have been developed in order to obtain larger specimens and make ER easier in the anatomically difficult situation in the oesophagus. Besides the ‘suck‐and‐cut' technique, for example (described in detail below), a double‐channel endoscope with a grasping forceps is used to improve ER in the oesophagus.14 In this method, the forceps is used to pull the target lesion through a snare that is introduced through the second working channel. The lesion is then resected with the loop. As a result of the large calibre of the instrument, it is often almost impossible to carry out difficult procedures in the oesophagus using a double‐channel endoscope, particularly at the oesophagogastric junction or in the inverted position.
ER with a suction device
The disadvantage of simple snare resection (also known as ‘strip biopsy') mentioned above, namely, that only small specimens can be obtained, was overcome with the development of the ‘suck‐and‐cut' technique. With this method, the mucosa and submucosa are sucked into a cap or tube, and the pseudopolyp created in this way is resected using a diathermy snare. In 93 consecutive patients with early gastric cancer, Tanabe et al.13 demonstrated that endoscopic suck‐and‐cut resection is more effective than strip biopsy with regard to the largest diameter of the resected specimen, the rate of en bloc resection, and the complication rate.
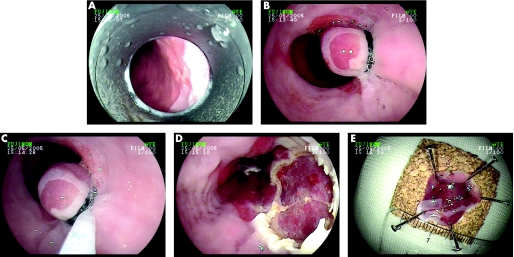
Inoue and Endo15 introduced the cap technique for the resection of early neoplastic lesions. In ER with the cap technique, a specially developed transparent plastic cap (e.g. Olympus MAJ‐295) is attached to the end of the endoscope. After submucosal injection under the target lesion, usually with a saline–epinephrine solution, the lesion is sucked into the cap and resected using a diathermy loop (e.g. Olympus SD‐221L‐25) that has previously been loaded onto a specially designed groove on the lower edge of the cap. Preloading of the loop is easily done in the gastric antrum by applying slight suction to the mucosa and carefully advancing the snare until it is placed exactly in the rim at the distal margin of the cap. Previous marking of the borders of the lesion with electrocautery is recommended, either using the tip of the snare or with an argon plasma coagulation probe, because injecting underneath a discrete neoplastic lesion often makes it difficult to identify the borders afterwards (fig 1).
Figure 1 Endoscopic resection.
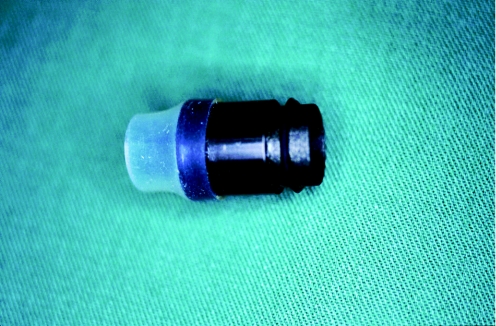
Another alternative to the suck‐and‐cut technique with the cap is using a ligation device of the type familiar from the treatment of oesophageal varices.16,21 With this method, the target lesion is sucked into the cylinder of the ligation device and a rubber band is then released to create a pseudopolyp that has the rubber band at its base. The endoscopist should wait a few seconds, letting the mucosa and submucosa totally prolapse into the cylinder, before releasing the rubber band. The main advantage of this method in comparison with cap resection is that previous submucosal injection is not necessary in ER with a ligation device. After this, the endoscope has to be withdrawn and reintroduced in order to remove the ligation cylinder and introduce the loop. In addition to single‐use devices, the ligation devices available include a reusable ligator,22 with which similar results can be achieved at reduced cost (fig 2; e.g. Euroligator, Mandel&Rupp, Germany; MR‐10042). Ligation devices with multiple rubber bands are also available to allow several ligations to be carried out in a single session without having to withdraw the endoscope. Another useful development is a ligation cylinder that has six rubber bands and a facility for advancing a snare through the working channel of a regular endoscope (e.g. Duette multiband mucosectomy kit CE0123; Cook Ireland Ltd., Limerick, Ireland).23 This enables the endoscopist to perform up to six resections without having to withdraw and reintroduce the endoscope.
Figure 2 The reusable Euroligator.
The question of whether suck‐and‐cut resection with the ligation or cap device is superior was recently answered by a prospective randomised trial.21 A total of 100 consecutive ER were carried out in 70 patients with early oesophageal cancer. Fifty resections were carried out using the reusable ligation device without previous injection, and 50 resections were performed using the cap technique with previous submucosal injection of a diluted saline–epinephrine solution. The main outcome criteria were the maximum diameter of the resected specimen, the resection area, and the complication rate. No significant differences between the two groups were observed after 24 hours in relation to the maximum diameter of the resected specimens and the resection area. There was only a slight advantage for the ligation group in patients who had had previous treatment. One minor bleeding incident occurred in each group, but no severe complications were seen. The mean diameter of the resected specimen was 16.4 SD 4.0 × 11 SD 3.1 mm in the ligation group compared with 15.5 SD 4.1 × 10.7 SD 2.7 mm in the cap group.
The major drawback of ER with the suck‐and‐cut technique appears to be that only small lesions with a diameter of less than 20 mm can be resected en bloc with tumour‐free lateral margins. Ulcerated lesions often have fibrosis attaching the submucosa to the lamina muscularis propria, resulting in failure of the lesion to lift. In these cases, ER is not advisable, or should only be performed with caution. Larger lesions can usually be resected completely using the piecemeal technique, but this method appears to be associated with a higher recurrence rate because of small neoplastic residues resulting from insufficient overlapping of the resection areas.24,25 In addition, en bloc resection allows more accurate histological evaluation of the neoplastic lesion, especially of the lateral and basal margins. A new resection technique, endoscopic submucosal dissection (ESD), was therefore developed.
