Abstract
Objective
To analyse the short and long term outcome of endoscopic stent treatment after bile duct injury (BDI), and to determine the effect of multiple stent treatment.
Design, setting and patients
A retrospective cohort study was performed in a tertiary referral centre to analyse the outcome of endoscopic stenting in 67 patients with cystic duct leakage, 26 patients with common bile duct leakage and 110 patients with a bile duct stricture.
Main outcome measures
Long term outcome and independent predictors for successful stent treatment.
Results
Overall success in patients with cystic duct leakage was 97%. In patients with common bile duct leakage, stent related complications occurred in 3.8% (n = 1). The overall success rate was 89% (n = 23). In patients with a bile duct stricture, stent related complications occurred in 33% (n = 36) and the overall success rate was 74% (n = 81). After a mean follow up of 4.5 years, liver function tests did not identify “occult” bile duct strictures. Independent predictors for outcome were the number of stents inserted during the first procedure (OR 3.2 per stent; 95% CI 1.3 to 8.4), injuries classified as Bismuth III (OR 0.12; 95% CI 0.02 to 0.91) and IV (OR 0.04; CI 0.003 to 0.52) and endoscopic stenting before referral (OR 0.24; CI 0.06 to 0.88). Introduction of sequential insertion of multiple stents did not improve outcome (before 77% vs after 66%, p = 0.25), but more patients reported stent related pain (before 11% vs after 28%, p = 0.02).
Conclusions
In patients with a postoperative bile duct leakage and/or strictures, endoscopic stent treatment should be regarded as the choice of primary treatment because of safety and favourable long term outcome. Apart from the early insertion of more than one stent, the benefit from sequential insertion of multiple stents did not become readily apparent from this series.
Bile duct injury (BDI) occurs in 0.2 to 1.4% of patients following laparoscopic cholecystectomy and is a severe surgical complication.1,2,3,4 BDI related morbidity is illustrated by increased hospital stay, poor long term quality of life and high rates of malpractice litigation.5,6,7,8 Although surgical reconstruction, mainly a hepaticojejunostomy, is a procedure associated with low mortality and low morbidity if performed in a tertiary centre, this is only indicated in selected patients with BDI; a population‐based study from the USA demonstrated the detrimental effect of BDI on survival in patients who underwent surgical reconstruction.9 The majority of biliary injuries, including cystic duct leakage, common bile duct (CBD) leakage or bile duct strictures, can be treated successfully in 70–95% of the patients by means of endoscopic or radiological interventions.10,11,12,13,14,15,16
It has been suggested that endoscopic treatment is associated with an increased risk of re‐stenosis and biliary cirrhosis followed by end‐stage liver disease. However, reliable data about the long term outcome of endoscopic management of BDI are scarce and predicting factors for successful outcome are unreported. Several years ago reports from uncontrolled studies indicated that a more aggressive type of dilation treatment in patients with bile duct strictures, based on the sequential insertion of multiple stents, may be associated with a more favourable outcome and this treatment policy has been adapted in our clinic since the end of 2001.17,18,19,20
The purpose of this study was to analyse the short and long term outcome of stent treatment in BDI patients (including liver function test after long term follow up) and to determine factors that are predictive for successful outcome in patients who are stented for a bile duct stricture. In addition, the outcomes of patients treated before and after the introduction of sequential insertion of multiple stents were compared.
Patients and methods
Between January 1990 and December 2005, 500 consecutive patients were referred after laparoscopic cholecystectomy to the departments of surgery, radiology and gastroenterology of the Academic Medical Center, the Netherlands, for treatment of BDI and were included into a prospective database. For the present study, patients were selected who were treated by endoscopic stenting before 1 April 2005 to allow for at least one year of follow up. Patients' medical charts were reviewed to analyse operation reports and clinical data, including the type of the initial cholecystectomy (and subsequent relaparotomy) and therapeutic interventions in the referring centre. The initial injury was classified according to the Amsterdam classification: type A, cystic bile duct leakage; B, CBD leakage; C, bile duct stricture and type D, complete transsection of the bile duct.21 Patients with type D injury included in the present study had already undergone surgical reconstruction in the referring centre and were referred for treatment of a stricture of the anastomosis. To investigate the outcome of stent treatment, three categories of injuries were defined: (1) cystic duct leakage, (2) CBD leakage and (3) bile duct strictures. The location of injury in patients with a bile duct stricture was classified according to Bismuth.22
Treatment protocol
Standard management at our institution before 2002 has been published previously.23 After obtaining a diagnostic cholangiogram and documentation of the site and extent of the injury, attempts were made to pass a hydrophilic guide wire and diagnostic catheter to the leak or stricture. If a stenosis was too tight to allow passage of the catheter, dilating catheters of gradually increasing diameter (4–7 French) were passed over the guide wire and through the stenosis and/or balloon dilation was applied. There was no standardised protocol concerning balloon dilation (usually 8 mm) or catheter dilation before stent placement. An Amsterdam‐type straight polyethylene stent was then inserted over the guide wire and catheter; thus bridging the location of the leakage or the stenosis. The treatment protocol specified the placement of two 10F stents if possible. For multiple stent insertion, an endoscopic sphincterotomy was performed to facilitate stent placement. In the case of a tight stenosis sometimes only a 7F or a single 10F stent could be placed. In these patients, the single stent was electively exchanged for two 10F stents after 6 weeks. The two stents were subsequently exchanged every 3 months to avoid cholangitis caused by clogging.
