Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease that affects up to one in 700 women in western populations (female to male ratio is approximately 10 to 1, with the disease typically presenting over the age of 40 years).1,2 PBC is characterised histologically by damage to, and eventual loss of, the biliary epithelial cells (BEC) lining small intrahepatic bile ducts. BEC loss is typically accompanied by a significant portal tract inflammatory infiltrate that is mixed in phenotype (T cells (CD4 and CD8, with the latter predominating in the periductal areas), B cells, macrophages, eosinophils and natural killer cells).3,4 Early descriptions of PBC emphasised the predominant role played by the progression of bile duct loss, accompanied by increasing portal tract and linking fibrosis leading to biliary cirrhosis, in the clinical expression of PBC, and described an aggressive and uniformly fatal condition.5 Increased awareness of the condition and, in particular, the availability of diagnostic tools such as serology has led to broader and earlier diagnosis.6 This has had the effect that we now recognise PBC far more frequently than was previously the case, and typically do so at an earlier stage in the disease process. Broadening of the diagnostic base has, in addition, led us to appreciate that there is a significant subgroup within the PBC population who have a low risk of disease progression and who are unlikely to develop end‐stage liver disease during a normal lifetime (but who remain at risk of developing the often life‐altering symptoms of the disease such as fatigue, which are seemingly unrelated to the severity of the underlying liver disease).2,7,8 The factors that determine individual risk of disease progression remain unclear, precluding us, at present, from targeting disease‐modifying therapies at “high‐risk” patients.
Study of the pathogenesis of PBC has focused, until relatively recently, almost entirely on immunological aspects of the disease.9 PBC was one of the first conditions in which the presence of autoantibodies in the serum was identified, and was one of the first conditions in which the antigen specificity of this autoreactive response was characterised.3 This led, quite naturally, to the view that PBC is an autoimmune disease. This led, again not unreasonably (but largely unsuccessfully), to a focus on developing and applying therapies aimed at modifying this increasingly well‐characterised autoreactive immune response. More recently, however, the true complexities of the processes seemingly contributing to PBC pathogenesis have begun to be appreciated and the exclusively autoimmune disease model has been challenged.
Pathological factors in PBC
PBC pathogenesis from an immune system perspective
PBC is characterised immunologically by the breakdown of immune self‐tolerance to highly conserved mitochondrial and nuclear antigens (table 1).3,10,11,12,13,14,15,16,17,18,19 The first autoreactive responses to be characterised in PBC were the serum antibody responses directed at antigens present on the inner mitochondrial membrane (antimitochondrial antibodies; AMA). AMA, which are present, often at very high titre, in over 95% of patients, are directed at the members of the 2‐oxoacid dehydroganese complex family of multi‐enzyme complexes. These complexes, which all play important roles in mitochondrial energetics, share a common multicomponent, multidomain structure. The dominant autoantibody response in PBC is directed at pyruvate dehydrogenase complex (PDC), although reactivity is seen to a lesser extent to all other 2‐oxoacid dehydroganese complex family members (table 1). Within PDC, reactivity is seen, in particular, to the dihydrolipoamide acetyltransferase (E2) and enzyme 3 binding protein (E3BP) components. Reactivity is seen to these two antigens alone in over 90% of PBC patients. It was appreciated from relatively early on that the antibodies reactive with these two enzymes are fully crossreactive and that they can block the function of both enzymes. The mechanistic explanation for these two, in retrospect key, observations is the reactivity seen to a highly conserved structural motif present within both PDC‐E2 and E3BP,11 which consists of the binding domain for a lipoic acid (LA) co‐factor that plays an essential role in enzyme function. The importance of LA in the autoantibody response to PDC is confirmed by the finding that LA itself forms part of the PDC‐E2 and PDC‐E3BP autoepitope.11,20,21
Table 1 Characteristic autoantibodies seen in primary biliary cirrhosis and their reported frequencies in populations.
| Antibody group | Antigen | Patient frequency (%) |
|---|---|---|
| Mitochondrial | PDC‐E210 | 95* |
| PDC‐E3BP10,11 | 95* | |
| PDC‐E1α12 | 40–60† | |
| PDC‐E1β12 | 10 | |
| OGDC‐E213 | 40–90†,‡ | |
| BCOADC‐E214 | 10‡ | |
| Nuclear | gp21015 | 10–40† |
| Sp10016 | 10–30† | |
| p6217 | 20–30† | |
| Centromere18 | 10 | |
| Lamin B receptor19 | 2 |
*Autoantibodies to pyruvate dehydrogenase complex (PDC)‐acetyltransferase (E2) and PDC‐enzyme 3 binding protein (E3BP) are fully crossreactive (reactivity is to the shared, highly conserved lipoic acid binding domain). The identical population frequency reflects the fact that these are, de facto, the same antibodies.
†Primary biliary cirrhosis population frequencies to these antibodies appear to show significant geographical variation and this is reflected in the varying reported frequencies.
‡2‐Oxoglutarate dehydrogenase (OGDC)‐E2, branched chain 2‐oxoacid dehydroganese (BCOADC)‐E2 and PDC‐E3BP share an apparent molecular weight on sodium dodecylsulphate–polyacrylamide gel electrophoresis in the range 50–52 kDa (with subtle intermammalian species differences that can be important depending on the source of the PDC used in immunoblots). This molecular weight similarity has led, in the past, to errors in the identification of antibodies by immunoblot.
Despite the importance of serum AMA in the history of PBC (and its ongoing importance in the serological diagnosis of the disease),22 the actual role it plays in disease pathogenesis appears to be limited (although a number of potential mechanisms, such as antibody‐directed cell cytotoxicity (itself relevant because of the upregulation of cell surface PDC seen on BEC in PBC)23 remain to be fully explored (fig 1A)).9 Observations in human infectious disease settings, in which serum IgG anti‐PDC can be identified in the apparent absence of liver damage,24 have suggested that the presence of AMA does not, in isolation, result in BEC damage (although the potential significance of differences in the IgG isotype of the resulting antibody response (PBC is characterised by a specific increase in responses of the the IgG3 isotype)25 again remains to be explored). More recently, it has been suggested that it may be non‐IgG isotypes of AMA (in particular IgA, which can undergo transcytosis in cells such as BEC, theoretically causing cytopathic effects through its intracellular interaction with PDC) that are pathogenetic. These models remain unconfirmed despite the demonstration that such transcytosis can indeed occur (fig 1A).26,27
Figure 1 (A) Potential immune effector mechanisms for biliary epithelial cell (BEC) damage in primary biliary cirrhosis (PBC). 1. IgA (and, potentially, IgM) transcytosing across BEC inducing cytopathy as a consequence of interactions with nascent pyruvate dehydrogenase complex (PDC) component peptides.27 2. Antimitochondrial antibody (AMA)‐mediated antibody‐directed cell cytotoxicity against BEC by killer cells. 3. BEC epithelial to mesenchymal transition potentially induced by transforming growth factor beta (TGFβ). In this model TGFβ could be released by cells of either immune or non‐immune system origin28,29 (the true role, if any of this pathway in BEC “loss” in PBC remains to be determined). 4. PDC‐acetyltransferase (E2)‐derived epitope directed cytotoxic T‐cell response.30 5. Actions of pro‐inflammatory cytokines released by CD4 (and CD8) T cells interacting with macrophages. (B) Potential non‐immune effector mechanisms for BEC damage in PBC. 1. Oxidant stress resulting from macrophage activation. 2. BEC senescence (which may, in turn, be augmented by oxidant stress).31 3. Cytotoxic effect of hypothetical environmental toxic trigger excreted into the bile.32,33 4. Cytopathic effect of postulated (but not confirmed) PBC‐associated beta‐retrovirus.34
Following the original identification and characterisation of AMA in PBC more recent studies have characterised apparently PBC‐specific antibodies reactive with highly conserved nuclear structures such as the gp210 and Sp100 antigens (giving rise to characteristic nuclear dot and perinuclear staining patterns on immunofluorescence).35 In a scenario redolent of AMA it has, again, been difficult to show any direct pathogenetic role for antinuclear antibodies in PBC (although there are emerging data to suggest that their presence is associated with a poorer clinical outcome).36 More recently it has been postulated that although AMA do not play a direct role per se in causing BEC damage in PBC, the B cells that are responsible for their release may have an additional important “upstream” pathogenetic role in priming the breakdown of T‐cell tolerance described below, which has a more plausible effector role.37 This observation has echoes in an emerging body of literature suggesting that B cells can, contrary to earlier beliefs, function as “professional” antigen presenting cells able to prime naive T‐cell responses.38 It is certainly the case that B cells in PBC have the surface activation phenotype required if they are indeed to function as antigen presenting cells.39
The potential for T‐cell autoreactive immune responses to contribute to bile duct injury in PBC (fig 1A) is suggested by a number of observations, not least the almost universal presence of activated T cells in the portal tract infiltrates surrounding affected bile ducts.3 Both CD4 and CD8 autoreactive T‐cell responses directed at self‐PDC‐E2 have now been extensively characterised in PBC patients (and have been found to be, importantly, entirely absent from normal and chronic liver disease controls).30,40,41,42 The key class I and class II restricted autoepitopes appear to be, once again, located within the LA binding domain of PDC‐E2 spanning the LA binding residue itself. Both CD4 and CD8 PDC‐reactive T‐cell responses are enriched within the liver and the CD8 response, which appears to be at its highest level in the early stages of the disease when bile duct loss appears to be at its height, is effective at lysing PDC‐E2 peptide pulsed target cells.30 These observations would all be compatible with a cytotoxic T‐cell‐mediated response as the final pathway for BEC loss in PBC (fig 1A).
