Abstract
Background/aims
An efficient cytolytic T cell function is essential for immune mediated rejection of colorectal cancer. However, the molecular mechanisms driving T cell mediated cancer rejection are still poorly understood. Here, we assessed the relevance of the T‐box transcription factor eomesodermin in colorectal cancer.
Methods/results
By analysing tissue probes from 88 different colorectal tumours, a significant (p<0.02) inverse correlation between eomesodermin expression in colorectal cancers and the presence of lymph node metastases could be shown, whereas no such correlation was noted for the master transcription factor of regulatory T cells, FoxP3 and CD8alpha expression. To evaluate whether this effect might be due to effects of eomesodermin on tumour infiltrating CD8 T cells, we subsequently analysed the regulated expression and function of this transcription factor in human T cells. Whereas overexpression of this factor induced perforin but not granzyme expression, siRNA mediated suppression of eomesodermin expression led to significantly reduced IFN‐γ production, perforin levels and cytolytic activity of CD8 T cells. Furthermore, TGF‐β and IL4 could be identified as important inducer of eomesodermin expression.
Conclusion
These data define for the first time a regulatory role of eomesodermin for CD8 T cell activity in humans. Our findings are consistent with a model in which eomesodermin expression in tumour infiltrating T cells regulates cytolytic functions of CD8 T cells via perforin expression. These data provide novel insights into control mechanisms governing the functional activity of human CD8 T lymphocytes via T‐box transcription factors in cancer.
Keywords: cytokines, eomesodermin, colorectal cancer
Colorectal cancer (CRC) presents the third most common cancer and the fourth most frequent cause of cancer deaths worldwide. People in developed countries especially have a relatively high risk of developing CRC with a lifetime incidence of about 5%.1 Therefore, considerable efforts have been made to improve prevention, early diagnosis and therapy of CRC by gaining insights into disease specific pathogenesis. The pivotal role of the immune system in CRC pathogenesis has been illuminated by various studies.2 Antitumour immunity presents a complex mechanism, involving a broad variety of distinct immunocompetent cells. Among these cells, cytolytic CD8 T lymphocytes are key players. CD8 T cells are able to lyse tumour cells specifically3 and strong evidence indicates that infiltration of primary tumours with CD8 T lymphocytes represents a strong favourable prognostic factor.2,4
The T‐box transcription factor eomesodermin was initially identified as a major regulator of mesodermal development in Xenopus and mice.5,6,7,8 Subsequently, this factor has been found to play an important part in the differentiation and activation of effector CD8 T cells as well.6 Ectopic expression of eomesodermin in murine cells led to enhanced expression of genes like perforin and granzyme‐B, which are associated with cytolytic capacity of CD8 T cells.6 These data suggested that eomesodermin has a pivotal role in controlling cytolytic activity of murine CD8 T lymphocytes.
Based on these observations in the murine system, we here analysed the functional role of eomesodermin in human CD8 T cells and its functional relevance in CRC. Silencing of eomesodermin in human CD8 T lymphocytes was strongly associated with reduced expression of perforin and decreased cytolytic activity. Furthermore, the absence of eomesodermin in tumour infiltrating lymphocytes correlated with enhanced lymph node metastasis of CRC. These data indicate a pivotal role of eomesodermin in human CD8 T cell activation with major relevance for CRC.
Methods
Cells
Human peripheral blood mononuclear cells from healthy volunteers were isolated using Ficoll‐Hypaque gradients. Cells were further purified using CD8 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), CD4 beads plus Detachabeads (Invitrogen, Karlsruhe, Germany) or CD25 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Naive CD8CD45RA T cells were further separated from human CD8 T cells by negative selection techniques using CD45RO monoclonal antibodies (PharMingen, Heidelberg) and immunomagnetic beads (Dynal). AK‐EBVB cells, kindly provided by Professor Wölfel (University of Mainz, Germany), were used as antigen presenting cells (APCs). For induction of transforming growth factor β (TGF‐β) induced CD4CD25FoxP3 regulatory cells (Ti‐Treg), freshly isolated CD4CD25− T cells were cultured in the presence of recombinant interleukin 2 (IL2) and TGF‐β1 (10 ng/ml; R&D, MI, USA) as described recently.9 In short, freshly isolated CD4CD25− T cells were plated in anti‐CD3 coated wells (0.04 µg/ml; BD Bioscience) in serum‐free medium in the presence of soluble anti‐CD28 (1 µg/ml; BD Bioscience), recombinant IL2 (200 U/ml; R&D) and recombinant TGF‐β1 (10 ng/ml; R&D). After five days of culture, these cells were magnetically isolated with CD25 microbeads and used in further experiments. To generate CD4CD25FoxP3− cells, cells were cultered in the absence of TGF‐β1. Naturally occuring regulatory CD4CD25 cells were seperated directly from freshly isolated human CD4 T cells by CD25 microbeads. Lamina propria mononuclear cells were isolated from colon specimens and further separated into CD4, CD8 and non‐T cells by magnetic CD4 and CD8 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Tissue of colorectal tumours
Tumour samples were obtained from 88 patients undergoing elective surgery for CRC. The tumour tissue originated from the centre of the tumour.
