Abstract
Background and aims
Butyrate oxidation by colonocytes is impaired in ulcerative colitis. This study examined the activity of enzymes involved in butyrate oxidation in ulcerative colitis.
Methods
Activities of mitochondrial acetoacetyl coenzyme A (CoA) thiolase, crotonase and β‐hydroxy butyryl CoA dehydrogenase were estimated spectrophotometrically in rectosigmoid mucosal biopsies from patients with ulcerative colitis and Crohn's colitis, and control subjects undergoing colonoscopy for colon cancer or rectal bleeding.
Results
The activity of mitochondrial acetoacetyl CoA thiolase was decreased by 80% in ulcerative colitis (3.4 (0.58) μmol/min/g wet weight, n = 30) compared with control (16.9 (3.5), n = 18) and with Crohn's colitis (17.6 (3.1), n = 12) (p<0.0001). The activity of two other mitochondrial butyrate oxidation enzymes—crotonase and β‐hydroxy butyryl CoA dehydrogenase—as well as of cytoplasmic thiolase was normal in ulcerative colitis. Mitochondrial thiolase activity in ulcerative colitis did not correlate with clinical, endoscopic or histological indices of disease severity. Mitochondrial thiolase activity was reduced in the normal right colon mucosa of patients with left‐sided ulcerative colitis. Enzyme kinetic studies revealed a lowered Vmax, suggesting inhibition at a site distinct from the catalytic site. Reduced thiolase activity in ulcerative colitis was returned to normal by exposure to 0.3 mM β‐mercaptoethanol, a reductant. Using normal colon mucosal biopsies, redox modulation of thiolase activity by hydrogen peroxide, a mitochondrial oxidant, could be shown. A significant increase in hydrogen peroxide formation was observed in ulcerative colitis biopsies.
Conclusion
A defect of mitochondrial acetoacetyl CoA thiolase occurs in ulcerative colitis. Increased reactive oxygen species generation in mitochondria of epithelial cells in ulcerative colitis may underlie this defect.
Despite considerable advances in our understanding of the inflammatory bowel diseases, the aetiology of ulcerative colitis remains unclear. Crohn's disease, which is characterised by transmural inflammation involving the small bowel and/or colon, is believed to result from defective innate immune recognition of luminal bacteria in a genetically predisposed individual.1 In ulcerative colitis, where inflammation is restricted to the mucosa of the colon, it has been suggested that a primary defect in the colonic epithelium resulting in the increased absorption of pro‐inflammatory molecules from the colonic lumen may underlie the development of disease.2,3 Defective colonic epithelial oxidation of butyrate in ulcerative colitis, first demonstrated by Roediger4 and confirmed by other investigators,5,6 has been implicated in the pathogenesis of ulcerative colitis. Butyrate, a short‐chain fatty acid, is formed from the bacterial fermentation of unabsorbed carbohydrates in the colonic lumen. It undergoes oxidative metabolism in the mitochondria of colonic epithelial cells to CO2 and ketone bodies and is the major source of energy, providing >70% of the energy requirements7 for the colonic epithelium, which cannot effectively utilise glucose as energy substrate.4 Colonocyte butyrate metabolism was defective in an animal model of colitis.8 Inhibition of butyrate oxidation in experimental animals resulted in mucosal inflammation similar to ulcerative colitis.9 Decreased ATP levels are evident in mucosa from ulcerative colitis patients.10 Diversion colitis occurring in the colon surgically excluded from the faecal stream, and therefore a model of butyrate deficiency, is successfully treated by the rectal infusion of butyrate and other short‐chain fatty acids.11 Thus, impaired butyrate oxidation is associated with the development of colitis in a variety of situations.
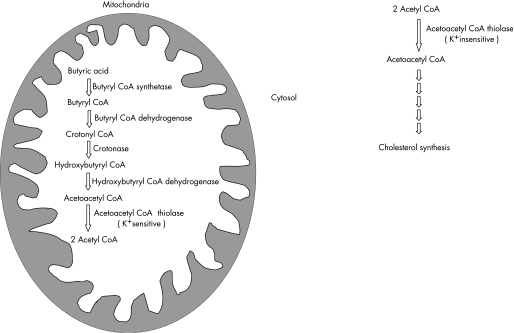
Oxidation of butyrate is carried out in mitochondria by a series of five enzymes (fig 1). An earlier study, which examined all these enzymes, failed to demonstrate any significant alteration in these enzymes in quiescent ulcerative colitis.12 Mitochondrial acetoacetyl coenzyme A (CoA) thiolase (EC 2.3.1.9) catalyses the last step in butyrate oxidation to acetyl CoA. In addition to being present in mitochondria, there is a second isoform of this enzyme in the cytosol, which is principally involved in cholesterol biosynthesis as well as ketone body synthesis.13 The two isoforms can be distinguished biochemically by the fact that the mitochondrial isoform is K+ sensitive while the cytoplasmic isoform is K+ insensitive. The earlier study of butyrate‐oxidising enzymes assayed acetoacetyl CoA thiolase, but did not specifically examine the mitochondrial isoform of the enzyme—that is, the study used the appropriate substrate but did not measure potassium‐sensitive thiolase activity. This differentiation has been shown to be important, as for example in children with repeated ketoacidotic episodes, in whom mitochondrial acetoacetyl CoA thiolase deficiency was not picked up by the normal assay, but a specific assay of potassium ion‐sensitive enzyme activity diagnosed the deficiency.14 The present study specifically examined the activity of mitochondrial potassium‐sensitive acetoacetyl CoA thiolase in mucosal biopsies from the colon of patients with active and quiescent ulcerative colitis and compared it with that in control subjects. In addition, studies were undertaken to determine the genesis of decreased enzyme activity in ulcerative colitis.