In a separate strand of work, animal modelling studies have suggested that the breakdown of tolerance to PDC at the T‐cell level is associated with the development of portal tract inflammation and bile duct changes with features redolent of early human PBC.43 Transfer studies from primed into naive animals suggest that the transfer of AMA alone is insufficient to induce bile duct lesions, whereas the transfer of activated splenocytes is able to achieve this effect.44 These preliminary transfer observations, together with the observation that the timing of bile duct lesion development mirrors the timing of the breakdown of T‐cell tolerance to self‐PDC,45 would all support a T‐cell pathogenesis model in PBC. Furthermore, the murine studies identified conservation of the LA binding domain structure across evolution and, in particular, immune crossreactivity at the level of LA itself, as a potential mechanism for the breakdown of immune self‐tolerance to PDC.46
Recent studies in autoimmune disease have highlighted a potentially significant role in the orchestration of target cell damage for a CD4 T‐cell subset characterised by release of the cytokine IL‐17.47 Development of the “Th17” cell subset (well characterised in the mouse and postulated to be present in the human) is induced in the joint presence of TGFβ and IL‐6, is suppressed by the actions of IL‐2, and is associated with the reciprocal regulation of IFN‐γ.47,48 Although “Th‐17” type CD4 T‐cell function remains to be studied in PBC there is indirect evidence to implicate cells of this phenotype. Evidence of upregulated TGF‐β signalling is present in the liver in PBC, and IL‐6 release is augmented (partly by the actions of hydrophobic bile salts).28,49 Studies in this area are clearly warranted.
Despite the evidence, however, some investigators in the field retain doubts about the precise extent of the role played by autoimmunity in the pathogenesis of PBC. Several observations have been used to argue against the autoimmune model. First, several of the “classic” features defining autoimmune diseases are absent in the case of PBC (table 2). Second, treatments that might be expected to modify autoreactive T‐cell responses significantly have proved disappointing in clinical trials in PBC. Third, there is difficulty in explaining the now widely recognised recurrence of PBC seen post‐transplantation, which can clearly occur across human leukocyte antigen (HLA) boundaries, by an HLA‐restricted process (table 3). Finally, there is the conceptual problem associated with an apparently highly tissue‐specific pathological process (BEC loss) being mediated by a T‐cell response directed at a ubiquitous self‐antigen.
Table 2 Classic characteristics of autoimmune disease aetiology and the extent to which primary biliary cirrhosis demonstrates those characteristics.
| Characteristic | Characteristic seen in PBC? | Comment |
|---|---|---|
| Autoreactive immune responses to defined autoantigens | Yes | PDC association is among the strongest seen in any autoimmune disease |
| Female predisposition | Yes | 10 : 1 Female to male ratio although classical disease occurs in men ruling out an obligate female association |
| HLA association | Yes | Significant associations but with atypical alleles and weaker than in other autoimmune diseases |
| Induction of disease by sensitisation with autoantigen | Possibly | Observation awaits replication |
| Response to immunosuppressive therapy | Possibly | Comprehensive trials equivocal. Probably effective in selected individuals with significant inflammatory component |
| Production of disease by adoptive transfer of autoreactive cells and/or antibody | Possibly | Preliminary data suggestive. As yet unconfirmed |
| Spontaneous animal model with identical antigen specificity | No | No confirmed spontaneous model |
| Both children and adults affected | No | Disease in children is anecdotal |
| Autoantigen distribution matches disease distribution | No | PDC is universal but disease is highly restricted in its distribution |
HLA, human leukocyte antigen; PBC, primary biliary cirrhosis; PDC, pyruvate dehydrogenase complex.
Table 3 Summary of the postulated upstream pathogenetic processes in primary biliary cirrhosis and their characteristics.
| Category | Mechanism/process/factor | Observation confirmed in repeat studies | Mechanism observed in majority of PBC patients | Mechanism able to explain post‐transplant disease recurrence across HLA boundaries | Mechanism able to explain tissue tropism of PBC |
|---|---|---|---|---|---|
| Immunological | AMA | Yes | Yes | Yes (ADCC) | No |
| ANA | Yes | No | ? (BEC surface expression not reported) | No | |
| AMA transcytosis | Yes | ? | Yes | Yes | |
| CD4 T‐cell response to PDC | Yes | Yes | No | ? | |
| CD8 T‐cell response to PDC | Yes | Yes | No | ? | |
| T‐cell response to nuclear antigen | No data yet | ? | No | ? | |
| ? TGFβ‐driven EMT | No | ? | Yes | ? | |
| Non‐immunological | Retrovirus | No | ? | Yes | Yes |
| Environmental toxin effect | Yes | ? | Yes | Yes | |
| Acclelerated BEC senescence | No | ? | Yes | Yes | |
| Oxidative stress | Yes | ? | Yes | Yes |
ADCC, antibody‐directed cell cytotoxicity; AMA, antimitochondrial antibody; ANA, antinuclear antibody; BEC, biliary epithelial cell; EMT, epithelial to mesenchymal transition; HLA, human leukocyte antigen; PBC, primary biliary cirrhosis; PDC, pyruvate dehydrogenase complex; TGFβ, transforming growth factor beta.
? Denotes the fact that this aspect of the process has not been studied in large enough study cohorts and/or that the appropriate mechanistic studies have not been performed.
The innate immune system has been less well studied in PBC. There are, however, emerging data suggesting that chronic activation of the innate immune system may occur. Peripheral blood mononuclear cells from PBC patients show an augmented response to Toll‐like receptor ligands, suggesting the potential for chronic high‐level inflammatory cytokine release.49 This observation mirrors findings suggesting increased levels of cytokine transcription within the liver in PBC. The presence of chronic activation of the innate immune system would fit with characteristic clinical features of PBC, such as fatigue and chronic sleep disturbance,50,51 which can, in other circumstances, occur as a consequence of chronic exposure to inflammatory cytokines.52,53 The mechanisms underpinning chronic inflammatory cytokine release are at present unclear, although observations concerning the release of high levels of such cytokines by cells, including BEC themselves,54,55 also after exposure to hydrophobic bile salts, suggest that this may be a consequence of cholestasis rather than a disease‐initiating event.