The maximum treatment period with two 10 F stents in situ was 12 months. The stents were not replaced if the bile duct was considered to be adequately dilated based on the following criteria as subjectively assessed by the endoscopist: (1) adequate dilation of the stenosis based on the cholangiograph appearance, (2) satisfactory drainage of contrast and (3) passage of an extraction balloon (12 to 15 mm) through the stenosis without encountering significant resistance. After 2002, sequential insertion of multiple stents was introduced at our institution. Since then an attempt was made to gradually insert an increasing number of stents with each successive endoscopic retrograde cholangiopancreatography (ERCP) procedure. Balloon dilation of the CBD stricture was performed to facilitate positioning of an extra endoprothesis in case of resistant and tight stenosis. Before new stents were inserted, “old” stents were generally removed first and a cholangiogram was obtained. All patients were treated prophylactically by intravenous administration of antibiotics. In the absence of cholangitis as an indication for the ERCP, no antibiotics were given after the procedure. Informed consent was obtained from all patients.
Outcome
Outcome parameters that were analysed included treatment related complications, the duration of stent treatment, the incidence of re‐stenosis after stent removal, the number of patients subsequently referred for reconstructive surgery and BDI related mortality. The success of stent treatment was calculated by considering the following patients as failures: patients who died because of a BDI related cause, patients referred for surgery and patients with a re‐stenosis after stent removal. Referral for surgery was based on the following indications: prolonged stenting (more than 1 year), patient's preferences and a newly developed stricture of a segmental duct.
To detect (silent) cholestasis after long term follow up, the outcome of available liver function tests, taken 2 years after stent removal, were obtained from general practitioners and referring hospitals. The liver function tests included alanine transaminase normal <45 IU/L; aspartate transaminase normal <40 IU/L; gamma‐glutamyl transpeptidase (gammaGT) normal <60 IU/L; and alkaline phosphatase (ALP) normal range 40–120 IU/L. Patients with either one liver function test parameter with a greater than twofold increase above normal, or patients with two or more liver function test parameters with any increase above normal, were considered as patients with plausible occult biliary pathology. Follow up was performed through regular outpatient visits and long term outcome was obtained by mail and telephone surveys to the general practitioner and the referring institutions.
Statistical analysis
Data from patient characteristics, management and outcome are outlined in numbers and percentages. Means with standard deviation or median values with minimum and maximum values are presented, whichever is appropriate. Comparison between groups was performed with a χ2 test, t test and Mann‐Whitney U test, when appropriate. To determine which variables were associated with successful stent treatment (p⩽0.1) univariate analysis was performed first using a binary logistic regression. To identify independent predictors for success, variables identified as significant in univariate analysis, without significant inter‐variable correlation, were subsequently included in a logistic regression analysis. Data analyses were performed using SPSS® software (SPSS, Chicago, Illinois, USA). A p value of <0.05 was considered statistically significant.
Results
Patient characteristics
Patients characteristics, type of cholecystectomy, initial injury and therapeutic interventions before referral are summarised (per group) in table 1. Although bile duct leakage was the initial injury in 119 patients (type A injury in 68 patients and type B injury in 51 patients), 26 of these patients developed a bile duct stricture during stent treatment in the referring centre. Therefore, 93 patients (46%) were referred for treatment of a leaking bile duct, whereas the majority of the patients (n = 110, 54%) were referred for the treatment of a bile duct stricture.
Table 1 Patients' characteristics and indication for referral. Bile leakage (n = 93) and bile duct stricture (n = 110).