Figure 1 Schematic illustrating the function of acetoacetyl coenzyme A (CoA) thiolase: butyrate is oxidised in the mitochondria by a series of five different enzymes. The mitochondrial isoform of acetoacetyl CoA thiolase catalyses the last step of the reaction, converting acetoacetyl CoA to two molecules of acetyl CoA. In contrast, the cytosolic form of acetoacetyl CoA thiolase functions predominantly in cholesterol metabolism, and is involved in the synthesis of acetoacetyl CoA from two molecules of acetyl CoA.
Methods
Subjects
Subjects were recruited from patients undergoing colonoscopy in the Department of Gastrointestinal Sciences. A total of 49 consecutive ulcerative colitis patients (31 male; mean age 39 years, range 14–64) who underwent colonoscopy as part of their clinical evaluation were included. The diagnosis of ulcerative colitis was established on the basis of clinical, endoscopic and histological criteria. Disease duration in ulcerative colitis patients ranged from 6 weeks to 19 years (median 3.31 years). All subjects were on treatment at the time of colonoscopy and biopsy; 30 received aminosalicylates or sulfasalazine (together grouped as ASAs) as sole therapy, while 19 received corticosteroids along with ASAs. Disease severity was graded as quiescent, mild, moderate or severe according to the Truelove and Witts criteria,15 and endoscopic severity was graded as quiescent, mild, moderate or severe.16 Histologically, disease activity was graded as quiescent (none), mild, moderate or severe depending on the severity of neutrophilic infiltration of the lamina propria and crypts. Colonic biopsies were also obtained from endoscopically normal mucosa of 49 control subjects (29 male; mean age 42.2 years, range 15–67) who were undergoing colonoscopy for investigation of bowel symptoms (38 subjects) or lower gastrointestinal bleeding (11). None of the above control subjects had evidence of mucosal inflammation on colonoscopy and biopsy. A second group of inflammatory control subjects with colonic Crohn's disease was also recruited (n = 12, eight male, mean age 37.1 years, range 23–60). In patients with Crohn's disease, biopsies were obtained from actively inflamed and ulcerated mucosa as well as from mucosa that appeared endoscopically normal in each patient. All patients with Crohn's colitis had active disease involving the colon at the time of study, while six additionally had involvement of the ileum. All subjects included in the study had colonoscopy only as clinically indicated and were recruited into the study at the time of endoscopy. Informed written consent was obtained. The study protocol and consent forms were approved by the Institutional Review Board of the Christian Medical College, Vellore.
Colonoscopy was performed after standard cleansing involving the administration of a PEG‐electrolyte solution (PEGLEC, Tablets India Ltd) until the bowel return was clear. Mucosal biopsy specimens were obtained, snap‐frozen in liquid nitrogen and stored at −80°C until use. Biopsies (3–4) were obtained from the rectosigmoid junction in order to avoid possible segmental differences in enzyme activity. In 11 patients with active left‐sided ulcerative colitis, biopsies were taken both from endoscopically involved rectosigmoid mucosa and from endoscopically normal areas more proximally in the colon.
Chemicals used and initial processing
Acetoacetyl CoA, CoA, crotonyl CoA and butyryl CoA were all obtained from Sigma‐Aldrich‐Fluka (Bangalore, India). Assays were performed spectrophotometrically using a Shimadzu UV‐visible 160A spectrophotometer (Shimadzu Co., Tokyo, Japan). Mucosal biopsies were processed for enzyme studies as follows.12 For study of β‐hydroxy butyryl CoA dehydrogenase, mucosa was homogenised using a 0.5 ml Teflon pestle homogeniser (Kontes 885752‐0018, Vineland, NJ, USA) in 50 mM phosphate buffer (pH 7.0) containing 0.3 mM EDTA, 200 μM phenylmethylsulphonyl fluoride (PMSF) and 2 mM dithiothreitol (DTT); for estimation of crotonase and thiolase activity, mucosa was homogenised in 10 mM Tris buffer (pH 7.4) containing 1 mM EDTA, 3 mM MgCl2 and 300 mM sucrose.
Thiolase assay (total and potassium‐sensitive)
To assess mitochondrial acetoacetyl CoA thiolase activity in the samples, enzyme activity was measured in the presence of 50 mM KCl (total activity) and in its absence (K+ replaced by an equivalent concentration of Na+). The latter was taken to represent cytosolic thiolase activity. Mitochondrial thiolase activity was calculated by subtracting the enzyme activity rate in the absence of K+ from that in the presence of K+. When measuring K+ activation, care was taken to ensure that neither substrates nor enzyme solutions contained K+, this ion being replaced by Na+. The rate of the reaction due to formation of acetyl CoA was assessed spectrophotometrically at 303 nm. The final concentration of the reaction mixture contained 50 μM CoA, 100 mM Tris‐HCl (pH 8.1), 25 mM MgCl2, 50 mM KCl and 10 μM acetoacetyl CoA.