Endoscopic submucosal dissection
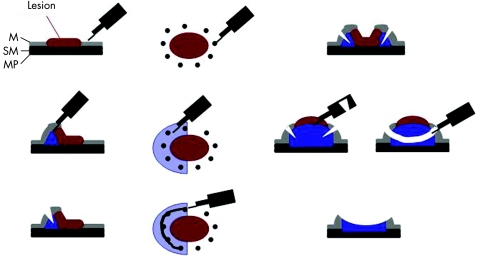
The ESD procedure in the treatment of early gastric cancer was first described by Hosokawa and Yoshida26 and Ono et al.27, with a method using an insulated‐tip knife to dissect the submucosal layer underneath the carcinoma in order to obtain a large resection specimen with the neoplasm resected en bloc (fig 3). Since the introduction of this method, several publications have reported on its use in patients with early gastric carcinoma. The size of the resected specimen obtained with ESD can extend to more than 10 cm in diameter, but this fascinating new method is associated with several problems and disadvantages. There is a substantial complication rate, including perforations requiring surgery, long procedure times of up to several hours, a slow learning curve and a high degree of operator dependency. Recent reports from Japan have described an en bloc R0 resection rate for gastric neoplasias of more than 90%, with a perforation rate of less than 5%.28 The data on ESD procedures in early oesophageal cancer are very limited. At present, there has only been one report on early squamous cell cancer29 and one on early cancer of the oesophagogastric junction,30 both from Japan.
Figure 3 The method of endoscopic submucosal dissection. M, mucosal layer; MP, proper muscle layer; SM, submucosa layer.
The ESD procedure
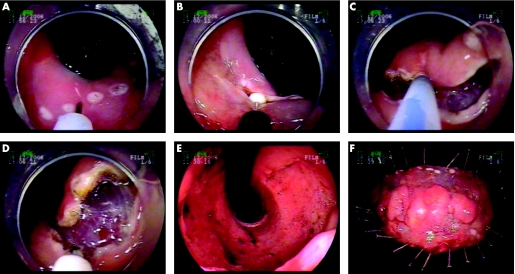
Once the borders of a neoplastic lesion have been adequately visualised, e.g. using chromoendoscopy, the borders are marked with electrocautery at a distance of a few millimetres from the carcinoma. After this, submucosal injection of fluid is carried out to elevate the lesion from the muscular layer, and the mucosa surrounding the lesion is circumferentially cut outside the markings, using a needle‐knife, for example. Finally, the submucosal connective tissue is dissected using a special knife (fig 4). Visible vessels can be coagulated, e.g. using a coagulation forceps, to prevent bleeding. The fluid used for submucosal injection can be a solution of hyaluronic acid with or without glycerol, or 20% glucose or hypertonic saline with epinephrine. Some endoscopists add a dye such as indigo carmine to the solution to facilitate visualisation of the submucosal layer. According to the results of recent studies, a solution of hyaluronic acid plus 10% glycerine plus 5% fructose appears to be the best injection solution in relation to the thickness of the submucosal fluid cushion produced and tissue damage caused by the solutions. The use of this solution has also been shown to result in excellent clinical outcomes.31
Figure 4 Endoscopic submucosal dissection of Barrett's oesophagus.
A wide variety of different knives are used for ESD, including the insulated‐tip knife, hook knife, flex knife, needle‐knife, flush knife and triangle‐tip knife. Flex knives were used in the published case series describing ESD in early oesophageal cancer.
ESD versus conventional ER
No studies comparing ESD and conventional ER in early oesophageal cancer have been published. A historical comparison of conventional ER and ESD in early gastric cancer32 showed that the en bloc resection rate and the completeness of the resection were significantly greater in lesions larger than 10 mm in diameter with ESD than with ER (63.6% versus 91.3% and 51.5% versus 85.9%, respectively). The methods used for conventional ER were strip biopsy with a double‐channel endoscope and grasping forceps, and suck‐and‐cut resection with a ligation device or cap. The required time for resection was significantly longer with ESD than with ER (84 versus 26 minutes), and the complication rate did not vary significantly between the two groups (4/125 versus 5/120 perforations). The study indicates that ESD may be superior to conventional EMR methods with regard to en bloc resection of gastric neoplasms, and particularly of large tumours, but it has several limitations, the major one being the comparison with a historical cohort in which the technique of conventional ER was not standardised. In addition, the high perforation rate in the conventional ER group, at more than 3%, is surprising and may have been caused by a selection bias. Prospective comparative trials are necessary to evaluate the acute outcome: operating time, rate of complete resection (R0), complications; as well as the long‐term results, in order to decide which method should be used in the future. This is required not only for early gastric cancer but also for early oesophageal carcinoma.
Indications for ER of early squamous cell carcinoma
ER should only be carried out in patients with squamous cell neoplasia (SCN) if the carcinoma is limited to the mucosal layer (fig 5). It has been shown that in these cancers, the risk of lymph‐node metastases is low.33,34,35,36 The mucosal layer of the oesophageal squamous epithelium can be divided into three layers. Intraepithelial cancers (m1; also termed carcinoma in situ) and cancers invading the proper mucosal layer are associated with almost no risk of lymph‐node metastases. The risk appears to be higher with cancers that have invaded the lamina muscularis mucosa (m3), in the range of 0–10%.33,37 A recent analysis of resection specimens in 464 consecutive patients with SCN showed that lymph‐node metastases was found in 0.0%, 5.6% and 18.0% of Tm1, m2 and m3 cancers and in 53.1% and 53.9% of sm1 and sm2/3 carcinomas, respectively.34 Surprisingly, the proportion of patients with m3 carcinoma and positive lymph nodes was relatively high, but further analysis showed that angiolymphatic invasion (L1) was a major risk factor for malignant lymph nodes (L1, 41.7%; L0, 10.3%). Multivariate analysis showed that m3/sm1 tumours, lymph vessel and venous invasion, and grade of differentiation were independent risk factors associated with lymph‐node metastases. By contrast, Tajima et al.33 did not find any malignant lymph nodes in 83 mucosal squamous cell cancers (m1–m3). The study suggested that cancers with an infiltration depth of more than 500 μm are also associated with an increased risk of positive lymph nodes. A recently introduced tumour characteristic that appears to be associated with a higher rate of lymph‐node metastasis is the presence of groups of dissociated dedifferentiated carcinoma cells at the invasive front. Chibana and co‐workers37 were able to show that early squamous cell cancers with confirmed lymph‐node metastasis had a significantly higher tumour cell dissociation score than those without metastatic lymph nodes.