| Leakage n = 93 (%) | Strictures n = 110 (%) | Total n = 203 (%) | |
|---|---|---|---|
| Gender, female | 60 | 79 | 139 (68.5) |
| Age at cholecystectomy,mean in years (SD) | 49 (15) | 49 (17) | 50 (16) |
| Indication for cholecystectomy | |||
| Symptomatic cholelithiasis | 74 (79.6) | 83 (75.5) | 157 (77.3) |
| Chronic cholecystitis | 8 (8.6) | 9 (8.2) | 4 (2.0) |
| Acute cholecystitis | 7 (7.5) | 10 (9.1) | 17 (8.4) |
| Cholecystectomy a froid | 2 (2.2) | 2 (1.8) | 17 (8.4) |
| Unknown | 2 (2.2) | 6 (5.5) | 8 (3.9) |
| Initial procedure | |||
| Laparoscopic cholecystectomy | 74 (79.6) | 50 (45.5) | 124 (61.1) |
| Laparoscopic cholecystectomy with conversion | 16 (17.2) | 47 (42.7) | 63 (31.0) |
| Open cholecystectomy | 3 (3.2) | 13 (11.8) | 16 (7.9) |
| Time of diagnosis | |||
| During initial operation | 6 (6.5) | 34 (30.9) | 40 (17.7) |
| In hospital | 43 (46.2) | 30 (27.3) | 73 (35.9) |
| After discharge | 42 (45.2) | 41 (37.3) | 83 (40.9) |
| Unknown | 2 (2.2) | 5 (4.5) | 7 (3.4) |
| Type of initial injury | |||
| Type A, cystic duct leakage | 67 (72.0) | 1* (0.9) | 68 (33.5) |
| Type B, bile duct leakage | 26 (28.0) | 25* (22.7) | 51 (25.1) |
| Type C, bile duct stricture | 0 | 46 (41.8) | 46 (22.7) |
| Type D, bile duct transection | 0 | 38 (34.5) | 38 (18.7) |
| Therapeutic intervention before referral | |||
| Repair during initial cholecystectomy | 4 (4.3) | 34 (30.9) | 38 (18.7) |
| Relaparotomy with repair | 1 (1.1) | 14 (12.7) | 15 (7.4) |
| Endoscopic stent insertion | 9 (9.7) | 28 (25.5) | 37 (18.2) |
| Endoscopic papillotomy | 11 (11.8) | 26 (23.6) | 20 (9.8) |
| PTC/D | 0 | 3 (2.7) | 3 (1.5) |
*After stent treatment for initial bile duct leakage, 26 patients developed a stricture during endoscopic stent treatment and were referred to our centre. PTCD, percutaneous transhepatic cholangiography/drainage.
Endoscopic treatment
The outcome of endoscopic treatment is listed in table 2 for patients with cystic duct leakage, CBD leakage and bile duct strictures.
Table 2 Endoscopic stenting in bile duct injury patients. Short term complications and duration of treatment.
| Cystic duct leakage n = 67 (%) | CBD leakage n = 26 (%) | CBD stricture n = 110 (%) | |
|---|---|---|---|
| Days between LC and referral, median (range) | 10 (1–40) | 15 (3–61) | 75 (4–2899) |
| Maximum stents inserted during procedure, median (range) | 1 (1–3) | 1 (1–4) | 2 (1–7) |
| Number of stent changes, median (range) | 2 (0–4) | 2 (1–5) | 4 (0–12) |
| Number of patients with a stent related complication | 11 (16.4) | 1 (3.8) | 36 (32.7) |
| Stent migration | 5 (7.5) | 0 | 21 (19.1) |
| Clogging | 5 (7.5) | 1 (3.8) | 15 (13.6) |
| Cholangitis | 1 (1.5) | 0 | 6 (5.5) |
| Fausse route | 0 | 0 | 4 (3.6) |
| Pain | 2 (3.0) | 0 | 18 (16.4) |
| Total duration of stents in situ, months | |||
| Mean (SD) | 1.7 (1.0) | 2.8 (3.0) | 11.5 (9.4) |
| Median, (range) | 1.5 (0.4–7.8) | 1.8 (0.7–11.2) | 11 (1–69) |
BDI, bile duct injury; CBD, common bile duct; LC, laparoscopic cholecystectomy.
Patients treated for cystic duct leakage were referred after a median of 10 days (range 1–40). Stent related complications occurred in 11 patients (16.4%) and were generally mild. The mean duration of stent treatment was 1.7 months. The overall success rate of endoscopic treatment in patients with cystic duct leakage was 97% (n = 65). Patients with CBD leakage were referred after a median of 15 days (range 3–61). Before stent treatment, 3 of the 26 patients had undergone percutaneous biliary drainage. During a mean duration of stent treatment of 2.8 months (range 1–11), clogging of the stent occurred in one patient (3.8%). One patient (3.8%) was subsequently referred for surgery after diagnosing a segmental duct stenosis during the first stent exchange. One frail patient (1%) died because of biliary sepsis and multi‐organ failure in the referring hospital after 2 months. During follow up, one patient (3.8%) developed a stenosis 6 months after stent removal (table 3). The overall success rate in patients with CBD leakage was 89% (n = 23).