The reaction velocities of mitochondrial thiolase in mucosal homogenates of control subjects and ulcerative colitis patients were determined using four different concentrations (ranging from 2.5 to 20 μM) of acetoacetyl CoA. The kinetic parameters were obtained by fitting initial velocity data to the Michaelis–Menten equation by non‐linear regression analysis.
β‐Hydroxy butyryl CoA dehydrogenase assay
This enzyme, involved in butyrate oxidation, was assayed in a reaction mixture consisting of final concentrations of 97 mM potassium phosphate, 0.09 mM acetoacetyl CoA, 0.1 mM β‐nicotinamide adenine dinucleotide reduced form, and mucosal homogenate. The rate of reaction resulting from the formation of nicotinamide adenine dinucleotide was assessed spectrophotometrically at 340 nm.17
Crotonase assay
Crotonase was assayed according to the method of Steinmann et al.18 The final concentration of the reaction mixture comprised 44 mM Tris‐HCl, 0.044% bovine albumin, 0.67 mM EDTA and 0.107 mM crotonyl CoA. The decrease in absorbance was assessed spectrophotometrically at 280 nm.
Effect of oxidative and nitrosative stress on potassium‐sensitive thiolase activity
The effect of oxidative stress on mitochondrial thiolase activity was determined by incubating the biopsy homogenate from control subjects with 0.2 mM H2O2 for 1 min at room temperature prior to assay of K+‐sensitive thiolase activity. In order to assess whether the oxidant‐induced alteration was reversible, thiolase activity was assayed after incubation with H2O2 for 1 min followed by incubation with 0.3 mM β‐mercaptoethanol for 1 min. The effect of nitrosative stress on enzyme activity was investigated by determining the effect of incubation for 30 min at 30°C with 1 mM sodium nitroprusside, an NO donor. Hydrogen peroxide (H2O2) release from colonic mucosal biopsies was measured using biopsies obtained from seven ulcerative colitis patients and seven control subjects with normal colonic mucosa, using the Amplex Red Hydrogen Peroxide assay kit (Invitrogen, India) in a fluorescence microplate reader (Victor3V, Perkin‐Elmer). The assay was done within an hour of collection of biopsy samples.
Statistical analysis
GraphPad Prism 3.02 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis as well as for graphing the data. All results are expressed as mean (SEM) in the text, while dispersion is shown using box plots (median and interquartile range for boxes, and maximum and minimum values for whiskers). The Mann–Whitney U test for non‐parametric data was used for determining the significance of differences between groups. A two‐tailed p value <0.05 was considered significant.
Results
Impaired colonic mucosal mitochondrial acetoacetyl CoA thiolase activity and unaltered cytoplasmic acetoacetyl CoA thiolase activity in ulcerative colitis
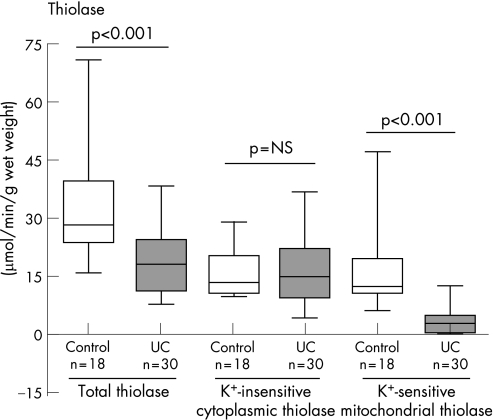
A marked and statistically significant decrease in mitochondrial (K+‐sensitive) acetoacetyl CoA thiolase activity was observed in ulcerative colitis mucosal biopsies (3.4 (0.58) μmol/min/g wet weight, n = 30) when compared with control (16.9 (3.5), n = 18) (p<0.001) (fig 2). On the other hand, cytoplasmic acetoacetyl CoA thiolase activity (measured in the absence of K+) was not significantly different between control (15.5 (1.18) μmol/min/g wet weight, n = 18) and ulcerative colitis (16.5 (1.63), n = 30) (fig 2).
Figure 2 Mitochondrial acetoacetyl coenzyme A (CoA) thiolase activity in colonic mucosa of patients with ulcerative colitis (ulcerative colitis) compared with controls. Box and whiskers plots show the median with interquartile range in the box and the extreme values in the whiskers. p Values shown were calculated using the Mann–Whitney U test.