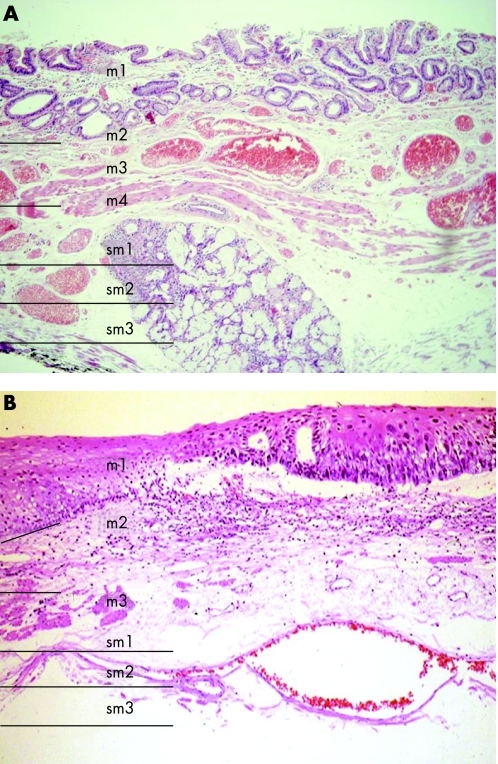
Figure 5 Histological appearance of Barrett's epithelium (A) and squamous cell epithelium (B) with the depth of penetration indicated.
In conclusion, cancers invading the lamina muscularis mucosa (m3) or the upper layer of the submucosa (<500 μm) have a higher risk of positive lymph nodes and should only be treated with ER if no further risk factors are present, such as a poor grade of differentiation, lymph vessel infiltration (L1), venous infiltration (V1), or a higher grade of tumour cell dissociation. In older patients with greater co‐morbidity, the surgical risk has to be balanced against the higher risk of lymph‐node metastases. Patients with an SCN invading the deeper layers of the submucosa (sm2, sm3) should always be treated using surgery or chemoradiotherapy. It remains unclear whether a combination of ER and chemoradiotherapy represents adequate treatment for patients who are at higher risk of lymph‐node metastases, and further studies are needed in order to answer this question.
Indications for ER of early Barrett's carcinoma
The indications for ER are HGIN and mucosal oesophageal cancer. Risk stratification should be carried out in accordance with known risk factors such as grade of differentiation, lymph vessel or venous infiltration and the infiltration depth of the carcinoma (m1–m3/m4). A second opinion from an experienced centre should always be sought in the case of borderline decisions.
The limitations for ER of early Barrett's cancers should be submucosal infiltration or infiltration of the lamina muscularis mucosa in combination with another risk factor such as poor tumour differentiation or lymph vessel and venous infiltration. Whether cancers limited to the upper submucosal layer (sm1) are eligible for ER in selected cases is not as yet clear. Surgical series have been able to show that patients with sm1 Barrett's cancer have a very low risk of metastatic lymph nodes, but larger series reporting on the endoscopic treatment of these patients are still lacking.38,39 A problem with this categorisation of the submucosal levels is the fact that the entire submucosal layer is not represented in ER specimens, often because of thermal destruction. The depth of infiltration in micrometers should therefore be measured, as suggested and widely accepted in squamous cell cancer. There is as yet a lack of published series including risk stratification for lymph‐node metastasis in relation to the measured infiltration depth.
Indications and contraindications of ER in patients with squamous cell cancer and Barrett's cancer are summarised in table 3.
Table 3 Indications and contraindications for endoscopic resection in early squamous cell and Barrett's cancer.
| Indication for ER | Intermediate indication* | Contraindication for ER | |
|---|---|---|---|
| Barrett's neoplasia | LGIN, HGIN, carcinoma, size <20 mm, no risk factors†, macroscopic type I, IIa, b, c | Adenocarcinoma >20 mm, multifocal adenocarcinoma, sm1 infiltration without risk factors | Sm2 tumour infiltration and deeper, sm1 cancer with one risk factor†, macroscopic type III |
| Squamous cell neoplasia | LGIN, HGIN, mucosal cancer, no risk factors†, macroscopic type I, IIa, b, c | Size >20 mm, multifocal cancer | Sm1 cancer and deeper, macroscopic type III |
LGIN, Low‐grade intraepithelial neoplasia; HGIN, high‐grade intraepithelial neoplasia.
*Endoscopic resection (ER) should only be performed in highly experienced centres or under study conditions.
†Risk factors: lymph vessel invasion (L1), venous infiltration (V1), poorly differentiated carcinoma (G3).
Staging procedures
Accurate staging is mandatory before ER of early oesophageal cancer. The most important part of the staging procedure is careful evaluation of the neoplasm and the borders of the lesion using a high‐resolution endoscope, and searching for multifocal neoplasia. In addition, the macroscopic type of the lesion has to be determined, as it has been shown that there is a significant correlation with infiltration depth. In squamous cell carcinomas, chromoendoscopy with iodine solution (1–2%) should be carried out in order to search for synchronous lesions.40 In Barrett's oesophagus, the recommendations regarding whether or not chromoendoscopy should be performed and what kind of dye should be used are not as clear as in SCN. A recent study demonstrated that chromoendoscopy with methylene blue is superior to the four‐quadrant biopsy method that has previously served as the gold standard.41 Additional chromoendoscopy methods such as indigo carmine staining or contrast enhancement with acetic acid, both with magnification endoscopy, and newer methods such as virtual chromoendoscopy (e.g. narrow band imaging or computed virtual chromoendoscopy), have shown promising results in small series, but conclusive recommendations cannot yet be made.42,43,44,45 Confocal endomicroscopy can probably help to decide whether a lesion is neoplastic or benign in vivo as demonstrated in a recently published paper, but further studies are needed to confirm these initial promising results.46 All these new endoscopy technologies can help in finding and assessing a lesion for its suitability for ER, but the most important factor is experience in the field of Barrett's oesophagus.
In addition, conventional EUS and EUS with miniprobe examination should be carried out in order to evaluate the depth of infiltration and the lymph node status of the tumour. It has been shown that the accuracy of T staging is limited, particularly for distinguishing between the important stages T1m and T1sm. Accuracy in diagnosing submucosal cancer only ranges from 33% to 85%.6,7,10 Underdiagnosis by EUS occurred in 12.5–67% of cases, especially in patients with incipient submucosal infiltration (sm1).6,7 EUS is highly accurate in differentiating T1 and T2 tumours.6,10 One way of solving this dilemma is to carry out diagnostic ER when infiltration of the lamina muscularis propria has been ruled out by EUS. If after diagnostic ER the resection specimen shows submucosal infiltration of the tumour, the patient can be referred for oesophageal resection. EUS is considerably superior to computed tomography for lymph‐node staging, as was recently shown in a study including 100 patients with early Barrett's cancer.10 Systemic metastasis is very rare in early oesophageal cancer, and abdominal ultrasonography should be performed to rule out metastatic disease before ER.