Table 3 Long term outcome of endoscopic stenting in BDI patients.
| Cystic duct leakage n = 67 (%) | CBD leakage n = 26 (%) | CBD stricture n = 110 (%) | |
|---|---|---|---|
| Mean years of follow up (SD) | 7.8 (3.5) | 7.7 (2.9) | 7.6 (3.7) |
| Number of patients referred for surgery | – | 1 (3.8) | 22 (20.0) |
| Indication for surgery | |||
| Prolonged stenting (>1 year) | – | – | 9 (8.2) |
| Patients preferences | – | – | 6 (5.4) |
| Re‐stenosis | – | – | 5 (2.7) |
| Stricture of segmental bile duct | – | 1 (3.8) | 2 (1.8) |
| Subsequent stenting for recurrence of stenosis | 1 (1.5) | 1 (3.8) | 6 (5.5) |
| Mortality related to BDI* | – | 1 (1.1) | 2 (1.8) |
| Successful endoscopic stenting | 65 (97.0) | 23 (88.5) | 81 (73.6) |
*One patient treated for a type B injury died 2 months after initial surgery in the referring centre because of severe sepsis. One patient treated for a bile duct stricture died 4 years after initial surgery because of a duodenum perforation caused by a migrated stent and one patient died 7 years after initial surgery because of liver cirrhosis caused by a persisting stenosis and alcohol abuse. BDI, bile duct injury; CBD, common bile duct.
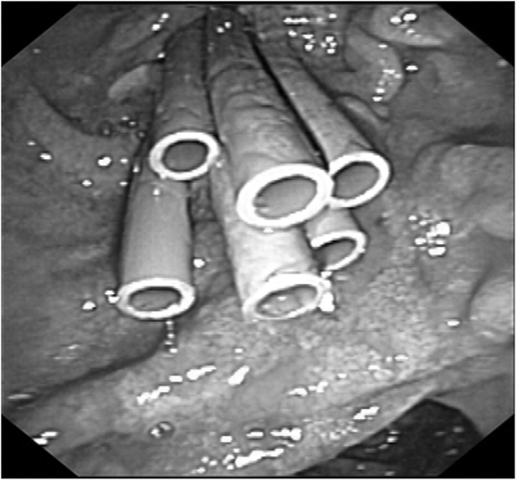
Patients with a bile duct stricture were referred after a median of 75 days (range 4–2899) (table 2). The median number of stents inserted during endoscopic treatment was two (range 1–7) (fig 1). In a median duration of stenting of 11 months (range 1–69), stent related complications occurred in 36 patients (33%). Most complications, such as stent migration (n = 21, 19%) and clogging (n = 15, 14%), were mild and managed by stent exchange. Eventually, 22 patients (20%) were referred for surgery after stent treatment had failed, after a median of 15 months, range 8–21 (table 3). The most common indication for surgery was ongoing stent dependency (n = 9) or patient preference (n = 6). Two patients (2%) died because of a BDI related cause. One patient died 4 years after initial cholecystectomy because of a perforation of the duodenum caused by a migrated stent placed for the treatment of a re‐stenosis. The other patient died 7 years after initial surgery because of liver cirrhosis caused by a persisting stenosis. Liver transplantation was not considered in this 62 year old patient because of ongoing alcohol abuse. During follow up, six patients (6%) developed a re‐stenosis after a median of 3.5 months (range 0.5–13 months) following stent removal. The overall success rate in patients treated for a bile duct stricture was 74% (n = 81).
Figure 1 Multiple stent treatment in patients with a bile duct stricture.
Liver function
Liver function tests taken two years after stent removal were available for 51 of the 110 patients (46%). No significant differences were found comparing this group and the group of patients from whom no tests were available. The following characteristics were compared: initial injury, location of injury, duration of treatment, number of inserted stents, complications and outcome (data not shown). The median duration between stent removal and the liver function test was 4.6 years (range 2–14). In 6 of the 51 patients (11.7%), stent treatment was unsuccessful based on the outcome of long term liver function tests. In three patients the ALP value exceeded twice the normal value. The values are explained by BDI related death in one patient and a hepaticojejunostomy combined with a right‐sided hepatectomy in a 40 year old patient with segmental hypotrophy because of persistent strictures. The third patient is followed at the outpatient clinic. Although the latest ALP value was 232 IU/L, no signs of biliary obstruction were found by ERCP. In the other three patients, a combination of high gammaGT and ALP was found. Two of these patients are followed at the outpatient clinic without suspicion of biliary outflow obstruction based on ultrasound and CT. In the third patient the high values are associated with alcohol abuses.
Multivariate analysis for successful treatment in patients with a bile duct stricture
Factors associated with successful treatment are summarised in table 4. An independent predictor for successful stent treatment for bile duct strictures was an increasing number of stents inserted during the first procedure (odds ratio (OR) 3.2, 95% confidence interval (CI) 1.2 to 8.3, p = 0.017). Independent predictors for failure were injuries classified as Bismuth III (OR 0.12, 95% CI 0.016 to 0.91, p = 0.04) and IV (OR 0.039, 95% CI 0.003 to 0.54, p = 0.015) and endoscopic stenting before referral (OR 0.24, 95% CI 0.06 to 0.88).