Crotonase and β‐hydroxy butyryl CoA dehydrogenase activity
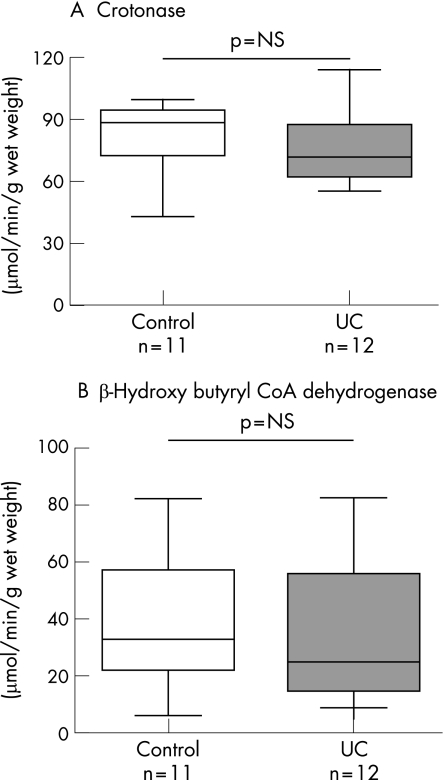
These two enzymes were also investigated in order to determine whether the reduction in mitochondrial thiolase activity was a non‐specific feature of epithelial cell damage in ulcerative colitis. The colonic mucosal activity of crotonase was very similar in ulcerative colitis (77.9 (5.5) μmol/min/g wet weight, n = 12) and control subjects (86.0 (6.1), n = 11) (p = NS). Similarly, the activity of β‐hydroxy butyryl CoA dehydrogenase was similar in ulcerative colitis (30.6 (7.9) μmol/min/g wet weight, n = 12) and control colonic mucosa (37.8 (0.03), n = 11) (p = NS) (fig 3).
Figure 3 Comparison of crotonase (A) and β‐hydroxy butyryl coenzyme A (CoA) dehydrogenase (B) activity in colonic mucosa of patients with ulcerative colitis (ulcerative colitis) and control subjects. The medians of the two groups were compared using the Mann–Whitney U test.
Mitochondrial acetoacetyl CoA thiolase activity in Crohn's colitis
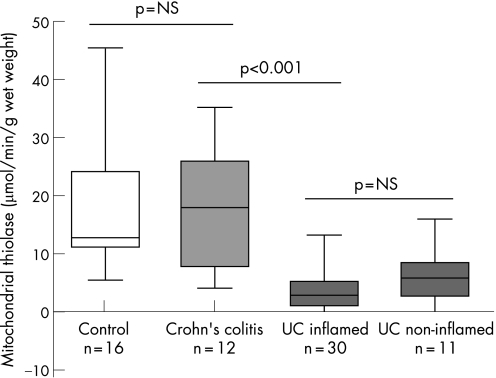
Crohn's colitis was used as an inflammatory control group to determine whether reduced mitochondrial acetoacetyl CoA thiolase activity was specific to ulcerative colitis or could be expected non‐specifically as a result of any mucosal damage or inflammation. Mitochondrial acetoacetyl CoA thiolase activity was not decreased in the colonic mucosa of patients with active Crohn's colitis (17.6 (3.1) μmol/min/g wet weight) (p = NS compared with controls) (fig 4). The activity of this enzyme was significantly different from that in ulcerative colitis patients.
Figure 4 Comparison of mitochondrial acetoacetyl coenzyme A (CoA) thiolase activity in Crohn's colitis (inflamed and non‐involved colonic mucosa) with that in controls and ulcerative colitis (ulcerative colitis) (inflamed and non‐involved colonic mucosa). Non‐involved colonic mucosa in ulcerative colitis was obtained from endoscopically normal right colon in patients with left‐sided ulcerative colitis. The activity in Crohn's colitis was similar to that in control subjects and significantly different from that in ulcerative colitis. There was no difference in activity between inflamed and non‐involved colonic mucosa in ulcerative colitis or between involved and non‐involved areas of the mucosa in patients with active Crohn's colitis.
Mitochondrial acetoacetyl CoA thiolase activity in right colon mucosa from patients with left‐sided ulcerative colitis
Mitochondrial thiolase activity was compared in mucosa from endoscopically and histologically normal right colon and involved mucosa from left colon in 11 patients with left‐sided ulcerative colitis. A significant decrease in mitochondrial thiolase activity was observed in the apparently normal colonic mucosa of patients with ulcerative colitis (5.9 (1.38) μmol/min/g wet weight, n = 11). In these patients, mitochondrial acetoacetyl CoA thiolase activity was comparable in apparently normal mucosa and endoscopically involved mucosa of the same patients (fig 4).
Mitochondrial acetoacetyl CoA thiolase activity is unrelated to disease severity in ulcerative colitis
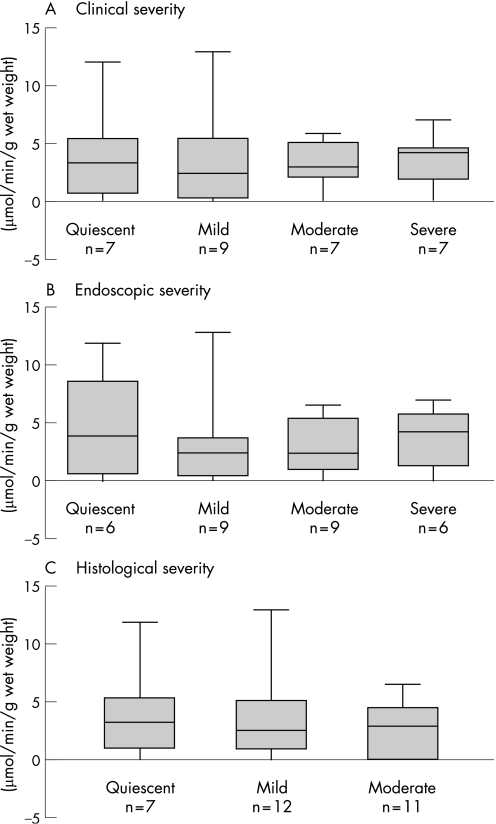
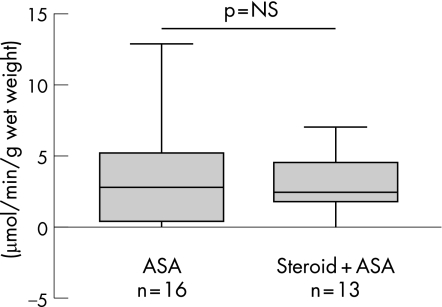
If the decrease in mitochondrial acetoacetyl CoA thiolase in ulcerative colitis was secondary to mucosal damage or inflammation, a gradation of enzyme activity would be expected in relation to disease severity. However, thiolase activity was similar whether the disease was quiescent, mild, moderate or severe, using clinical and endoscopic indices of disease severity as well as a histological index of disease activity which measures neutrophil infiltration semi‐quantitatively (fig 5). The nature of current treatment—that is, ASAs and/or immunosuppressive agents—did not significantly influence mitochondrial acetoacetyl CoA thiolase activity (fig 6).