PBC pathogenesis from a target cell perspective
Until relatively recently BEC were regarded as passive “victims” in the PBC pathogenetic pathway (a conventional effector cell/target cell immunological paradigm). It is becoming increasingly clear that the nature of the BEC response to harmful processes and, in particular, the balance between damage and proliferative repair is both critical in the development of bile duct lesions and may provide important opportunities for the development of new approaches to treatment (fig 2).
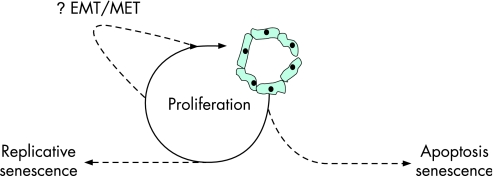
Figure 2 The balance between damage and proliferative repair in the expression of ductopenia in primary biliary cirrhosis. Irreversible biliary epithelial cell (BEC) “loss” through, for example, apoptosis or oxidant‐stress‐induced senescence is counterbalanced by a homeostatic proliferative response. Ultimately, ongoing proliferation will result in replicative senescence and failure of homeostasis (cellular “exhaustion”). The recently identified process of epithelial to mesenchymal transition (EMT), in which BEC appear to undergo transition to a fibroblast phenotype could, theoretically, result in both further ductopenia and paradoxically in proliferative homeostasis if the resulting mesenchymal cells themselves undergo proliferation and subsequent mesenchymal to epithelial transition (MET; reversion to the epithelial phenotype possibly driven by hepatocyte growth factor function). Studies in this area are required.
The earliest observations identified (perhaps unsurprisingly in light of the then prevailing immune model for disease pathogenesis) apoptosis as a key mechanism for BEC loss.56,57,58,59,60,61 The identification of markers suggestive of T‐cell cytotoxicity, such as perforin and granzyme in PBC liver,62 together with the data suggesting PDC‐E2‐derived epitope restricted CD8 T‐cell killing by PBC patient‐derived clones, supported this model of immune effector cell‐mediated BEC apoptosis as a key pathogenetic step. Although BEC apoptosis undoubtedly occurs the temporal association between BEC apoptosis and cytotoxic T‐cell responses appears more complex than has previously been appreciated. Studies of apoptotic markers in PBC suggest that apoptosis is actually at its peak in the middle stages of the disease (stage II–III) rather than in the very earliest stages when, intuitively, the BEC‐directed cytotoxic T‐cell response might be predicted to be at its peak.63,64 Moreover, hydrophobic bile salts of the type known to be progressively retained in the liver in cholestatic liver disease are, themselves, pro‐apoptotic.65,66 These observations raise the possibility that, rather than being a feature of the cardinal anti‐BEC process, apoptosis may represent, in part at least, a secondary process occurring as a consequence of cholestatic bile salt retention and/or failed replicative homeostasis. This model gives rise, of course, to the key question as to the cause of the primary BEC loss if apoptosis is a secondary phenomenon. The concept of apoptosis as a secondary process in PBC also gives rise to an interesting potential implication for the anti‐PDC response. Data from our group have suggested that apoptosis may be a cause of the aberrant cell‐surface expression of PDC seen on PBC BEC.23,67 If, as has previously been postulated, aberrant PDC processing and expression contribute to its immunogenicity, and this altered processing occurs as a consequence of apoptosis,68 it raises the possibility that the anti‐PDC immune response seen in PBC is actually also a secondary phenomenon, which, although potentially contributing to the expression of the disease, is not its initiating feature.
Emerging data suggest intriguing alternative potential mechanisms for BEC loss in PBC. Observations in both animal models of biliary obstruction, and to a more limited extent in PBC itself, have suggested that one such mechanism is the process of EMT in which epithelial cells undergo phenotypic “reprogramming” (fig 1A).28,29,69,70,71 Phenotypic studies demonstrate an intermediate stage in which both epithelial (CK19‐9, E‐cadherin) and mesenchymal (S100A4, Vimentin, αSMA) markers are expressed, with the eventual loss of epithelial features and acquisition of a full mesenchymal functional and surface expression phenotype. This model is attractive as an explanation for the “loss” of BEC and their “replacement” by fibroblasts. Studies using cultured BEC have demonstrated that EMT can be driven by TGFβ, an observation that is supported by the presence of markers of TGFβ signalling such as nuclear pSMAD 2/3 in BEC in PBC liver.29 Critically, the induction of EMT in cultured BEC can be reversed by hepatocyte growth factor (HGF; which is, in turn, able to reverse liver fibrosis secondary to bile duct ligation and ameliorate PBC‐like bile duct lesions in chronic graft versus host disease).71,72 The observation that HGF is itself expressed by BEC in affected bile ducts in PBC73 is in keeping with a dynamic balance model in which the net extent to which ductopenia develops reflects the degree to which homeostatic BEC protective/reparative processes such as EMT reversal by physiological HGF are able to counterbalance productopenic processes such as EMT and/or apoptosis (fig 2).70 Studies in tubular renal disease, in which EMT plays a prominent role, have identified other potential therapeutic approaches that may be of relevance for use in PBC. These include the TGFβ antagonising cytokine bone morphogenetic protein 774,75 and rapamycin,76 an agent already demonstrated to attenuate liver fibrosis in the bile duct ligated rat model.77
A further potential mechanism for BEC loss in PBC has been suggested with the demonstration that senescence of BEC is both a feature of PBC lesions in liver biopsy samples and can be induced in cultured BEC by oxidative stress (a phenomenon previously demonstrated to be present in situ in PBC patients; fig 1B).31,78 Senescence‐associated markers such as β‐galactosidase and p16INK4 and p21WAF1/CIP were found to be present in the majority of a representative sample of PBC patient livers and can be induced in culture by the actions of hydrogen peroxide.31 The in‐situ changes were closely associated with the presence of myeloperoxidase‐positive inflammatory cells suggesting the potential for senescence to occur as a consequence of chronic inflammation. As yet the full role played by cellular senescence in disease pathogenesis has not been explored, but the apparent role of inflammation oxidative stress in driving the process gives rise to at least the theoretical potential for therapeutic intervention using anti‐inflammatory and anti‐oxidant medication approaches. It should be noted, however, that neither of these approaches has previously been shown to be of particular benefit in empirical trials of therapy in PBC.
Potential pathogenetic factors in PBC
There is now a broad consensus in the field that, regardless of the precise nature of the pathways responsible for BEC damage in PBC, susceptibility to the disease in individual patients results from a combination of environmental trigger(s) acting on a genetically susceptible individual.
Environmental factors
A significant role for environmental factors in the triggering of PBC is suggested by the demonstration, using formal cluster analysis, of disease “hot spots” in the north‐east of England.32 Geographical approaches, and studies of individual potential risk factors, have highlighted diverse and potentially interesting putative PBC triggers. The original UK cluster analysis suggested that PBC was most frequently seen in former industrial and/or coal mining areas. Furthermore, a recent study from New York has suggested high levels of disease in the environs of highly toxic federal waste disposal sites.33 Taken together, these observations raise the possibility of a chemical environmental factor, potentially associated with contaminated land, which could either trigger disease (fig 1A) or cause disease through a direct toxic effect (potentially explaining the tissue tropism of PBC if the toxin or toxins are excreted into the bile (and therefore concentrated in the biliary tree); fig 1B). A potential mechanism for the triggering of disease by environmental factors is suggested by the key role played by LA in the immunogenicity of PBC. Homologues of LA, which could be encountered in the environmental setting and which are crossreactive with AMA in terms of in‐vitro reactivity with AMA (such as 6‐bromohexanoic acid), appear to be able to induce AMA in experimental animals.79,80 Unpublished work from our group has suggested that xenobiotic agents such as 6‐bromohexanoic acid can enter the physiological post‐translational lipoylation enzyme pathway in the mitochondria and be incorporated into PDC components in place of LA, thereby generating potentially immunogenic “altered‐self” (one of the postulated mechanisms for the breakdown of immune self‐tolerance to PDC in PBC; fig 3). Although these models are potentially very exciting in terms of the opportunity they offer for the identification of the first true environmental chemical trigger for a human autoimmune disease, they have, as yet, not translated into clinical practice and there is currently no advice that should be given to people concerned about the development of PBC (such as family members of affected patients) regarding potentially hazardous environmental exposure.