Table 4 Univariate and multivariate analysis for successful stent treatment for bile duct strictures.
| Factors | Total n = 110 (%) | Univariate analysis* | p Value | Multivariate analysis | p Value |
|---|---|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95%CI) | ||||
| Gender, female | 79 (72%) | 2.3 (0.94 to 5.7) | 0.07 | 3.1 (1.0 to 9.5) | 0.05 |
| Initial procedure | |||||
| Open cholecystectomy | 13 (12%) | – | |||
| Laparoscopic cholecystectomy | 50 (45%) | 2.7 (0.78 to 9.5) | 0.15 | 1.4 (0.28 to 6.5) | 0.70 |
| Laparoscopy with conversion | 26 (24%) | 6.4 (1.4 to 29.5) | 0.017 | 5.6 (0.79 to 39.6) | 0.084 |
| Laparoscopy with conversion after BDI | 21 (19%) | 7.0 (1.4 to 36.0) | 0.020 | 7.3 (0.97 to 55.3) | 0.053 |
| Location of injury | |||||
| Bismuth I | 17 (15%) | – | |||
| Bismuth II | 64 (58%) | 0.52 (0.10 to 2.6) | 0.43 | 0.55 (0.87 to 3.6) | 0.53 |
| Bismuth III | 23 (21%) | 0.17 (0.03 to 0.94) | 0.042 | 0.12 (0.016 to 0.91) | 0.040 |
| Bismuth IV | 6 (5%) | 0.07 (0.01 to 0.63) | 0.018 | 0.039 (0.003 to 0.54) | 0.015 |
| Endoscopic stenting before referral | 28 (25%) | 0.44 (0.17 to 1.1) | 0.07 | 0.24 (0.06 to 0.88) | 0.032 |
| Number of stents inserted during first procedure | range (1–4) | 2.1 (0.97 to 4.4) | 0.06 | 3.19 (1.22 to 8.32) | 0.017 |
| Maximum number of stents inserted† | range (1–7) | 1.4 (0.96 to 2.2) | 0.08 |
Values in parentheses are percentages or 95% CIs. Factors analysed in univariate analysis with a
p value >0.1 include age, time of diagnose, type of injury according to the Amsterdam Classification, primary repair, surgical interventions before referral, and time interval between cholecystectomy and referral.
CI denotes confidence interval. *Binary logistic regression. †Factor not included in multivariate analysis due to significant correlation (p<.001) with number of stents inserted during first procedure.
Progressive stent treatment
The introduction of sequential insertion of multiple stents in the present series is shown in table 5. A significant increase in the maximum number of stents inserted during treatment is shown if patients treated before 2002 are compared with patients treated after 2002. Between both periods the median number of inserted stents increased from two to four (p<.001). However, the number of patients who were treated successfully did not improve significantly (77% before vs 67% after, p = 0.25). The deciding factor in this non‐significant decrease in patients successfully treated by endoscopy was the number of patients referred for subsequent surgery, which increased from 16% to 28% (p = 0.15). The introduction of sequential insertion of multiple stents did not affect the re‐stenosis rates (5% in both groups) or the duration of treatment (11 months in both groups). Since the introduction of multiple stenting, significantly more patients report stent related pain with an increase from 11% to 28% (p = 0.02).
Table 5 Changes in number of stents and outcome after introducing multiple stent treatment in 2002 for patients with bile duct strictures.
| Total | |||
|---|---|---|---|
| Before n = 74 (%) | After n = 36 (%) | p Value | |
| Number of stents | |||
| Maximum number of stents inserted first procedure, median (range) | 1 (1–3) | 2 (1–4) | 0.017* |
| Maximum number of stents inserted, median (range) | 2 (1–4) | 4 (1–7) | <0.001* |
| Primary outcome | |||
| Patients with unsuccessful stent treatment | 17 (23.0) | 12 (33.3) | 0.25 |
| BDI related mortality | 2 (2.7) | 0 | 0.32 |
| Re‐stenosis after stent removal | 4 (5.4) | 2 (5.6) | 0.97 |
| Referred for surgery | 12 (16.2) | 10 (27.8) | 0.15 |
| Secondary outcome | |||
| Duration of stent treatment, months, median (range) | 11 (1–69) | 10 (1–30) | 0.82* |
| Patients with one or more stent related complications | 21 (28.4) | 15 (41.6) | 0.16 |
| Number of patients with stent related pain | 8 (10.8) | 10 (27.7) | 0.024 |
*Mann‐Whitney U test. BDI, bile duct injury.