Figure 5 Mitochondrial acetoacetyl coenzyme A (CoA) thiolase activity in ulcerative colitis patients classified according to disease severity based on (A) clinical (B) endoscopic or (C) histological criteria of activity. None of the biopsies showed evidence of severe histological activity. None of the differences was statistically significant.
Figure 6 Effect of treatment type on mitochondrial acetoacetyl coenzyke A (CoA) thiolase activity in colonic mucosa of ulcerative colitis patients treated with aminosalicylates (ASAs) alone or ASAs and steroids. No significant difference was noted.
Kinetics of thiolase activity in ulcerative colitis
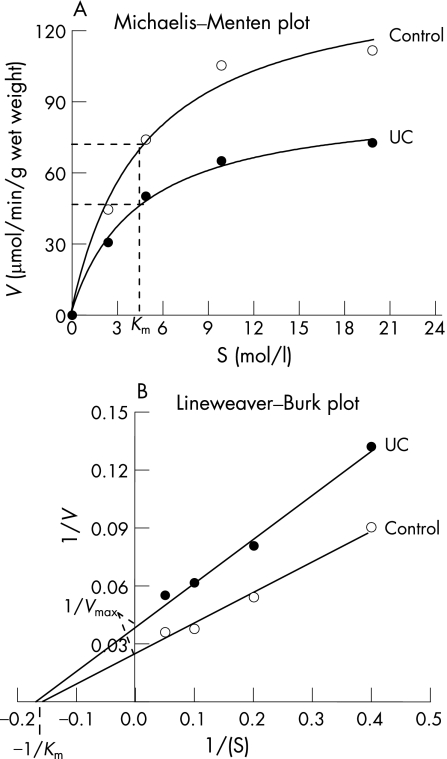
To determine the mechanism of inhibition of mitochondrial acetoacetyl CoA thiolase activity in ulcerative colitis patients, kinetic analysis was carried out using mucosal homogenates of control subjects and ulcerative colitis patients. Apparent Km and Vmax values were determined using Lineweaver–Burk plots. Under the conditions of the standard assay, a hyperbolic profile was found between the substrate concentration and the enzyme activity in both control and ulcerative colitis patients. As shown in fig 7, the Vmax was significantly decreased in ulcerative colitis (22.6) compared with control (36.14), but the Km in both groups was essentially identical.
Figure 7 Kinetic analysis of mitochondrial acetoacetyl coenzyme (CoA) thiolase activity using a Michaelis–Menten plot (A) and Lineweaver–Burk plot (B). The plot suggests an inhibition of the enzyme at a site different from the catalytic site. Assays were performed in the presence of different concentrations (2.5–20 μM) of substrate. ulcerative colitis, ulcerative colitis.
Reversibility by β‐mercaptoethanol of reduced thiolase activity
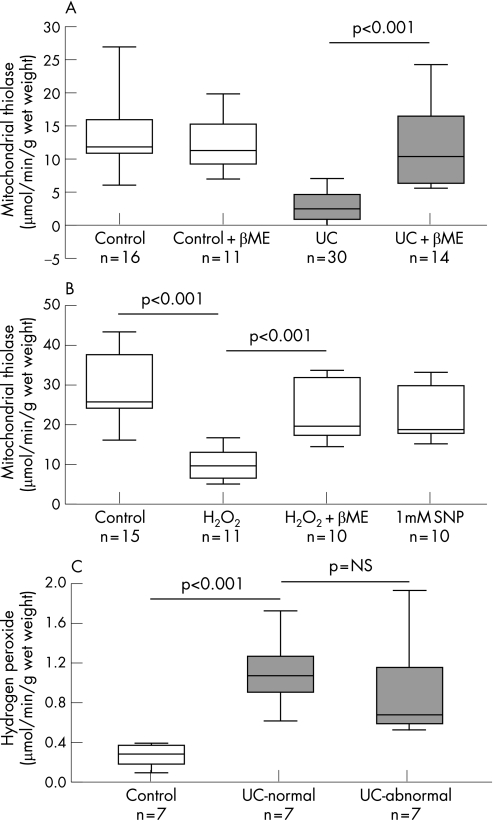
In order to test whether the thiolase enzyme could have been modified by reactive oxygen species, the biopsy homogenates were treated with 0.3 mM β‐mercaptoethanol, a reductant. This resulted in full recovery of mitochondrial acetoacetyl CoA thiolase to levels seen in control biopsies (fig 8A). In contrast, β‐mercaptoethanol had no significant effect on mitochondrial acetoacetyl CoA thiolase activity in mucosal homogenates from control patients. This suggests that the reduced mitochondrial acetoacetyl CoA thiolase activity in ulcerative colitis involves reversible oxidative modification of critical sulphydryl residues on the protein.