Table 5 Publications on endoscopic resection for early Barrett's neoplasia.
| First author, ref. | Patients (n) | Resection technique | Complications (ER‐related) | Complete response | Follow‐up (months) | Recurrence rate |
|---|---|---|---|---|---|---|
| Ell16 | 64 (3 HGIN, 61 MC) | ER‐L | Minor bleeding 12.5% | 82.5% | 12 | 14% |
| Nijhawan62 | 17 (4 HGIN, 13 MC) | L&C, ER‐L (7 PDT, 2 OP) | 0% | 100% | 14.6 | 0% |
| Buttar63 | 17 (7 BE/LGIN/HGIN, 10 MC/SMC) | ER‐L + PDT | Minor bleeding 6%, | 94% | 13 | 0% |
| strictures 30% | ||||||
| May58 | 115 (19 HGIN, 95 MC, 11 SMC) | 66 ER‐L, 32 PDT, 9 | Minor bleeding 7.5% | 98% | 31 | 30% |
| ER + PDT, 3 APC | Strictures 4.5% | |||||
| Seewald59 | 12 (3 BE/LGIN, 5 HGIN, 4 MC) | Circumferential L&C | Strictures 17%, | 100% | 9 | 0% |
| minor bleeding 33% | ||||||
| Giovannini48 | 21 (12 HGIN, 9 MC) | Semicircumf. L&C | Bleeding 19% | 86% | 18 | 11% |
| Behrens64 | 44 HGIN | 14 ER‐L 27 PDT | Minor bleeding 9.3% | 97.7% | 38 | 17.1% |
| Conio65 | 39 (5 LGIN, 27 HGIN, 2 MC, 5 SMC) | ER‐C | Bleeding 10.3% | 94 | 34.9 | 3% |
| Peters66 | 33 (3 BE, 8 HGIN, 15 MC, 7 SMC) | ER‐C | Minor bleeding 46% | 79% | 19 | 19% |
| Peters60 | 39 (3 BE, 1 LGIN, 18 HGIN, 12 MC, 3 SMC) | Circumferential ER‐C | Perforation 2.6% | 95% | 11 | 0% |
| Major bleeding 2.6% | ||||||
| Strictures 26% | ||||||
| Ell19 | 100 MC | ER‐L | Minor bleeding 10% | 98% | 36.7 | 11% |
| Pech67 | 304 (45 HGIN, 239 MC, 20 SMC) | 215 ER‐L, 72 PDT, 12 | Minor bleeding 11% | 86% | 69.5 | 21% (tumour‐related deaths, 0.7%) |
| ER‐L + PDT, 5 APC | Major bleeding 0.6% | |||||
| Strictures 3.3% |
APC, argon plasma coagulation; BE, Barrett's oesophagus; ER tube, endoscopic resection with a tube; ER‐C, endoscopic resection with cap device; ER‐L, endoscopic resection with ligation device; ESD, endoscopic submucosal dissection; HGIN, high‐grade intraepithelial neoplasia; L&C, lift and cut; LGIN, low‐grade intraepithelial neoplasia; MC, mucosal carcinoma; OP, surgery; PDT, photodynamic therapy; SMC, submucosal carcinoma.
Clinical results
Early squamous cell carcinoma
Endoscopic treatment of SCN was first performed by endoscopists in Asia,47 but western centres have also reported successful ER in these patients in recent years.53,54,56,57 The first report on ER treatment for early squamous cell carcinoma was published in the early 1990s.15 In 1997, Takeshita et al.47 reported in the western literature for the first time on their experience in a larger group of patients undergoing ER for intraepithelial neoplasias (n = 9) and mucosal (n = 43) as well as submucosal (n = 4) early squamous cell carcinomas.46 The resections were carried out using the suck‐and‐cut technique. Successful removal of the lesion was possible in one session in 25% of cases; the remaining patients required more than one ER. After a follow‐up period of three years, 53 patients were in complete remission, three had died of other causes, and one patient with submucosal invasion was receiving radiotherapy. Similarly good results were presented by Narahara et al.49 In 21 patients, a total of 25 mucosal carcinomas were successfully treated using ER after the injection of a saline solution under the lesion. No major complications occurred. A recent report from Japan has presented data on the problem of metachronous lesions after successful ER of squamous cell carcinoma. In 116 patients with 165 neoplastic lesions, the recurrence rate was 20% after a median follow‐up period of 35 months. Patients with multiple Lugol‐voiding lesions after iodine staining had a 3.1‐fold higher risk of metachronous neoplasia in comparison with those without Lugol‐voiding lesions. In addition, piecemeal resection was also associated with some risk of recurrence.50
The largest series in western countries have been published by our own group.53,54 In the first publication with acute and mid‐term results, we were able to demonstrate that ER is a safe and effective treatment in patients with HGIN and mucosal cancer, but that patients with submucosal invasion had an unfavourable outcome because of other underlying diseases such as liver cirrhosis and malignant tumours in other locations.53 A complete response was achieved in 36 of 39 patients (92%), but the rate of metachronous lesions observed during a mean follow‐up period of 29.7 (SD 14.3) months was 16.7%. In a very recent publication,54 we were able to identify independent risk factors for the recurrence of metachronous lesions. In a series including 65 patients (HGIN, n = 12; mucosal cancer, n = 53), a complete response was achieved with ER in 62 patients (95.4%), but neoplasia recurred in 26% after 39.3 (SD 22.8) months.54 Multivariate analysis revealed multifocal lesions as an independent risk factor for recurrence, with a relative risk of 4.1 (95% CI, 1.28 to 13.3), whereas piecemeal resection was not a risk factor for recurrence.
A recent publication by Fujishiro and co‐workers29 has reported on ESD in 58 patients with early oesophageal squamous cell carcinoma. Complete resection in relation to the histological criteria (R0 resection) was possible in 45 of 58 patients (78%). Complications observed in the series included perforations in 6.9% and strictures in 16% of cases. All of the perforations were managed conservatively. The results of ER in SCC are summarised in table 4.