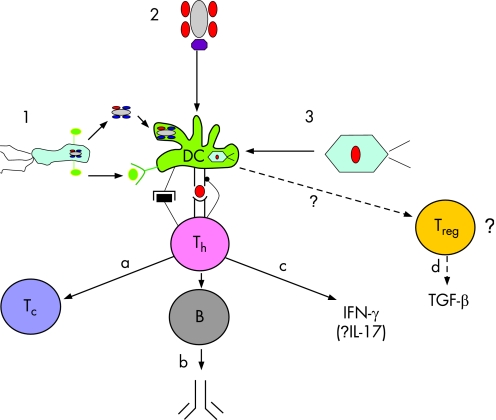
Figure 3 Potential mechanisms for, and sequelae of, the breakdown of immune self‐tolerance to pyruvate dehydrogenase complex (PDC) in the pathogenesis of primary biliary cirrhosis (PBC). 1. Induction of a response to bacterial PDC, which is cross‐reactive with self‐epitopes (denoted in red). In this postulated model the antigen‐presenting cell (represented here as a dendritic cell; DC) is activated by bacterial Toll‐like receptor ligand (e.g. lipopolysaccharide, denoted in green) through cell surface Toll‐like receptors). 2. Response to self‐PDC modified by environmental xenobiotic (purple lozenge). This is an “altered‐self” model. 3. Response to a virus containing a protein that shows molecular mimicry with self‐PDC. In each of these models (most particularly models 1 and 2) activated B cells could replace dendritic cells as antigen presenting cells. The potential effector mechanisms that could result from this breakdown of tolerance are: (a) cytotoxic T cells; (b) anti‐PDC antibody (potentially able to mediate biliary epithelial cell (BEC) damage through antibody‐directed cell cytotoxicity or BEC transcytosis; (c) inflammatory cytokines (IFN‐γ and potentially IL‐17, although data are at present lacking); (d) release (potentially) of TGFβ by FOXP3+ CD103+ regulatory phenotype T cells arising in response to the primary autoreactive T‐cell response. There are data to suggest the presence of transforming growth factor beta (TGF‐β) signalled effects in the liver in PBC, but the identity of the cells responsible for TGF‐β release/activation remains unclear.
The second form of environmental trigger that has been proposed in PBC is exposure to infectious organisms. From the earliest observations that bacteria contain PDC components fully crossreactive with the mammalian form it has been proposed that exposure to these homologues could trigger crossreactive immunity (fig 3). Furthermore, more recent data suggesting that Toll‐like receptor ligands induce an augmented inflammatory response in PBC would fit with bacteria both providing crossreactive antigen and a pro‐inflammatory environment theoretically able to break tolerance.49,81 Although attractive, this model has little direct evidence to support it and there remain to be any objective data, obtained either from prospective follow‐up cohorts or through case–control epidemiological approaches, confirming a role for bacteria in triggering PBC. An alternative infectious aetiology has recently been proposed with the identification of a human retrovirus in both liver tissue and draining lymph nodes from PBC patients that has the capacity to infect cultures inducing marked phenotypic change.34 Retroviral infection could cause BEC damage either through a direct viral cytopathic effect or through crossreactivity between viral protein and self‐PDC (a “molecular mimicry” model; fig 1 and fig 3). In the latter scenario the surface upregulation of “PDC” on BEC would reflect expression of the viral crossreactive protein (although virus‐induced apoptosis would represent another explanation for the phenomenon). Although theoretically attractive in terms of its capacity to explain key phenomena in PBC (such as disease recurrence that can occur extremely rapidly after transplantation; table 3),28 well performed follow‐up studies have failed to replicate the key findings of that study and it is too early, in the absence of such confirmation, to think in terms of antiviral therapy.82
Genetic factors
A high concordance rate is seen for PBC in monozygotic twins. This observation, together with a significantly increased incidence of PBC seen in the first‐degree relatives of probands, would argue for a genetic component to PBC susceptibility.83,84 Moreover, the increased incidence of autoimmune disease in both PBC patients and their families would support this susceptibility being expressed at least partly via immunoregulatory genes.85 Despite the strong supportive evidence for a genetic contribution to PBC pathogenesis, relatively limited progress has been made in identifying susceptibility loci. The genetic associations for which there is the strongest supporting evidence in PBC are with major histocompatibility complex‐encoded genes.86,87,88,89,90,91,92,93,94,95,96,97,98 There is now a broad consensus that PBC is associated with the DRB1*08 family of alleles. Significant variation is seen, however, between different ethnic groups. Association is seen with the DRB1*0801‐containing haplotype in populations of European origin (DRB1*0801–DQA1*0401–DQB1*0402). In contrast, in populations of Asian origin an association is seen with the DRB1*0803 allele (table 3). A protective association has been described with DRB1*11 and DRB1*13 but, once again, significant population differences are seen. Although associations with other loci have been described in individual populations, the vast majority have not been replicated (an example being the CTLA‐4 association originally described by our group). A discussion of these associations and the potential factors underpinning the failure to replicate associations is beyond the scope of this review and the issue has been addressed in detail elsewhere.99,100 To date, none of the genetic associations identified in PBC have proved sufficiently strong to be useful clinically in the prediction of disease risk.
Table 4 Human leukocyte antigen associations in primary biliary cirrhosis.
| Allele | Nature of association | Reproducible | Population group | Frequency range in PBC |
|---|---|---|---|---|
| DRB1*0801† | Susceptibility | Yes | Northern & southern European, USA Caucasian, Canadian86,87,88,89,90 | 12–18% |
| DRB1*0803 | Susceptibility | Yes | Japanese91,92,93 | 30–35% |
| DQA1*0401† | Susceptibility | Yes | Northern & southern European, USA Caucasian87,88,89,90 | 14–18% |
| DQB1*0402† | Susceptibility | Yes | Northern & southern European, USA Caucasian86,87,88,89 | 11–21% |
| DRB1*0701 | Susceptibility | No | Chinese94 | 29% |
| DRB1*13 | Protection | Yes | Northern & southern European87 | 10–14% |
| DRB1*1101 | Protection | Yes | Southern European & USA87,95,96 | 7–28% |
| DPB1*0301 | Susceptibility | No | Northern European97 | 50% |
| DPB1*0501 | Susceptibility | No | Japanese98 | 85% |
PBC, primary biliary cirrhosis.
†Alleles within disease associated‐haplotypes.
The strongest inherited trait associated with PBC is, of course, with female sex. This gives rise to obvious and intriguing questions as to which potential mechanisms might explain a strong, but not obligate (approximately 10% of PBC patients are men who have clinical features seemingly identical to those seen in female patients) female preponderance. A role for fetal microchimerism has been proposed but not proved. Moreover, the clinical observation of disease in men and nulliparous women, and a lack of association between parity and disease frequency would argue against such a mechanism. An alternative mechanistic explanation for the female predominance of PBC has been proposed following the observation of an increased frequency of X chromosome monosomy in PBC (and other female autoimmune disease patients).101 It has been suggested that this increase in X chromosome monosomy may lead to haploinsufficiency for specific X‐linked genes thereby increasing disease predisposition. At the tissue level cholangiocytes from PBC patients in the earliest disease stages (but not normal controls) express oestrogen receptors102 and polymorphisms in oestrogen receptor genes have been shown to be associated with the disease in some populations.103 Oestrogen signalling has been proposed to play a role in the homeostatic proliferative response of cholangiocytes outlined in fig 2, and agents able to modulate oestrogen receptor‐mediated responses (such as tamoxifen) have been proposed as novel, BEC homeostasis targeting, therapies.104 As yet, this potentially interesting therapeutic approach has not undergone formal clinical trial assessment.