Figure 8 Redox modulation of mitochondrial acetoacetyl coenzyme A (CoA) thiolase activity in ulcerative colitis (ulcerative colitis). (A) In ulcerative colitis biopsies, β‐mercaptoethanol (βME) reversed the defect in enzyme activity, suggesting a reversible oxidative modification of a critical sulphydryl residue(s) on the enzyme. (B) In control biopsies, addition of hydrogen peroxide (H2O2) inhibited mitochondrial acetoacetyl CoA thiolase activity; this was reversed by β‐mercaptoethanol; there was no inhibition of enzyme activity in the presence of sodium nitroprusside (SNP). These findings indicate modulation of the enzyme by reactive oxygen species but not by reactive nitrogen species. (C) Hydrogen peroxide formation by colonic mucosal biopsies from control subjects and patients with left‐sided ulcerative colitis (inflamed and non‐involved mucosa) indicating increased generation in ulcerative colitis.
Redox modulation of thiolase activity
Several enzymes with catalytic or regulatory cysteine residues have been shown to be reversibly inactivated by H2O2, a physiological oxidant that is generated in mitochondria. In order to determine whether mitochondrial acetoacetyl CoA thiolase is redox regulated, homogenates from control patients were incubated in the presence of 200 μM H2O2. As shown in fig 8B, the addition of a bolus of H2O2 significantly decreased enzyme activity. We further investigated whether H2O2‐dependent inactivation could be reversed by reductant, by first incubating the mucosal homogenate with H2O2 and then incubating it in the presence of 0.3 mM β‐mercaptoethanol. In the presence of the reductant, H2O2‐induced inactivation of the enzyme was significantly reversed (fig 8B). Redox modulation may be mediated by both reactive oxygen species and reactive nitrogen species. In order to examine the role of nitrosative stress in redox modulation of mitochondrial acetoacetyl CoA thiolase, mucosal homogenates from control subjects were incubated with 1 mM sodium nitroprusside, a well‐known NO donor. No significant difference in the activity of mitochondrial acetoacetyl CoA thiolase was observed in the mucosal homogenate from the control samples treated in the presence or absence of sodium nitroprusside, suggesting that the enzyme is not modulated by reactive nitrogen species (fig 8B). A significant increase in H2O2 formation was observed in colonic mucosal biopsies obtained from ulcerative colitis patients when compared with controls (fig 8C). The increase in H2O2 was noted in both actively inflamed (1.11 (0.12) mmol/min/mg protein) and endoscopically normal (0.97 (0.18)) mucosa of patients with left‐sided ulcerative colitis compared with control biopsies (0.27 (0.04)) (p = 0.006).
Discussion
This study identifies, for the first time, a specific defect in a mitochondrial butyrate oxidative enzyme in patients with ulcerative colitis and provides a basis for the hitherto unexplained observation that butyrate oxidation is impaired in ulcerative colitis. Mitochondrial acetoacetyl CoA thiolase, which catalyses the important last step of butyrate oxidation, was significantly impaired in the colonic mucosa of patients with ulcerative colitis. The impairment was observed in the majority of patients, regardless of disease span or medical history. Thiolases are distributed extensively, and subserve important metabolic activities in different tissues. In the colonic epithelium, the mitochondrial acetoacetyl CoA thiolase is a key link in the chain of butyrate oxidation. Impairment of this isoform may not have a major physiological significance in other tissues, where alternative forms of the enzyme (eg, oxoacyl/ketoacyl CoA thiolase located in mitochondria) may compensate. However, the oxoacyl/ketoacyl CoA thiolase is specific for carbon chain length from C6 and above,19,20,21 while butyrate is a C4 fatty acid and can only be metabolised by the acetoacetyl CoA thiolase. Therefore, in the colonocyte, where butyrate is the preferred fuel for energy production, mitochondrial acetoacetyl CoA thiolase deficiency is likely to lead to defective butyrate oxidation and cell death.