Table 4 Publications on endoscopic resection for early oesophageal squamous cell carcinoma.
| First author, ref. | Patients (n) | Resection technique | Complications | Complete response | Follow‐up (months) | Recurrences/metachronous lesions |
|---|---|---|---|---|---|---|
| Takeshita47 | 56 (HGIN 9, MC 43, SMC 4) | ER‐C | Minor bleeding 3.6% | 100% | 39 | 0% |
| Stricture 3.6% | ||||||
| Perforation 1.8% | ||||||
| Giovannini48 | 14 | L&C | Minor bleeding 5% | 90.4% | 20 | 21.4% |
| Narahara49 | 21 | L&C | Minor bleeding 24% | 100% | 24 | 0% |
| Shimizu50 | 82 (74 MC, 8 SMC) | ER‐C, ER tube | n.a. | 100% | 25 | 17% (1 tumour‐related death) |
| Nomura51 | 51 | ER tube | n.a. | 100% | 18 | 8% |
| Shimizu52 | 26 (SMC) | n.a. | 0% | n.a. | 45 | 2 tumour‐related deaths |
| Pech53 | 39 (HGIN 10, MC 19, SMC 10) | ER‐L, ER‐C | Minor bleeding 7.5%, stricture 7.5% | 92% | 29.7 | 16.7% |
| Katada17 | 116 MC | L&C, ER‐C | n.a. | 100% | 35 | 20% |
| Fujishiro29 | 43 | ESD | Strictures 16%, perforation 6.9% | 100% | 17 | 2.3% |
| Pech54 | 65 (HGIN 12, MC 53) | ER‐L, ER‐C | Minor bleeding 3%, strictures 23% | 95.4% | 39.3 | 26% (2 tumour‐related deaths) |
APC, argon plasma coagulation; ER tube, endoscopic resection with a tube; ER‐C, endoscopic resection with cap device; ER‐L, endoscopic resection with ligation device; ESD, endoscopic submucosal dissection; HGIN, high‐grade intraepithelial neoplasia; L&C, lift and cut; LGIN, low‐grade intraepithelial neoplasia; MC, mucosal carcinoma; OP, surgery; PDT, photodynamic therapy; SMC, submucosal carcinoma.
In conclusion, ER of squamous cell cancers limited to the epithelial and proper mucosal layer (m1 and m2) has proved to be safe and effective in several studies. Patients with cancer infiltration into the muscularis mucosa or the proper submucosa should only be treated with ER if no further risk factors for lymph‐node metastasis are present.
Early neoplasia arising in Barrett's oesophagus
The first report on ER in 64 patients with early carcinoma or HGIN arising in Barrett's oesophagus was published in 2000.16 Complete remission was achieved with ER in 82.5% of cases (97% in the low‐risk group and 59% in the high‐risk group) in the study. During a mean follow‐up period of 12 months, recurrences or metachronous lesions were observed in 14% of patients, who underwent successful endoscopic re‐treatment.
In a further study by our group,21 115 patients with HGIN (n = 19) and early Barrett's carcinoma (n = 96) were treated with ER (n = 70), PDT (n = 32), a combination of the two (n = 10), or APC (n = 3). Complete remission was achieved in 98% of the patients, but metachronous neoplasia was found in 31% during a mean follow‐up period of 34 months.
Recurrences or metachronous neoplasia have been shown to be the major problem with endoscopic therapy in early Barrett's neoplasia, although successful repeat endoscopic treatment is possible in almost all patients. The reasons for the high rate of recurrence appear to be a percentage of undetected neoplasia in the residual Barrett's segment after treatment and, more importantly, the fact that the residual Barrett's metaplasia appears to have an increased risk of malignant transformation as a result of genetic abnormalities not influenced by the endoscopic treatment. Several attempts have therefore been made to reduce the rate of recurrent malignancy after successful treatment. Circumferential ER to eradicate the entire Barrett's mucosa at risk was recently introduced.59 Endoscopic treatment was carried out in 12 patients with HGIN or mucosal carcinoma. The complete Barrett's segment was resected in one to five sessions, with a median of five ER per session (range 1–19). Complications occurred in six cases (four cases of bleeding and two strictures), all of which were managed endoscopically. The median follow‐up in this small series was nine months, and no recurrences were observed.
Another study by Giovannini et al.48 also investigated the concept of complete circumferential ER of the entire Barrett's segment. Twelve patients with HGIN and nine with mucosal cancer were included in the study. ER was carried out in two sessions. In the first session, the lesion and the surrounding half of the Barrett's segment was removed by ER. In a second session one month later, the other half of the segment was resected to prevent stricture formation, which was observed in none of the patients in the series. Complete remission of cancer was achieved in 18 cases (86%); the three remaining patients underwent surgery (n = 1) or chemoradiotherapy (n = 2). Complete removal of the Barrett's epithelium was only possible in 75% of cases, and malignancy recurred in two patients (11%) during a mean follow‐up period of 18 months. Similar results were achieved by the Amsterdam group:60 complete eradication of early neoplasia was achieved in all 37 patients treated in a median number of three sessions, and complete removal of all Barrett's mucosa was achieved in 33 patients (89%). Symptomatic stenoses occurred in 26% of patients; further complications observed were one perforation and one case of delayed bleeding, all managed endoscopically. No recurrences had been observed after 11 months.
In conclusion, circumferential ER of the whole Barrett's segment appears to be an interesting approach in order to prevent recurrences or metachronous lesions after successful eradication of malignancy. Major problems include the high stricture rate and the fact that despite this radical treatment, there is relevant residual Barrett's epithelium, with a recurrence rate of up to 11%. In addition, the follow‐up periods and patient numbers were too limited in these studies for final conclusions to be drawn.
It is not yet clear whether ablative therapy with APC might be capable of reducing the recurrence rate. A retrospective analysis by our own group,18 including 210 patients successfully treated with endoscopic therapy, showed that patients who underwent ablation of the residual non‐neoplastic Barrett's epithelium using APC had a significantly lower recurrence rate than those without ablation after a mean follow‐up of 65.5 months: 34 of 102 patients (33.3%) without ablation developed a metachronous lesion in comparison with 19 of 108 patients (17.6%) who received ablative therapy (p = 0.002), and not performing ablation after complete remission of neoplasia was a risk factor for recurrence, with a relative risk of 0.4 (p = 0.0003; CI 0.26 to 0.66). The limitations of that study are its retrospective design and the fact that the Barrett's segment was not completely ablated in all patients in the APC group. Prospective trials are ongoing to investigate this issue.