Models for the pathogenesis of PBC
In what way do these exciting emerging data lead us to modify our existing views regarding disease pathogenesis?
The concept of “upstream” and “downstream” pathogenetic processes in PBC
One important implication of the emerging data regarding the cycle of augmented BEC damage innate immune system activation, and, ultimately, fibrosis that appears to occur as a consequence of cholestasis, is that we should consider the disease as occurring in sequential phases (fig 4). In this model “upstream” refers to the originating pathological process responsible for early BEC loss and the resulting initiation of ductopenia and cholestasis. “Downstream” processes are the further cytopathic, pro‐inflammatory and fibrotic mechanisms that occur as a consequence of cholestasis and the sequelae of the homeostatic proliferative BEC response (such as replicative senescence). One important implication of this model is that there are elements to the pathogenesis of PBC that are likely to be unique to PBC (the upstream elements) and elements that are likely to be common to many ductopenic cholestatic diseases (the downstream elements; fig 5). It may also help us to rationalise our approach to therapy by suggesting the potential value of combination approaches, which address both downstream processes (probably the principal site of action of ursodeoxycholic acid) and upstream mechanisms. The dual process model may also explain the apparent lack of efficacy of immunomodulatory drugs in PBC. Agents such as prednisolone would be expected, in this model, to have their principal effects on upstream mechanisms. They have, however, largely been used in clinical trials that were restricted to relatively advanced, symptomatic, disease patients in whom downstream processes might be expected to have come to predominate.
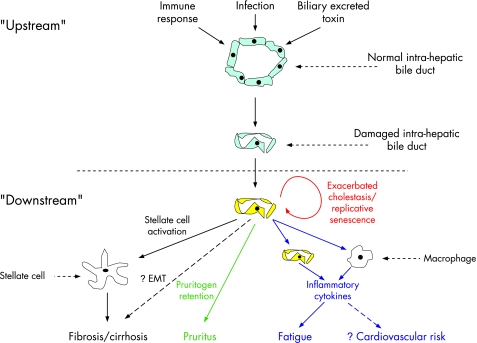
Figure 4 Sequential model for primary biliary cirrhosis pathogenesis. In this model one (or more) processes causes biliary epithelial cell damage, which in turn leads to ductopenia (“upstream” phase). The cholestasis that results from this effect induces a subsequent series of secondary effects, the nature of which is independent of the cause of the upstream process (“downstream” phase). The sequelae include biliary fibrosis and the characteristic clinical features of cholestasis. There is growing interest in a more generic effect of cholestasis on clinical outcomes (including cardiovascular morbidity and mortality) that may result from pro‐inflammatory effects. EMT, Epithelial to mesenchymal transition.
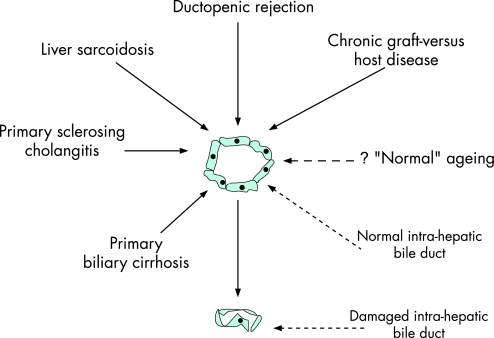
Figure 5 Application of the proposed unique upstream/common downstream model for the pathogenesis of cholestatic liver diseases to the broader issue of the pathogenesis of the vanishing bile duct syndromes.
Improvement in treatment of both upstream and downstream processes would be of real value in both PBC and other ductopenic cholestatic liver disease. Improved treatment of upstream processes is an obvious goal as it offers the hope of curative treatment. There are, however, two major problems inherent in targeting upstream mechanisms in PBC. The first is our ongoing lack of understanding of the precise mechanism(s) responsible for BEC damage initiation, which means we remain unsure as to the process we are actually targeting (fig 1). The second is the relatively late stage in the disease process (in cell biology terms) at which the disease is diagnosed (existing concepts of disease stage and progression largely relate to the degree of fibrosis development (an exclusively downstream process) rather than the degree of BEC loss and balancing regeneration). Effective upstream process‐directed treatment is likely to require very early disease diagnosis if it is to have any chance of success. In light of the potential limits of upstream process‐directed treatment, there is real value in our continuing to optimise treatment aimed at ameliorating downstream damage. Given the important role played by bile salt effects (in particular those mediated by retained hydrophobic bile salts) in progressive ductopenic and fibrosis development, an improvement in bile salt‐directed therapy represents a major opportunity in PBC treatment.
“Upstream” pathogenetic processes in PBC
Although improved treatment of downstream mechanisms in PBC will bring undoubted clinical benefit in PBC, we must find ways to arrest and reverse the upstream processes if we are ever to approach the long‐term goal of curing the disease. We can now integrate recent findings regarding pathogenetic processes in PBC into three theoretical pathogenetic models, the last of which represents a unifying model.
The immune model for BEC damage
The balance of evidence in PBC remains strongly in favour of an autoimmune process in which autoreactive effector mechanisms are directed at epitopes within self‐PDC‐E2 expressed normally or aberrantly by BEC (fig 1A, fig 3). There is a strong evidence base to support both “altered‐self” and molecular mimicry mechanisms for the breakdown of tolerance to self‐PDC, with crossreactivity between the lipoic acid co‐factor and environmental xenobiotics, between bacterial and self‐PDC and between self‐PDC and viral protein all potentially playing important roles. Animal modelling data would argue that a crossreactive B‐cell response induced to xenobiotic modified self‐PDC can, through a process of epitope spreading driven by antigen‐specific crossreactive B cells, translate into the breakdown of T‐cell tolerance responsible for the effector T‐cell mechanisms thought to be directly responsible for BEC loss. The clinical implication of this observation is that immunomodulatory approaches to therapy should play a role in the very earliest stages of PBC. The key outstanding paradoxes inherent in the immune‐mediated model of PBC pathogenesis are the tissue tropism of the disease (why is an immune response mounted to a ubiquitous antigen such as PDC in a tissue‐specific manner?), the previously highlighted lack of a response seen to immunomodulatory therapies, and the mechanism whereby disease recurrence can occur post‐transplant. The potential role played by liver‐excreted xenobiotics in the earliest stages of the immune pathogenesis model would potentially provide an explanation for the first of these issues.
The cytopathic model for BEC damage
An alternative pathogenetic model is that non‐immune‐mediated cytopathic processes are responsible for BEC damage and eventual ductopenia. Potential mechanisms for this would include the direct cytopathic effects of viruses that are trophic to BEC, a process of BEC apoptosis secondary to environmental factors excreted in the bile, and BEC senescence (fig 1B). The potentially directly cytopathic models would certainly be compatible with the clinical observation of recurrent PBC occurring in occasional cases very rapidly after transplant and across HLA boundaries (table 2). In the direct cytopathy model, the characteristic autoreactive immune responses seen in PBC would be regarded as epiphenomena arising as a consequence of the BEC cytopathic process and as playing no role in mediating or accelerating BEC damage. Major progress is likely to be made in the next few years in exploring directly cytopathic processes that may affect BEC in PBC and which may be amenable to therapeutic intervention.