The studies described here demonstrate that the decrease in acetoacetyl CoA thiolase activity is specific and not a reflection of epithelial cell damage that occurs in ulcerative colitis. Decreased thiolase activity was not accompanied by reduced activity of other butyrate oxidation enzymes such as crotonase or β‐hydroxy butyrate. Reduced enzyme activity not only characterised the ulcerated mucosa in patients with left‐sided ulcerative colitis, but was also found in the apparently normal mucosa of the right colon in these patients. The level of enzyme activity did not correlate with the type of treatment that the patient was receiving at the time of colonoscopy. Finally, in a disease control group consisting of Crohn's colitis, no impairment of mitochondrial acetoacetyl CoA thiolase activity could be detected, suggesting that impaired enzyme activity was not just a response to colonic inflammation. These findings, taken together with earlier in vitro and in vivo studies demonstrating impaired butyrate oxidation in ulcerative colitis,4,5,6,22 suggest that the demonstrated defect in mitochondrial acetoacetyl CoA thiolase activity is important in the pathogenesis of ulcerative colitis. An earlier study investigated the role of butyrate oxidation enzymes in quiescent ulcerative colitis and concluded that there was no defective activity of any of the butyrate oxidation enzymes in ulcerative colitis.12 That study did not attempt to measure mitochondrial acetoacetyl CoA thiolase activity separately from total acetoacetyl CoA thiolase activity, which can be done on the basis of potassium sensitivity as in this study. It has been shown, in a different clinical context,14 that it is important to test specifically for the K+‐sensitive acetoacetyl CoA thiolase activity in order not to miss defects in this enzyme. The present study specifically examined K+‐sensitive acetoacetyl CoA thiolase activity and showed it to be defective. A review of the earlier study showed that a minor decrease in K+‐insensitive thiolase activity was actually detected in ulcerative colitis compared with controls, but it was attributed to age differences between cases and controls.12 Although there was a significant difference in total thiolase activity between ulcerative colitis and control subjects in our study as shown in fig 2, specific measurement of potassium‐dependent acetoacetyl CoA thiolase highlighted the gross reduction in enzyme activity in ulcerative colitis.
These studies examined a possible mechanism behind the selective impairment of mitochondrial acetoacetyl CoA thiolase activity and demonstrated that colonic mitochondrial acetoacetyl CoA thiolase activity in control subjects was potentially redox modulated, and that the impaired thiolase activity in ulcerative colitis could be restored to normal by addition of a reductant. Thiolase has several cysteine residues involved in regulatory as well as catalytic function.13 Of the many modifications of cysteine's sulphydryl groups, sulphenic acids and disulphide bonds are reversible upon treatment with reducing agents such as mercaptoethanol or glutathione.23,24,25 The redox status of these thiol groups is important in many cellular functions including regulation of structure and activity of enzymes.26 β‐Mercaptoethanol was able to restore enzyme activity to normal levels in ulcerative colitis, suggesting that thiol groups in acetoacetyl CoA thiolase may undergo oxidative modification in ulcerative colitis patients. This suggests that the reduced mitochondrial acetoacetyl CoA thiolase activity in ulcerative colitis involves a reversible oxidative modification of a critical sulphydryl residue(s) on the protein. This also suggests that the observed impairment in activity is not due to decreased expression or synthesis of the enzyme or a gene mutation at the active or regulatory site. The enzyme kinetic studies showed a lowered Vmax and unaltered Km of the enzyme, supporting the possibility of a thiol modification and suggesting that thiol status of this thiolase is a determinant of its catalytic and regulatory competency.
Ulcerative colitis is characterised by evidence of oxidant injury to the colonic mucosa as well as a depletion of mucosal antioxidant defences.27,28,29 H2O2 is an oxidant that is continuously produced in cells, and 90% of this is produced by mitochondria as a toxic by‐product of electron transport chain activity.30 Our studies showed that low concentrations of exogenously added H2O2 were able to modulate mitochondrial acetoacetyl CoA thiolase activity. As expected, there was an increase in H2O2 formation in both inflamed and non‐inflamed biopsy specimens from patients with ulcerative colitis. We speculate that increased generation of reactive oxygen species may occur in the mitochondria of colonic epithelial cells in ulcerative colitis and lead to oxidative modification of several cysteine residues at or near the active site of the enzyme, which is located in the mitochondria in very close proximity to the electron transport chain. This explanation would imply that an increased generation of oxygen free radicals (possibly H2O2) in mitochondria occurs in ulcerative colitis epithelial cells even in quiescent disease and that it also occurs in the apparently normal mucosa of left‐sided ulcerative colitis patients. In Crohn's colitis as well, increased mucosal H2O2 may be expected due to increased generation from neutrophils and inflammatory cells in the lamina propria. However, this perhaps does not have the same effect as increased H2O2 generation within epithelial cells, which may inhibit thiolase activity in mitochondria. A “radical induction” theory of ulcerative colitis has recently been proposed,31 which hypothesises that the initial event in ulcerative colitis is an increased generation of H2O2 from mitochondria. If this were to happen primarily within epithelial cells, it is then possible to attribute all pathogenic events subsequent to this. Although the theory is not substantiated by this or earlier studies, it clearly will bear further investigation.
In conclusion, these studies demonstrated a specific impairment of mitochondrial acetoacetyl CoA thiolase activity in ulcerative colitis, which will explain the “energy deficiency state” that characterises the disease. The origin of this defect, and in particular the role in its localised generation of reactive oxygen species within the mitochondria of the colonic epithelial cells, deserves further study.
Acknowledgements
This study was supported by ad hoc research grant no. 5/4/3‐2/2001‐NCD‐II from the Indian Council of Medical Research, New Delhi, India. The Wellcome Trust Research Laboratory received infrastructural support from the Department of Science & Technology, Government of India under the FIST programme. The authors thank Professor KA Balasubramanian for useful discussions.
Abbreviations
ASA - aminosalicylate
CoA - coenzyme A
Footnotes
Competing interests: None.