Until 2006, no long‐term results for ER in patients with early Barrett's neoplasia were available. A very recent study by our group has now provided excellent long‐term results for ER in 100 consecutive patients with low‐risk mucosal Barrett's cancer.19 Complete remission was achieved in 99% of cases, and the five‐year overall survival rate was 98%. None of the patients died of Barrett's neoplasia in the study, and minor bleeding occurred in 11 cases. Metachronous lesions were observed in only 11% after 36.7 months.
This study of ER in a highly selected cohort of patients with low‐risk Barrett's carcinoma does not reflect the general population of patients with Barrett's neoplasia, but clearly underlines the safety and efficacy of the method in a highly specialised centre. The results with regard to the complete response rate and recurrence rate are slightly poorer in patients with larger neoplasms or submucosal cancer. The success rates are also excellent in these patients, however, as underlined by the largest series we have published,67 including 304 patients with HGIN, mucosal cancer, and submucosal cancer. A total of 215 patients underwent ER, 72 patients had PDT, and 12 had a combination of the two. After ER, bleeding was observed in 11% and strictures in 3.3%, but it was possible to manage all of the complications endoscopically. Eighty‐six per cent of patients achieved a complete response after endoscopic therapy, and metachronous lesions were found in 21% during a mean follow‐up period of 69.5 months. Tumour‐related deaths occurred in two inoperable patients (0.66%). This is the first study providing five‐year follow‐up data in a large patient cohort with early Barrett's carcinoma at different stages (HGIN, mucosal cancer and submucosal cancer), and it shows excellent results. Almost all metachronous lesions or recurrences were again treated successfully with ER.
There is almost no experience with ESD in patients with early Barrett's neoplasia. In a smaller series published by Kakushima et al.,61 ESD was performed in 30 patients with tumours of the oesophagogastric junction; only four of the patients had early Barrett's cancer. The average maximum diameters of the lesions and resected specimens were 22.4 mm and 40.6 mm, respectively. The R0 resection rate was 97% (29 of 30). Histology revealed lymph vessel invasion in five patients and submucosal invasion deeper than 500 μm also in five cases.
As already described in relation to surgery, this type of treatment should only be carried out in very experienced hospitals with a high annual volume of patients with Barrett's neoplasia. Endoscopic treatment of neoplastic lesions is only one part of the overall management strategy. Optimal detection and delineation of neoplastic lesions requires high‐resolution endoscopes, a high degree of experience in recognising what are often very subtle mucosal abnormalities, and experience in the use and interpretation of chromoendoscopy and other diagnostic techniques (such as acetic acid staining and virtual chromoendoscopy).
Conclusions
ER should be accepted as the treatment of choice in most patients with HGIN and mucosal carcinoma in the oesophagus. The various points in favour of endoscopic resection and against surgical resection are summarised in table 1. Several series have reported excellent acute results with ER for squamous cell carcinoma and Barrett's adenocarcinoma in comparison with oesophageal resection, which in the United States is associated with a 30‐day mortality rate that ranges from 8.4% in large‐volume centres up to 20.3% in centres with low experience (surgeons who carry out less than two oesophagectomies per year). The fact that 55% of oesophagectomies in the United States are carried out in low‐volume centres3,4 underlines the seriousness of this problem. In the case of early squamous cell carcinoma, the mortality rate after surgical resection appears to be clearly higher, as a result of the co‐morbidity in these patients.68 There are, however, no prospective studies comparing ER and surgery to draw final conclusions on this issue. Thoracoscopic oesophagectomy is a new and less invasive surgical option with reduced mortality and morbidity than open surgery, but still carries a substantial complication and mortality rate.20,69 The mortality and complication rate is still significantly higher than with ER but minimally invasive oesophageal resection could be an alternative to radical surgery in selected cases.69
With regard to the long‐term outcome, only one large series in early Barrett's cancer and three in patients with squamous cell carcinoma have been published.17,19,47,59 It is important to emphasise that a close follow‐up programme is crucial for surveillance of the residual Barrett's oesophagus. To improve the acceptance of endoscopic treatment, further prospective trials with long‐term data are necessary. Randomised trials comparing ER with surgery would be desirable, but there are two major reasons why this type of study would be almost impossible. First, it would be hard to find patients willing to agree to random selection, particularly in view of the excellent long‐term results with endoscopic treatment. In addition, to obtain significant results with regard to the key parameter of tumour‐related survival, more than 200 patients would have to be included in each group.
To obtain resection specimens that are large enough, ER in the oesophagus should only be carried out using the suck‐and‐cut technique, either with the cap or the ligation device. ESD appears to be an attractive new treatment method not only for early gastric cancer but also for patients with early oesophageal malignancy. This method is able to provide complete en bloc resection of larger neoplastic lesions, but experience is so far very limited and the complication rate is relatively high in comparison with conventional ER. The same requirements in terms of hospital volume that are made for surgeons should also be made for endoscopists diagnosing and treating patients with early oesophageal cancers, and a special level of experience should be mandatory.
Footnotes
Conflict of interest: None declared.