A consensus multistage model for PBC pathogenesis
The intriguing final possibility is that all the highlighted processes play a role in the mechanism of expression of PBC, and that the apparent involvement of different processes in different patients simply reflects different timepoints in the disease development pathway. In this consensus model the earliest effect is a directly cytopathic process that induces BEC morphological change either as an associated process or part of the homeostatic mechanism designed to retain BEC function. Failure of homeostasis in the earliest stages (which might typically be expected to occur before clinical presentation) is followed by the progressive development of ductopenia. BEC damage through apoptosis and/or related processes then alters PBC‐E2 expression patterns (as exemplified by the upregulation of PBC‐E2 seen in apoptotic cells), which, through exposure to the immune system of an altered form of self‐PBC‐E2, promotes a secondary immune phase with a BEC tropism. This leads to a second stage of immune‐mediated ductopenia in which immune‐mediated apoptosis becomes prominent. In the tertiary, downstream, phase of the disease progressive ductopenia and resulting cholestasis cause hydrophobic bile salt retention and BEC replicative senescence, with direct further consequential cytopathic effects. Such a model would have major implications for our approach to therapy in PBC, not least because it would imply that the appropriate therapeutic mechanism would depend entirely on the stage of the disease process in which patients are treated. Therapeutic intervention opportunities are likely to be most marked in the very earliest stages of the disease, which will raise important practical questions regarding disease identification and early diagnosis, particularly as serological disease markers, by this model a mid‐stage phenomenon, could lose their value.
Acknowledgements
The author acknowledges research grant support from the Medical Research Council and Liver North.
Footnotes
Conflict of interest: None declared.
References
- 1.Kaplan M M, Gershwin M E. Primary biliary cirrhosis. N Engl J Med 20053531261–1273. [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Chetwynd A, Newman W L.et al Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow‐up for up to 28 years. Gastroenterology 20021231044–1051. [DOI] [PubMed] [Google Scholar]
- 3.Yeaman S J, Kirby J A, Jones D E J. Autoreactive responses to pyruvate dehydrogenase complex in the pathogenesis of primary biliary cirrhosis. Immunol Rev 2000174238–249. [DOI] [PubMed] [Google Scholar]
- 4.Terasaki S, Nakanuma Y, Yamazaki M.et al Eosinophilic infiltration of the liver in primary biliary cirrhosis: a morphological study. Am J Gastroenterol 199317206–212. [PubMed] [Google Scholar]
- 5.Sherlock S, Scheuer P J. The presentation and diagnosis of 100 patients with primary biliary cirrhosis. N Engl J Med 1973289674–678. [DOI] [PubMed] [Google Scholar]
- 6.Prince M I, Jones D E J. Primary biliary cirrhosis: new perspectives in diagnosis and treatment. Postgrad Med J 200076199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton J L, Bhala N, Burt J.et al Characterisation of the associations and impact of symptoms in primary biliary cirrhosis using a disease specific quality of life measure. J Hepatol 200644776–782. [DOI] [PubMed] [Google Scholar]
- 8.Jones D E J, Bhala N, Burt J.et al Four year follow‐up of fatigue in a geographically‐defined primary biliary cirrhosis patient cohort. Gut 200655536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones D E J. Pathogenesis of primary biliary cirrhosis. J Hepatol 200339639–648. [DOI] [PubMed] [Google Scholar]
- 10.Yeaman S J, Fussey S P, Danner D J.et al Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet 1988i1067–1070. [DOI] [PubMed] [Google Scholar]
- 11.Palmer J M, Jones D E J, Quinn J.et al Characterisation of the autoantibody responses to recombinant E3 binding protein (protein X) of pyruvate dehydrogenase in primary biliary cirrhosis. Hepatology 19993021–26. [DOI] [PubMed] [Google Scholar]
- 12.Fussey S P M, Bassendine M F, Fittes D.et al The E1a and b subunits of the pyruvate dehydrogenase complex are M2“d” and M2“e” autoantigens in primary biliary cirrhosis. Clin Sci 198977365–368. [DOI] [PubMed] [Google Scholar]
- 13.Moteki S, Leung P S, Dickson E R.et al Epitope mapping and reactivity of autoantibodies to the E2 component of 2‐oxoglutarate dehydrogenase complex in primary biliary cirrhosis using recombinant 2oxoglutarate dehydrogenase complex. Hepatology 199623436–444. [DOI] [PubMed] [Google Scholar]
- 14.Leung P S, Chuang D T, Wynn R M.et al Autoantibodies to BCOADC‐E2 in patients with primary biliary cirrhosis recognize a conformational epitope. Hepatology 199522505–513. [PubMed] [Google Scholar]
- 15.Bandin O, Courvalin J ‐ C, Poupon R.et al Specificity and sensitivity of gp210 autoantibodies detected using an enzyme‐linked immunoabsorbent assay and a synthetic polypeptide in the disagnosis of primary biliary cirrhosis. Hepatology 1996231020–1024. [DOI] [PubMed] [Google Scholar]
- 16.Szostecki C, Will H, Netter H J. Autoantibodies to the nuclear Sp100 protein in primary biliary cirrhosis and associated diseases: eitope specificity and immunoglobulin class distribution. Scand J Immunol 199236555–564. [DOI] [PubMed] [Google Scholar]
- 17.Wesierska‐Gadek J, Hohenuer H, Hitchman E.et al Autoantibodies against nucleoporin p62 constitute a novel marker of primary biliary cirrhosis. Gastroenterology 1996110840–847. [DOI] [PubMed] [Google Scholar]
- 18.Hansen B U, Eriksson S, Lindgren S. High prevalence of autoimmune liver disease in patients with multiple nuclear dot, anti‐centromere, and mitotic spindle antibodies. Scand J Gastroenterol 199126707–713. [DOI] [PubMed] [Google Scholar]
- 19.Courvalin J ‐ C, Lassoued K, Worman H J.et al Identification and characterisation of autoantibodies against the nuclear envelope lamin B receptor form patients with primary biliary cirrhosis. J Exp Med 1990172961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn J, Diamond A G, Palmer J M.et al Lipoylated and unlipoylated domains of human PDC‐E2 as autoantigens in primary biliary cirrhosis: significance of lipoate attachment. Hepatology 1993181384–1391. [PubMed] [Google Scholar]
- 21.Bruggraber S F, Leung P S, Amano K.et al Autoreactivity to lipoate and a conjugated form of lipoate in primary biliary cirrhosis. Gastroenterology 20031251705–1713. [DOI] [PubMed] [Google Scholar]
- 22.Jones D E J. Primary biliary cirrhosis. Autoimmunity 200437325–328. [DOI] [PubMed] [Google Scholar]
- 23.Joplin R, Gordon L, Lindsay J.et al Membrane dihydrolipoamide acetyltransferase (E2) on human biliary epithelial cells in primary biliary cirrhosis. Lancet 199233993–94. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Wiebel M, Engelhart S.et al Sera from patients with tuberculosis recognise the M2a‐epitope (E2‐subunit of pyruvate dehydrogenase complex) specific for primary biliary cirrhosis. Clin Exp Immunol 199392308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torgano G, Vecchi M, Podda M.et al Primary biliary cirrhosis is associated with specific changes in liver IgG‐bearing cell sub‐populations. J Hepatol 199522545–550. [DOI] [PubMed] [Google Scholar]
- 26.Palmer J M, Doshi M, Kirby J.et al Secretory autoantibodies in primary biliary cirrhosis. Clin Exp Immunol 20001221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynoso‐Paz S, Leung P S C, Van de Water J.et al Evidence for a locally driven mucosal response and the presence of mitochondrial antigens in saliva in primary biliary cirrhosis. Hepatology 20003124–29. [DOI] [PubMed] [Google Scholar]