References
- 1.Watanabe T, Kitani A, Strober W. NOD2 regulation of Toll‐like receptor responses and the pathogenesis of Crohn's disease. Gut 2005541515–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibson P R, Muir J G. Reinforcing the mucus: a new therapeutic approach for ulcerative colitis? Gut 200554900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson P, Rosella O, Nov R.et al Colonic epithelium is diffusely abnormal in ulcerative colitis and colorectal cancer. Gut 199536857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roediger W E. The colonic epithelium in ulcerative colitis: an energy‐deficiency disease? Lancet 19802712–715. [DOI] [PubMed] [Google Scholar]
- 5.Chapman M A, Grahn M F, Boyle M A.et al Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut 19893573–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramakrishna B S, Roberts‐Thomson I C, Pannall P R.et al Impaired conjugation of phenols by the colonic mucosa in active and quiescent ulcerative colitis. Gut 19913246–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roediger W E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 198021793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad M S, Krishnan S, Ramakrishna B S.et al Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut 200046493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roediger W E, Nance S. Metabolic induction of experimental ulcerative colitis by inhibition of fatty acid oxidation. Br J Exp Pathol 198667773–782. [PMC free article] [PubMed] [Google Scholar]
- 10.Kameyama J, Narui H, Inui M.et al Energy level in large intestinal mucosa in patients with ulcerative colitis. Tohoku J Exp Med 1984143253–254. [DOI] [PubMed] [Google Scholar]
- 11.Harig J M, Soergel K H, Komorowski R A.et al Treatment of diversion colitis with short‐chain‐fatty acid irrigation. N Engl J Med 198932023–28. [DOI] [PubMed] [Google Scholar]
- 12.Allan E S, Winter S, Light A M.et al Mucosal enzyme activity for butyrate oxidation; no defect in patients with ulcerative colitis. Gut 199638886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton B. The oxoacyl‐coenzyme A thiolases of animal tissues. Biochem J 1973132717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang G X, Fukao T, Rolland M O.et al Mitochondrial acetoacetyl‐CoA thiolase (T2) deficiency: T2‐deficient patients with “mild” mutation(s) were previously misinterpreted as normal by the coupled assay with tiglyl‐CoA. Pediatr Res 20045660–64. [DOI] [PubMed] [Google Scholar]
- 15.Truelove S C, Witts L J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 195521041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutter M, Saunders B, Wilkinson K.et al Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004126451–459. [DOI] [PubMed] [Google Scholar]
- 17.Lynen F, Wieland O. Beta keto reductase. Methods Enzymol 19551566–573. [Google Scholar]
- 18.Steinman H M, Hill R L. Bovine liver crotonase (enoyl coenzyme A hydratase). EC 4.2.1.17 L‐3‐hydroxyacyl‐CoA hydrolyase. Methods Enzymol 197535136–151. [DOI] [PubMed] [Google Scholar]
- 19.Middleton B. 3‐Ketoacyl‐CoA thiolases of mammalian tissues. Methods Enzymol 197535128–136. [DOI] [PubMed] [Google Scholar]
- 20.Seubert W, Lamberts I, Kramer R.et al On the mechanism of malonyl‐CoA‐independent fatty acid synthesis. I. The mechanism of elongation of long‐chain fatty acids by acetyl‐CoA. Biochim Biophys Acta 1968164498–517. [DOI] [PubMed] [Google Scholar]
- 21.Staack H, Binstock J F, Schulz H. Purification and properties of a pig heart thiolase with broad chain length specificity and comparison of thiolases from pig heart and Escherichia coli. J Biol Chem 19782531827–1831. [PubMed] [Google Scholar]
- 22.Roediger W E. Colonic bicarbonate output as a test of disease activity in ulcerative colitis. J Clin Pathol 198437704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claiborne A, Yeh J I, Mallett T C.et al Protein‐sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry 19993815407–15416. [DOI] [PubMed] [Google Scholar]
- 24.Giles G I, Tasker K M, Jacob C. Oxidation of biological thiols by highly reactive disulfide‐S‐oxides. Gen Physiol Biophys 20022165–72. [PubMed] [Google Scholar]
- 25.Giles G I, Tasker K M, Collins C.et al Reactive sulphur species: an in vitro investigation of the oxidation properties of disulphide S‐oxides. Biochem J 2002364579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izbicka‐Dimitrijevic E, Gilbert H F. Multiple oxidation products of sulfhydryl groups near the active site of thiolase I from porcine heart. Biochemistry 1984234318–4324. [DOI] [PubMed] [Google Scholar]
- 27.Bhaskar L, Ramakrishna B S, Balasubramanian K A. Colonic mucosal antioxidant enzymes and lipid peroxide levels in normal subjects and patients with ulcerative colitis. J Gastroenterol Hepatol 199510140–143. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie S, Baker M, Buffinton G.et al Evidence of oxidant‐induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest 199698136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan E A, Rao S, Burdick J S.et al Glutathione in the chronic inflammatory disorders of the human colon. Nutr Res 199717463–473. [Google Scholar]
- 30.Balaban R, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005120483–495. [DOI] [PubMed] [Google Scholar]
- 31.Pravada J. Radical induction theory of ulcerative colitis. World J Gastroenterol 2005112371–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]