Discussion
The present series demonstrate that endoscopic stent treatment has a favourable outcome in patients with biliary injury because of laparoscopic cholecystectomy, with a low morbidity and a mortality rate of 0.5%. The overall success rate in patients with CBD leakage rate was 89% with a median duration of stent treatment of 1.8 months. In patients with a bile duct stricture, the overall success rate was 74% with a median duration of stenting of 11 months. Independent predictors for successful stent treatment in patients with a bile duct stricture were an increasing number of stents inserted during the first ERCP procedure, no previous stent treatment in the referring centre and injuries located under the biliary bifurcation. Although multiple stent insertion during the first procedure appears beneficial in patients with bile duct strictures, sequential insertion of multiple stents over time did not improve the overall success but was associated with more patients reporting stent related pain.
The incidence of postoperative bile leakage after laparoscopic cholecystectomy is assumed to be 1–3%.24,25 ERCP gives the opportunity to locate the injury and classify the extent of the lesion. Cystic duct leakage and leakage of the CBD can be treated by stent insertion and preserving the biliary sphincter, with a reported success rate over 90%.10,11,12,14,21,26,27 In the present series, 68 patients were referred because of a cystic duct leakage. It is noteworthy that in 16 of the 68 cases a re‐laparotomy was performed before patients were referred for endoscopic treatment. Nine patients underwent relaparotomy for drainage of fluid collections and seven patients underwent relaparotomy for exploration of the biliary anatomy. Importantly, if the injury initially had been assessed adequately by means of cholangiography (magnetic resonance cholangiopancreatograpy, ERCP or percutaneous transhepatic cholangiography) these unnecessary relaparotomies could have been prevented.
Stricture formation after endoscopic stenting for bile duct leakage is a surgery related complication resulting from concomitant ischaemic damage, tissue loss, local inflammation and scarring.28,29 In the present series, 26 of 119 patients with an initial leakage developed a stricture and were subsequently referred for treatment.
The incidence of postoperative bile duct strictures after laparoscopic cholecystectomy is estimated between 0.2 and 0.5%.30 Since several studies found similar outcome after surgical or endoscopic treatment for biliary strictures, nowadays most patients are initially treated by endoscopic treatment because surgery remains available when endoscopy fails but not vice versa.31 In the present series of patients with a postoperative stricture, short term complications occurred in 36 of the 110 patients (33%). This rate is on the higher end of the 9–40% range for complication rates in previously published series.18,23,30,31,32 However, previous reports did not report the number of patients with stent related pain. The incidence of stent clogging, which occurred in 14% in the present series, was less than the previously reported incidence of 27–40%.31,32 The incidence of cholangitis, which occurred in 6% in the present series, was previously reported in 7–9% of the patients treated for bile duct strictures.23,33 Strict adherence to the treatment protocol with stent exchange every 3 months probably reduced the cholangitis risk, especially in cases with only a single stent. The motive to perform a hepaticojejunostomy is mainly stent dependency (8%) after a prolonged period of stenting and patient's preference (5%).
The analysis in the present series identified predicting factors for successful outcome in patients with a bile duct stricture: injury below the biliary bifurcation, no previous stenting and an increasing number of stents inserted during the first procedure. Although previous groups suggested the influence of several of these factors on outcome, those series were too small to perform a multivariate analysis.17,18,23 With univariate analysis, two interesting factors showed a positive effect on outcome: an increasing number of stents inserted during the first ERCP procedure and an increasing number of maximum stents inserted during treatment. It is likely that both these factors are strongly correlated. Table 5 shows no additional benefit after the introduction of sequential insertion of multiple stents in a consecutive series of patients treated for bile duct strictures. The lack of success of sequential insertion of multiple stents cannot be explained by patient and injury characteristics, as no significant change was observed between both periods. The only probable explanation is the unwillingness of patients to proceed with endoscopic treatment because of pain. Although multiple stent treatment was associated with excellent outcome in previous series, the present results suggest that insertion of multiple stents is primarily beneficial in a patients in whom this was applied (or could be achieved) early in the course of treatment.18,20,34 Consequently, it may be worthwhile to adapt the procedure and timing of stent insertion in such a way that emphasis is put on early dilation rather than introducing many stents at any given time during treatment. Such a protocol could consist of the placement of two stents during the initial procedure when feasible and adding one stent per 4 weeks up to a maximum of four. If the latter has been achieved, there seems no need to exchange stents every 3 months as obstruction because of stent clogging in case of multiple stents is exceptional, even after many months. If treatment related pain occurs, a prompt response with prescription of analgesics is indicated. This relieves pain, preserves patient satisfaction and should ensure patients' adherence to the endoscopic dilation protocol.