- 28.Robertson H, Kirby J A, Yip W W.et al Biliary epithelial‐mesenchymal transtion in post‐transplantation recurrence of primary biliary cirrhosis. Hepatology 200745977–981. [DOI] [PubMed] [Google Scholar]
- 29.Rygiel K A, Robertson H, Burt A D.et al Demonstration of the transition of intrahepatic biliary epithelial cells to fibroblasts during chronic inflammatory liver diseases. J Hepatol 200644S241 [Google Scholar]
- 30.Kita H, Matsumura S, He X.et al Quantitative and functional analysis of PDC‐E2‐specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest 20021091231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki M, Ikeda H, Haga H.et al Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol 2005205451–459. [DOI] [PubMed] [Google Scholar]
- 32.Prince M I, Chetwynd A, Diggle P.et al The geographical distribution of primary biliary cirrhosis in a well‐defined cohort. Hepatology 2001341083–1088. [DOI] [PubMed] [Google Scholar]
- 33.Ala A, Stanca C M, Bu‐Ghanim M.et al Increased prevalence of primary biliary cirrhosis near Superfund waste sites. Hepatology 200643525–531. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Shen S, Guo L.et al Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc Natl Acad Sci U S A 20031008454–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Invernizzi P, Selmi C, Ranftler C.et al Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis 200525298–310. [DOI] [PubMed] [Google Scholar]
- 36.Wesierska‐Gadek J, Penner E, Battezzati P M.et al Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology 2006431135–1144. [DOI] [PubMed] [Google Scholar]
- 37.Robe A J, Kirby J A, Jones D E J.et al A key role for autoreactive B‐cells in the breakdown of T‐cell tolerance to the primary biliary cirrhosis associated autoantigen pyruvate dehydrogenase complex. Hepatology 2005411106–1112. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill S K, Shlomchik M J, Glant T.et al Antigen‐specific B cells are required as APCs and autoantibody‐producing cells for induction of severe autoimmune arthritis. J Immunol 20051743781–3788. [DOI] [PubMed] [Google Scholar]
- 39.Kikucki K, Lian Z X, Yang G X.et al Bacterial CpG induces hyper‐IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology 2005128304–312. [DOI] [PubMed] [Google Scholar]
- 40.Jones D E J, Palmer J M, Yeaman S J.et al T‐cell responses to the components of pyruvate dehydrogenase complex in primary biliary cirrhosis. Hepatology 199521995–1002. [PubMed] [Google Scholar]
- 41.Shimoda S, Van de Water J, Ansari A.et al Identification and precursor frequency analysis of a common T‐cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest 19981021831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kita H, Lian Z, Van de Water J.et al Identification of HLA‐A2‐restricted CD8+ cytotoxic T‐cell responses in primary biliary cirrhosis: T‐cell activation is augmented by immune complexes cross‐presented by dendritic cells. J Exp Med 2002195113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones D E J, Palmer J M, Bennett K.et al Investigation of a mechanism for accelerated breakdown of immune‐tolerance to the primary biliary cirrhosis associated autoantigen, pyruvate dehydrogenase complex. Lab Invest 200282211–219. [DOI] [PubMed] [Google Scholar]
- 44.Jones D E J, Robe A J, Kirby J A.et al Adopotive transfer of self‐PDC‐reactive T‐cells into naive mice induces portal tract and bile duct changes characteristic of primary biliary cirrhosis (PBC). J Hepatol 200644S10 [Google Scholar]
- 45.Jones D E J, Palmer J M, Yeaman S J.et al Breakdown of tolerance to pyruvate dehydrogenase complex in experimental autoimmune cholangitis a murine model of primary biliary cirrhosis. Hepatology 19993065–70. [DOI] [PubMed] [Google Scholar]
- 46.Palmer J M, Robe A J, Burt A D.et al Covalent modification as a mechanism for the breakdown of immune tolerance to pyruvate dehydrogenase complex in the mouse. Hepatology 2004391583–1592. [DOI] [PubMed] [Google Scholar]
- 47.Afzali B, Lombari G, Lechler R I.et al The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 200714832–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockinger B. Good for goose, but not for gander: Il‐2 interferes with Th17 differentiation. Immunity 200726278–279. [DOI] [PubMed] [Google Scholar]
- 49.Mao T K, Lian Z ‐ X, Selmi C.et al Altered monocyte responses to defined TLR ligands in patient with primary biliary cirrhosis. Hepatology 200542802–808. [DOI] [PubMed] [Google Scholar]
- 50.Goldblatt J, Taylor P J S, Lipman T.et al The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology 20021221235–1241. [DOI] [PubMed] [Google Scholar]
- 51.Newton J L, Gibson J G, Tomlinson M.et al Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology 20064491–98. [DOI] [PubMed] [Google Scholar]
- 52.Friedman E M, Hayney M S, Love G D.et al Social relationships, sleep quality, and interleukin‐6 in ageing women. Proc Natl Acad Sci (U S A) 200510218757–18762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vgontzas A N, Papanicolaou D A, Bixler E O.et al Sleep apnoea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance and hypercytokinemia. J Clin Endocrinol Metabol 2000851151–1158. [DOI] [PubMed] [Google Scholar]
- 54.Lamireau T, Zoltowska M, Levy E.et al Effects of bile acids on biliary epithelial cells: proliferation, cytotoxicity and cytokine secretion. Life Sci 2002721401–1411. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama T, Komori A, Nakamurs M.et al Human intrahepatic biliary epithelial cells function in innate immunity by producing IL‐6 and IL‐8 via the TLR4‐NFkB and‐MAPK signaling pathways. Liver Int 200626467–476. [DOI] [PubMed] [Google Scholar]
- 56.Kuroki T, Seki S, Kawakita N.et al Expression of antigens related to apoptosis and cell proliferation in chronic nonsuppurative destructive cholangitis in primary biliary cirrhosis. Virchows Arch 1996429119–129. [DOI] [PubMed] [Google Scholar]
- 57.Harada K, Ozaki S, Gershwin M E.et al Enhanced apoptosis relates to bile duct loss in primary biliary cirrhosis. Hepatology 1997261399–1405. [DOI] [PubMed] [Google Scholar]
- 58.Graham A M, Dollinger M M, Howie S E.et al Bile duct cells in primary biliary cirrhosis are “primed” for apoptosis. Eur J Gastroenterol Hepatol 199810540–541. [DOI] [PubMed] [Google Scholar]
- 59.Harada K, Furubo S, Ozaki S.et al Increased expression of WAF1 in intrahepatic bile ducts in primary biliary cirrhosis relates to apoptosis. J Hepatol 200134500–506. [DOI] [PubMed] [Google Scholar]
- 60.Odin J A, Huebert R C, Casciola‐Rosen L.et al Bcl‐2‐dependent oxidation of pyruvate dehydrogenase‐E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest 2001108223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tinmouth J, Lee M, Wanless I R.et al Apoptosis of biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. Liver 200222228–234. [DOI] [PubMed] [Google Scholar]
- 62.Martinez O M, Villanueva J C, Gershwin M E.et al Cytokine patterns and cytotoxic mediators in primary biliary cirrhosis. Hepatology 199521113–119. [PubMed] [Google Scholar]
- 63.Ballardini G, Guidi M, Susca M.et al Bile duct cell apoptosis is a rare event in primary biliary cirrhosis. Dig Liver Dis 200133151–156. [DOI] [PubMed] [Google Scholar]
- 64.Onori P, Alvaro A, Floreani A.et al Activation of the IGF1 system characterizes cholangiocyte survival during progression of primary biliary cirrhosis. J Histochem Cytochem 200755327–334. [DOI] [PubMed] [Google Scholar]