References
- 1.Hölscher A H, Bollschweiler E, Schneider P M.et al Early adenocarcinoma in Barrett's oesophagus. Br J Surg 1997841470–1473. [PubMed] [Google Scholar]
- 2.Thomas P, Doddoli C, Neville P.et al Esophageal cancer resection in the elderly. Eur J Cardiothorac Surg 199611941–946. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer J D, Siewers A E, Finlayson E V.et al Hospital volume and surgical mortality in the United States. N Engl J Med 20023461128–1137. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer J D, Stukel T A, Siewers A E.et al Surgeon volume and operative mortality in the United States. N Engl J Med 20033492117–2127. [DOI] [PubMed] [Google Scholar]
- 5.Pech O, May A, Gossner L.et al Barrett's esophagus: endoscopic resection. Gastrointest Endosc Clin North Am 200313505–512. [DOI] [PubMed] [Google Scholar]
- 6.May A, Guenter E, Roth F.et al Accuracy of staging in oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut 200453634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuccaro G, Jr, Rice T W, Vargo J J.et al Endoscopic ultrasound errors in esophageal cancer. Am J Gastroenterol 2005100601–606. [DOI] [PubMed] [Google Scholar]
- 8.Heeren P A, van Westreenen H L, Geersing G J.et al Influence of tumor characteristics on the accuracy of endoscopic ultrasonography in staging cancer of the esophagus and esophagogastric junction. Endoscopy 200436966–971. [DOI] [PubMed] [Google Scholar]
- 9.Lightdale C J, Kulkarni K G. Role of endoscopic ultrasonography in the staging and follow‐up of esophageal cancer. J Clin Oncol 2005234483–4489. [DOI] [PubMed] [Google Scholar]
- 10.Pech O, May A, Günter E.et al The impact of endoscopic ultrasound and computed tomography on the TNM staging of early cancer in Barrett's esophagus. Am J Gastroenterol 20061012223–2229. [DOI] [PubMed] [Google Scholar]
- 11.Kelly S, Harris K M, Berry E.et al A systematic review of the staging performance of endoscopic ultrasound in gastro‐oesophageal carcinoma. Gut 200149534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soehendra N, Binmoeller K F, Bohnacker S.et al Endoscopic snare mucosectomy in the esophagus without any additional equipment: a simple technique for resection of flat early cancer. Endoscopy 199729380–383. [DOI] [PubMed] [Google Scholar]
- 13.Tanabe S, Koizumi W, Kokutou M.et al Usefulness of endoscopic aspiration mucosectomy as compared with strip biopsy for the treatment of gastric mucosal cancer. Gastrointest Endosc 199950819–822. [DOI] [PubMed] [Google Scholar]
- 14.Noda M, Kobayashi N, Kanemasa H.et al Endoscopic mucosal resection using a partial transparent hood for lesions located tangentially to the endoscope. Gastrointest Endosc 200051338–343. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Endo M. A new simplified technique of endoscopic esophageal mucosal resection using a cap‐fitted panendoscope. Surg Endosc 19936264–265. [DOI] [PubMed] [Google Scholar]
- 16.Ell C, May A, Gossner L.et al Endoscopic mucosal resection of early cancer and high‐grade dysplasia in Barrett's esophagus. Gastroenterology 2000118670–677. [DOI] [PubMed] [Google Scholar]
- 17.Katada C, Muto M, Manabe T.et al Local recurrence of squamous‐cell carcinoma of the esophagus after EMR. Gastrointest Endosc 200561219–225. [DOI] [PubMed] [Google Scholar]
- 18.Pech O, May A, Gossner L.et al The effect of ablation of non‐neoplastic Barrett's epithelium on recurrence rate of high grade dysplasia and early Barrett's cancer after endoscopic therapy: an analysis in 219 patients. Presented at Digestive Disease Week; 19–24 May 2007, Washington, DC.
- 19.Ell C, May A, Pech O.et al Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc 2007653–10. [DOI] [PubMed] [Google Scholar]
- 20.Braghetto I, Csendes A, Cardemil G.et al Open transthoracic or transhiatal esophagectomy versus minimally invasive esophagectomy in terms of morbidity, mortality and survival. Surg Endosc 2006201681–1686. [DOI] [PubMed] [Google Scholar]
- 21.May A, Gossner L, Behrens A.et al A prospective randomized trial of two different suck‐and‐cut mucosectomy techniques in 100 consecutive resections in patients with early cancer of the esophagus. Gastrointest Endosc 200358167–175. [DOI] [PubMed] [Google Scholar]
- 22.Ell C, May A, Wurster H. The first reusable multiple‐band ligator for endoscopic hemostasis of variceal bleeding and mucosal resection. Endoscopy 199931738–740. [DOI] [PubMed] [Google Scholar]
- 23.Soehendra N, Seewald S, Groth S.et al Use of modified multiband ligator facilitates circumferential EMR in Barrett's esophagus (with video). Gastrointest Endosc 200663847–852. [DOI] [PubMed] [Google Scholar]
- 24.Muto M, Miyamoto S, Hosokawa A.et al Endoscopic mucosal resection in the stomach using the insulated‐tip needle‐knife. Endoscopy 200537178–182. [DOI] [PubMed] [Google Scholar]
- 25.Oka S, Tanaka S, Kaneko I.et al Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 200664877–883. [DOI] [PubMed] [Google Scholar]
- 26.Hosokawa K, Yoshida S. [Recent advances in endoscopic mucosal resection for early gastric cancer.] (In Japanese. ) Gan To Kagaku Ryoho 199825476–483. [PubMed] [Google Scholar]
- 27.Ono H, Kondo H, Gotoda T.et al Endoscopic mucosal resection for treatment of early gastric cancer. Gut 200148225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onozato Y, Ishihara H, Iizuka H.et al Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy 200638980–986. [DOI] [PubMed] [Google Scholar]
- 29.Fujishiro M, Yahagi N, Kakushima N.et al Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 20064688–694. [DOI] [PubMed] [Google Scholar]
- 30.Kakushima N, Yahagi N, Fujishiro M.et al Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy 200638170–174. [DOI] [PubMed] [Google Scholar]
- 31.Fujishiro M, Yahagi N, Nakamura M.et al Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high‐molecular‐weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 200663243–249. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe K, Ogata S, Kawazoe S.et al Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc 200663776–782. [DOI] [PubMed] [Google Scholar]
- 33.Tajima Y, Nakanishi Y, Ochiai A.et al Histopathologic findings predicting lymph node metastasis and prognosis of patients with superficial esophageal carcinoma: analysis of 240 surgically resected tumors. Cancer 2000881285–1293. [PubMed] [Google Scholar]
- 34.Eguchi T, Nakanishi Y, Shimoda T.et al Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol 200619475–480. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima Y, Nagai K, Miyake S.et al Evaluation of an indicator for lymph node metastasis of esophageal squamous cell carcinoma invading the submucosal layer. Jpn J Cancer Res 200293305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araki K, Ohno S, Egashira A.et al Pathologic features of superficial esophageal squamous cell carcinoma with lymph node and distal metastasis. Cancer 200294570–575. [DOI] [PubMed] [Google Scholar]