Asymptomatic re‐stricturing, subclinical cholestasis and secondary biliary cirrhosis after endoscopic treatment for bile duct strictures remain a concern when stents have long been removed. As previous studies showed that restenosis after 2 years is less likely, the present study analysed the long term liver function by liver function tests taken at least 2 years after stent removal.23 In the vast majority of patients, normal liver function tests were obtained. In 6 out of the 51 patients with plausible liver function abnormalities there was no evidence for occult bile duct strictures. Liver function tests were not available for all patients. It could be suggested, therefore, that our analysis has a potential bias and restenosis could have developed during follow up in the remaining 54% of the patients. However, this is not likely because these patients had similar features (injury, treatment and outcome characteristics) as patients in whom liver function tests were available.
Kuzela and colleagues evaluated liver function after sequential insertion of multiple stents for biliary strictures in 43 patients.17 Liver function tests were within the normal range in all 43 patients after a mean follow up of 16 months. The applied progressive stent strategy in their series was associated with a short term complication rate of 12%, and interestingly no re‐stenosis occurred during follow up.
From our results we can confirm that it is highly unlikely that patients will develop a re‐stenosis more than 2 years after stent removal. Therefore, we suggest that patients are followed at the outpatient clinic or by their family practitioner for a period of 2 years following stent removal with regular testing of liver function. After 2 years, follow up can be discontinued and patients should be re‐evaluated only in case of symptoms.
Conclusions based on the present series should be interpreted with caution. Although the largest study available to date, it consists of a heterogeneous population of BDI patients referred to a tertiary centre and results may not be easily generalised to other centres. However, by providing detailed information per patient group (eg stricture level) and performing multivariate analysis, factors predictive for successful outcome were identified that potentially may aid others with the clinical management of these patients.
In summary, BDI is a severe surgical complication that should be evaluated and treated by a multidisciplinary team consisting of surgeons, interventional endoscopists and interventional radiologists. Endoscopic stenting in BDI patients is associated with low morbidity and excellent long term outcome in case of biliary leakage and good outcome in case of biliary strictures. Consequently, endoscopic stenting is the choice of primary treatment in the majority of BDI patients. Surgery should only be undertaken when endoscopic treatment fails. Based on the outcome of this study, the timing of ERCP procedures and insertion of stents may need to be reconsidered in order to optimise treatment success in case of a biliary stricture. The latter should be addressed in a prospective study comparing standard dilation treatment (maximal number of two stents) with a modified protocol of early sequential insertion of multiple stents.
Abbreviations
ALP - alkaline phosphatase
BDI - bile duct injury
CBD - common bile duct
ERCP - endoscopic retrograde cholangiopancreatograpy
gammaGT - gamma glutanyl transpeptidase
Footnotes
Competing interests: None.
References
- 1.Giger U F, Michel J M, Opitz I.et al Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22,953 consecutive cases from the swiss association of laparoscopic and thoracoscopic surgery database. J Am Coll Surg 2006203(5)723–728. [DOI] [PubMed] [Google Scholar]
- 2.Richardson M C, Bell G, Fullarton G M. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg 199683(10)1356–1360. [DOI] [PubMed] [Google Scholar]
- 3.Calvete J, Sabater L, Camps B.et al Bile duct injury during laparoscopic cholecystectomy: myth or reality of the learning curve? Surg Endosc 200014(7)608–611. [DOI] [PubMed] [Google Scholar]
- 4.MacFadyen B V, Jr, Vecchio R, Ricardo A E.et al Bile duct injury after laparoscopic cholecystectomy. The United States experience. Surg Endosc 199812(4)315–321. [DOI] [PubMed] [Google Scholar]
- 5.Savader S J, Lillemoe K D, Prescott C A.et al Laparoscopic cholecystectomy‐related bile duct injuries: a health and financial disaster. Ann Surg 1997225(3)268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerma D, Rauws E A, Keulemans Y C.et al Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: a prospective analysis. Ann Surg 2001234(6)750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore D E, Feurer I D, Holzman M D.et al Long‐term detrimental effect of bile duct injury on health‐related quality of life. Arch Surg 2004139(5)476–481. [DOI] [PubMed] [Google Scholar]
- 8.Kern K A. Malpractice litigation involving laparoscopic cholecystectomy. Cost, cause, and consequences. Arch Surg 1997132(4)392–397. [DOI] [PubMed] [Google Scholar]