- 65.Rodrigues C M P, Fan G S, Ma X M.et al A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest 19981012790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology 200236525–531. [DOI] [PubMed] [Google Scholar]
- 67.Macdonald P, Palmer J M, Kirby J A.et al Apoptosis as a mechanism for cell surface expression of the autoantigen pyruvate dehydrogenase complex. Clin Exp Immunol 2004136559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumura S, Van de Water J, Kita H.et al Contribution to antimitochondrial antibody production: cleavage of pyruvate dehydroganse complex‐E2 by apoptosis‐related proteases. Hepatology 20023514–22. [DOI] [PubMed] [Google Scholar]
- 69.Lee J M, Dedhar S, Kalluri R.et al The epithelial‐mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006172973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wasilenko S, Mason A L. New insights from recurrent primary biliary cirrhosis in liver transplantation: the paradox of BEComing a fibroblast. Hepatology 200745837–840. [DOI] [PubMed] [Google Scholar]
- 71.Xia J ‐ L, Dai C, Michalopoulos G K.et al Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol 20061681500–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuroiwa T, Iwasaki T, Imado T.et al Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft‐versus‐host disease. Arthr Res Ther 20068R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasaki M, Ikeda H, Kataoka H.et al Augmented expression of hepatocyte growth factor activator inhibitor type 1 (HAI‐1) in intrahepatic small bile ducts in primary biliary cirrhosis. Virchows Arch 2006449462–471. [DOI] [PubMed] [Google Scholar]
- 74.Zeisberg M, Hanai J, Sugimoto H.et al BMP‐7 counteracts TGF‐B1‐induced epithelial‐to‐mesenchymal transition and reverses chronic renal injury. Nat Med 20039964–967. [DOI] [PubMed] [Google Scholar]
- 75.Zeisberg M, Shah A A, Kalluri R. Bone morphogenetic protein‐7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem 20052808094–8100. [DOI] [PubMed] [Google Scholar]
- 76.Wu M ‐ J, Wen M ‐ C, Chiu Y ‐ Y.et al Rapamycin attenuates unilateral ureteral obstruction‐induced renal fibrosis. Kidney Int 2006692029–2036. [DOI] [PubMed] [Google Scholar]
- 77.Biecker E, Gottardi A D, Neef M.et al Long‐term treatment of bile‐duct‐ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther 2005313952–961. [DOI] [PubMed] [Google Scholar]
- 78.Aboutwerat A, Pemberton P W, Smith A.et al Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta 20031637142–150. [DOI] [PubMed] [Google Scholar]
- 79.Long S A, Quan C, Van de Water J.et al Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol 20011672956–2963. [DOI] [PubMed] [Google Scholar]
- 80.Leung P S, Quan C, Park O.et al Immunization with a xenobiotic 6‐bromohexanoate bovine serum albumin conjugate induces anti‐mitochondrial antibodies. J Immunol 20031705326–5332. [DOI] [PubMed] [Google Scholar]
- 81.Jones D E J, Palmer J M, Burt A D.et al Bacterial motif DNA as an adjuvant for the breakdown of immune self‐tolerance to pyruvate dehydrogenase complex. Hepatology 200236679–686. [DOI] [PubMed] [Google Scholar]
- 82.Selmi C, Ross S R, Ansari A A.et al Lack of immunological or molecular evidence for a role of mouse mammary tumor retrovirus in primary biliary cirrhosis. Gastroenterology 2004127493–501. [DOI] [PubMed] [Google Scholar]
- 83.Selmi C, Mayo M J, Bach N.et al Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology 2004127485–492. [DOI] [PubMed] [Google Scholar]
- 84.Jones D E J, Watt F E, Metcalf J V.et al Familial primary biliary cirrhosis reassessed: a geographically‐based population study. J Hepatol 199930402–407. [DOI] [PubMed] [Google Scholar]
- 85.Watt F E, James O F W, Jones D E J. Patterns of autoimmunity in PBC patients and their families. Q J Med 200497397–406. [DOI] [PubMed] [Google Scholar]
- 86.Underhill J, Donaldson P, Bray G.et al Susceptibility to primary biliary cirrhosis is associated with the HLA‐DR8‐DQB1*0402 haplotype. Hepatology 1992161404–1408. [DOI] [PubMed] [Google Scholar]
- 87.Donaldson P T, Baragiotta A, Henneghan M.et al HLA class II polymorphisms: alleles, genotypes, haplotypes and amino acid sequences in primary biliary cirrhosis: a large scale study. Hepatology 200644667–674. [DOI] [PubMed] [Google Scholar]
- 88.Mullarkey M E, Stevens A M, McDonnell W M.et al Human leukocyte antigen class II alleles in caucasian women with primary biliary cirrhosis. Tiss Antigens 200565199–205. [DOI] [PubMed] [Google Scholar]
- 89.Wassmuth R, Depner F, Danielsson A.et al HLA class II markers and clinical heterogeneity in Swedish patients with primary biliary cirrhosis. Tiss Antigens 200259381–387. [DOI] [PubMed] [Google Scholar]
- 90.Stone J, Wade J A, Cauch‐Dudek K.et al Human leukocyte antigen class II associations in serum antimitochondrial antibodies (AMA)‐positive and AMA‐neagative primary biliary cirrhosis. J Hepatol 2002368–13. [DOI] [PubMed] [Google Scholar]
- 91.Oguri H, Oba S, Ogino H.et al Susceptibility to primary biliary cirrhosis is associated with human leucocyte antigen DRB1*0803. Int Hepatol Comm 19942263–270. [Google Scholar]
- 92.Mukai T, Kimura A, Ishibashi H.et al Association of HLA‐DRB1*0803 and *1602 with susceptibility to primary biliary cirrhosis. Int Hepatol Comm 19953207–212. [Google Scholar]
- 93.Onishi S, Sakamaki T, Maeda T.et al DNA typing of HLA class II genes; DRB1*0803 increases the susceptibility of Japanese to primary biliary cirrhosis. J Hepatol 1994211053–1060. [DOI] [PubMed] [Google Scholar]
- 94.Liu H Y, Deng A M, Zhou Y.et al Analysis of HLA polymorphisms in Chinese patients with primary biliary cirrhosis. Hepatobil Pancreatic Dis Int 20055129–132. [PubMed] [Google Scholar]
- 95.Gores G J, Moore S B, Fisher L D.et al Primary biliary cirrhosis: association with class II major histocompatibility complex antigens. Hepatology 19877889–892. [DOI] [PubMed] [Google Scholar]
- 96.Invernizzi P, Battezzati P M, Crosignani A.et al Peculiar HLA polymorphisms in Italian patients with primary biliary cirrhosis. J Hepatol 200338401–406. [DOI] [PubMed] [Google Scholar]
- 97.Mella J G, Roschmann E, Maier K P.et al Association of primary biliary cirrhosis with the allele HLA‐DPB1*0301. Hepatology 199521398–402. [DOI] [PubMed] [Google Scholar]
- 98.Seki T, Kiyosawa K, Ota M.et al Association of primary biliary cirrhosis with human leukocyte antigen DPB1*0501. Hepatology 19931873–78. [PubMed] [Google Scholar]
- 99.Jones D E J, Donaldson P T. Genetic factors in the pathogenesis of primary biliary cirrhosis. Clin Liver Dis 20037841–864. [DOI] [PubMed] [Google Scholar]
- 100.Invernizzi P, Selmi C, Mackay I R.et al From bases to basis: linking genetics to causation in primary biliary cirrhosis. Clin Gastroenterol Hepatol 20053401–410. [DOI] [PubMed] [Google Scholar]
- 101.Invernizzi P, Miozzo M, Battezzati P M.et al Frequency of monosomy X in women with primary biliary cirrhosis. Lancet 2004363533–535. [DOI] [PubMed] [Google Scholar]
- 102.Alvaro D, Invernizzi P, Onori P.et al Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J Hepatol 200441905–912. [DOI] [PubMed] [Google Scholar]
- 103.Lakatos L P, Bajnok E, Hegedus D.et al Vitamin D receptor, oestrogen receptor‐alpha gene and interleukin‐1 receptor antagonist gene polymorphisms in Hungarian patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol 200214733–740. [DOI] [PubMed] [Google Scholar]
- 104.Invernizzi P, Alvaro D, Crosignani A.et al Tamoxifen in treatment of primary biliary cirrhosis. Hepatology 2004391175–1176. [DOI] [PubMed] [Google Scholar]