- 37.Chibana Y, Fujii S, Ichikawa K.et al Tumor cell dissociation score highly correlates with lymph node metastasis in superficial esophageal carcinoma. J Gastroenterol Hepatol 2005201371–1378. [DOI] [PubMed] [Google Scholar]
- 38.Buskens C J, Westerterp M, Lagarde S M.et al Prediction of appropriateness of local endoscopic treatment for high‐grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 200460703–710. [DOI] [PubMed] [Google Scholar]
- 39.Stein H J, Feith M, Bruecher B L D M.et al Early esophageal squamous cell and adenocarcinoma: pattern of lymphatic spread and prognostic factors for long term survival after surgical resection. Ann Surg 2005242566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pech O, May A, Rabenstein T.et al Endoscopic resection in neoplastic lesions of the oesophagus. Langenbecks Arch Surg 2003388421–424. [DOI] [PubMed] [Google Scholar]
- 41.Gossner L, Pech O, May A.et al Comparison of methylene blue‐directed biopsies and four‐quadrant biopsies in the detection of high‐grade intraepithelial neoplasia and early cancer in Barrett's oesophagus. Digest Liver Dis 200638724–729. [DOI] [PubMed] [Google Scholar]
- 42.Sharma P, Weston A P, Topalovski M.et al Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett's oesophagus. Gut 20035224–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman A, Kiesslich R, Bender A.et al Acetic acid‐guided biopsies after magnifying endoscopy compared with random biopsies in the detection of Barrett's esophagus: a prospective randomized trial with crossover design. Gastrointest Endosc 2006641–8. [DOI] [PubMed] [Google Scholar]
- 44.Pohl J, May A, Rabenstein T.et al Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy 20073980–83. [DOI] [PubMed] [Google Scholar]
- 45.Kara M A, Ennahachi M, Fockens P.et al Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett's esophagus by using narrow band imaging. Gastrointest Endosc 200664155–166. [DOI] [PubMed] [Google Scholar]
- 46.Kiesslich R, Gossner L, Goetz M.et al In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Herpatol 20064979–987. [DOI] [PubMed] [Google Scholar]
- 47.Takeshita K, Tani M, Inoue H.et al Endoscopic treatment of early oesophageal or gastric cancer. Gut 199740123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giovannini M, Bories E, Pesenti C.et al Circumferential endoscopic mucosal resection in Barrett's esophagus with high‐grade intraepithelial neoplasia or mucosal cancer: preliminary results in 21 patients. Endoscopy 200436782–787. [DOI] [PubMed] [Google Scholar]
- 49.Narahara H, Iishi H, Tatsuta M.et al Effectiveness of endoscopic mucosal resection with submucosal saline injection technique for superficial squamous carcinomas of the esophagus. Gastrointest Endosc 200052730–734. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu Y, Tsukagoshi H, Fujita M.et al Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest Endosc 200154190–194. [DOI] [PubMed] [Google Scholar]
- 51.Nomura T, Boku N, Ohtsu A.et al Recurrence after endoscopic mucosal resection for superficial esophageal cancer. Endoscopy 200032277–280. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu Y, Tsukagoshi H, Fujita M.et al Long‐term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc 200256387–390. [DOI] [PubMed] [Google Scholar]
- 53.Pech O, Gossner L, May A.et al Endoscopic resection of superficial esophageal squamous‐cell carcinomas: western experience. Am J Gastroenterol 2004991226–1232. [DOI] [PubMed] [Google Scholar]
- 54.Pech O, May A, Gossner L.et al Curative endoscopic therapy in patients with early esophageal squamous‐cell carcinoma or high‐grade intraepithelial neoplasia. Endoscopy 20073930–35. [DOI] [PubMed] [Google Scholar]
- 55.Makuuchi H. Endoscopic mucosal resection for early esophageal cancer: indication and techniques. Dig Endosc 19968175–179. [Google Scholar]
- 56.Soehendra N, Binmoeller K F, Bohnacker S.et al Endoscopic snare mucosectomy in the esophagus without any additional equipment: a simple technique for resection of flat early cancer. Endoscopy 199729380–383. [DOI] [PubMed] [Google Scholar]
- 57.Giovannini M, Bernardini D, Moutardier V.et al Endoscopic mucosal resection (EMR): results and prognostic factors in 21 patients. Endoscopy 199931698–701. [DOI] [PubMed] [Google Scholar]
- 58.May A, Gossner L, Pech O.et al Local endoscopic therapy for intraepithelial high‐grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute‐phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002141085–1091. [DOI] [PubMed] [Google Scholar]
- 59.Seewald S, Akaraviputh T, Seitz U.et al Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high‐grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc 200357854–859. [DOI] [PubMed] [Google Scholar]
- 60.Peters F P, Kara M A, Rosmolen W D.et al Stepwise radical endoscopic resection is effective for complete removal of Barrett's esophagus with early neoplasia: a prospective study. Am J Gastroenterol 20061011449–1457. [DOI] [PubMed] [Google Scholar]
- 61.Kakushima N, Yahagi N, Fujishiro M.et al Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy 200638170–174. [DOI] [PubMed] [Google Scholar]
- 62.Nijhawan P K, Wang K K. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high‐grade dysplasia within Barrett's esophagus. Gastrointest Endosc 200052328–332. [DOI] [PubMed] [Google Scholar]
- 63.Buttar N S, Wang K K, Lutzke L S.et al Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett's esophagus. Gastrointest Endosc 200154682–688. [DOI] [PubMed] [Google Scholar]
- 64.Behrens A, May A, Gossner L.et al Curative treatment for high‐grade intraepithelial neoplasia in Barrett's esophagus. Endoscopy 200537999–1005. [DOI] [PubMed] [Google Scholar]
- 65.Conio M, Repici A, Cestari R.et al Endoscopic mucosal resection for high‐grade dysplasia and intramucosal carcinoma in Barrett's esophagus: an Italian experience. World J Gastroenterol 2005116650–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters F P, Kara M A, Rosmolen W D.et al Endoscopic treatment of high‐grade dysplasia and early stage cancer in Barrett's esophagus. Gastrointest Endosc 200561506–514. [DOI] [PubMed] [Google Scholar]
- 67.Pech O, Behrens A, May A.et al Curative endoscopic therapy for Barrett's early cancer and high grade dysplasia: long‐term results in 304 patients [abstract]. Gastrointest Endosc 200663AB84 [Google Scholar]
- 68.Holscher A H, Bollschweiler E, Schroder W.et al Prognostic differences between early squamous‐cell and adenocarcinoma of the esophagus. Dis Esophagus 199710179–184. [DOI] [PubMed] [Google Scholar]
- 69.Smithers B M, Gotley D C, Martin I.et al Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007245232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]