- 9.Flum D R, Cheadle A, Prela C.et al Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA 2003290(16)2168–2173. [DOI] [PubMed] [Google Scholar]
- 10.Foutch P G, Harlan J R, Hoefer M. Endoscopic therapy for patients with a post‐operative biliary leak. Gastrointest Endosc 199339(3)416–421. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkman D J, Carr‐Locke D L, Lichtenstein D R.et al Postsurgical bile leaks: endoscopic obliteration of the transpapillary pressure gradient is enough. Am J Gastroenterol 199590(12)2128–2133. [PubMed] [Google Scholar]
- 12.Prat F, Pelletier G, Ponchon T.et al What role can endoscopy play in the management of biliary complications after laparoscopic cholecystectomy? Endoscopy 199729(5)341–348. [DOI] [PubMed] [Google Scholar]
- 13.Misra S, Melton G B, Geschwind J F.et al Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: a decade of experience. J Am Coll Surg 2004198(2)218–226. [DOI] [PubMed] [Google Scholar]
- 14.Kaffes A J, Hourigan L, De L N.et al Impact of endoscopic intervention in 100 patients with suspected postcholecystectomy bile leak. Gastrointest Endosc 200561(2)269–275. [DOI] [PubMed] [Google Scholar]
- 15.de Reuver P R, Busch O R, Rauws E A.et al Long term results of a primary end to end anastomosis in peroperative detected bile duct injury. J Gastrointest Surg 200711(3)296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauws E A, Gouma D J. Endoscopic and surgical management of bile duct injury after laparoscopic cholecystectomy. Best Pract Res Clin Gastroenterol 200418(5)829–846. [DOI] [PubMed] [Google Scholar]
- 17.Kuzela L, Oltman M, Sutka J.et al Prospective follow‐up of patients with bile duct strictures secondary to laparoscopic cholecystectomy, treated endoscopically with multiple stents. Hepatogastroenterology 200552(65)1357–1361. [PubMed] [Google Scholar]
- 18.Costamagna G, Pandolfi M, Mutignani M.et al Long‐term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc 200154(2)162–168. [DOI] [PubMed] [Google Scholar]
- 19.Dumonceau J M, Deviere J, Delhaye M.et al Plastic and metal stents for postoperative benign bile duct strictures: the best and the worst. Gastrointest Endosc 199847(1)8–17. [DOI] [PubMed] [Google Scholar]
- 20.Draganov P, Hoffman B, Marsh W.et al Long‐term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc 200255(6)680–686. [DOI] [PubMed] [Google Scholar]
- 21.Bergman J J, van den Brink G R, Rauws E A.et al Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 199638(1)141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bismuth H, Majno P E. Biliary strictures: classification based on the principles of surgical treatment. World J Surg 200125(10)1241–1244. [DOI] [PubMed] [Google Scholar]
- 23.Bergman J J, Burgemeister L, Bruno M J.et al Long‐term follow‐up after biliary stent placement for postoperative bile duct stenosis. Gastrointest Endosc 200154(2)154–161. [DOI] [PubMed] [Google Scholar]
- 24.Barkun A N, Rezieg M, Mehta S N.et al Postcholecystectomy biliary leaks in the laparoscopic era: risk factors, presentation, and management. McGill Gallstone Treatment Group. Gastrointest Endosc 199745(3)277–282. [DOI] [PubMed] [Google Scholar]
- 25.Woods M S, Traverso L W, Kozarek R A.et al Characteristics of biliary tract complications during laparoscopic cholecystectomy: a multi‐institutional study. Am J Surg 1994167(1)27–33. [DOI] [PubMed] [Google Scholar]
- 26.Hartle R J, McGarrity T J, Conter R L. Treatment of a giant biloma and bile leak by ERCP stent placement. Am J Gastroenterol 199388(12)2117–2118. [PubMed] [Google Scholar]
- 27.Sandha G S, Bourke M J, Haber G B.et al Endoscopic therapy for bile leak based on a new classification: results in 207 patients. Gastrointest Endosc 200460(4)567–574. [DOI] [PubMed] [Google Scholar]
- 28.Stewart L, Robinson T N, Lee C M.et al Right hepatic artery injury associated with laparoscopic bile duct injury: incidence, mechanism, and consequences. J Gastrointest Surg 20048(5)523–530. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt S C, Settmacher U, Langrehr J M.et al Management and outcome of patients with combined bile duct and hepatic arterial injuries after laparoscopic cholecystectomy. Surgery 2004135(6)613–618. [DOI] [PubMed] [Google Scholar]
- 30.Davids P H, Rauws E A, Coene P P.et al Endoscopic stenting for post‐operative biliary strictures. Gastrointest Endosc 199238(1)12–18. [DOI] [PubMed] [Google Scholar]
- 31.Davids P H, Tanka A K, Rauws E A.et al Benign biliary strictures. Surgery or endoscopy? Ann Surg 1993217(3)237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tocchi A, Mazzoni G, Liotta G.et al Management of benign biliary strictures: biliary enteric anastomosis vs endoscopic stenting. Arch Surg 2000135(2)153–157. [DOI] [PubMed] [Google Scholar]
- 33.Costamagna G, Shah S K, Tringali A. Current management of postoperative complications and benign biliary strictures. Gastrointest Endosc Clin N Am. 2003;13(4):635–48, ix [DOI] [PubMed]
- 34.Familiari L, Scaffidi M, Familiari P.et al An endoscopic approach to the management of surgical bile duct injuries: nine years' experience. Dig Liver Dis 200335(7)493–497. [DOI] [PubMed] [Google Scholar]