Cloning of eomesodermin cDNA
Full length m‐eomesodermin cDNA was synthesised by Operon Chem and cloned into the EcoRI site of the pcDNA3.1 vector (Invitrogen, Karlsruhe, Germany).
Transient transfection of primary CD8 T lymphocytes
Human CD8 T cells were transfected with eomes‐siRNA (small interfering RNA) (Qiagen, Hilden, Germany), murine GFP‐siRNA (control‐siRNA) (Qiagen), eomesodermin /pcDNA3.1 vector or pmaxGFP vector (Amaxa, Cologne, Germany), using the Human T Cell NucleofectorKit (Amaxa).
Semiquantitative real time polymerase chain reaction (RT‐PCR)
mRNA was isolated from human T cells using the RNeasy Micro Kit (Qiagen; including DNase digestion) and transcribed reversely into cDNA using the StratScript First‐Strand cDNA synthesis system (Stratagene, CA, USA).
Primers used for semiquantitative PCR:
Eomesodermin: 5′‐CTA GGG ACT TGT GTA AAA AGC‐3′ and
5′‐GAG CCC‐TCA AAG ACC CAG A‐3′
tg eomesodermin : 5′‐TAC CAG AAC ACC GAC ATC AC‐3′ and
5′‐TCC TCT CGC CGT TGT AGT AT‐3′
Actin: 5′‐CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG‐3′
5′‐TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA‐3′
Perforin: 5′‐CCA GCA ATG TGC ATG TGT CT‐3′ and
5′‐CCG AAC AGC AGG TCG TTA AT‐3′
IFN‐γ: 5′‐GGT CAT TCA GAT GTA GCG GA‐3′ and
5′‐TCT TCG ACC TTG AAA CAG CA‐3′
Granzyme‐B: 5′‐GGG GAA GCT CCA TAA ATG TCA CCT‐3′ and
5′‐TAC ACA CAA GAG GGC CTC CAG AGT‐3′
PCR products were analysed on 1% agarose gels.
Quantitative real time PCR
Real time PCR was performed by using the QuantiTec SYBR Green PCR Kit (Qiagen, Hilden) in combination with specific primers (Qiagen, Hilden) for human eomesodermin, human CD8a and human HPRT or with Absolute QPCR Mixes (Abgene, Epsom, UK) in combination with specific primer‐probe sets for human T‐bet (Qiagen, Hilden), human FoxP3 (Applied Biosystem, CA, USA) or human HPRT (Applied Biosystem, CA, USA). PCR reaction was performed in an iCycler (Bio‐Rad, CA, USA).
Calculation of relative amounts of the target:
ΔCT = CTtarget− CTHPRT
ΔΔCT = ΔCTn − ΔCTreference
Relative amount of target gene = 2−ΔΔCT or 2−ΔCT, as specified in Results.
ELISA
The ELISA was performed using the BD OptEIA kit for IFN‐γ (ΒD Bioscience, CA, USA).
In situ hybridisation
A 877 bp fragment of human eomesodermin was amplified by RT‐PCR from CD8 T cell RNA and cloned into the pSC‐A vector (Invitrogen, Karlsruhe) using the StrataClone PCR Cloning kit (Stratagene, CA, USA). N‐terminus of eomesodermin fragment was flanked by the T7 promoter and C‐terminus by the T3 promter. Antisense RNA and sense RNA (negative control) were generated by using promoter specific RNA polymerase. To generate a 33P αUTP labelled RNA probe, transcription was performed with the MAXIscript in vitro transcription kit (Ambion, CA, USA). In situ hybridisation was performed using the mRNAlocator in situ hybridisation kit (Ambion, CA, USA). Haematoxylin counterstained slides were analysed by microscopy.
51Chromium release assay
51Chromium release assay was performed as described recently.10 AK‐EBVB cells were used as target cells at an effector/target ratio of 90:1. Determination of specific chromium release: Percent specific 51Cr release = (experimental release – spontaneous release) × 100/(maximum release – spontaneous release).
Statistical analysis
Tests for significance of differences were made by t tests using the program Excel.
Results
Inverse correlation between eomesodermin expression and the presence of lymph node metastases in colorectal cancer
As eomesodermin has been suggested to play an important part in controlling CD8 T cell activity, we assessed its potential regulatory role for metastasis in CRC patients. Firstly, we aimed at detection of eomesodermin mRNA by in situ hybridisation. As shown in figure 1A, there was a marked variability of eomesodermin mRNA expression in colonic tissue from patients with CRC. While about 50% of the patients showed similar or even increased levels of eomesodermin in comparison to normal control tissue, the other patients showed a markedly reduced or even absent eomesodermin mRNA expression consistent with a potential regulatory role of this T‐box transcription factor in colorectal cancer.
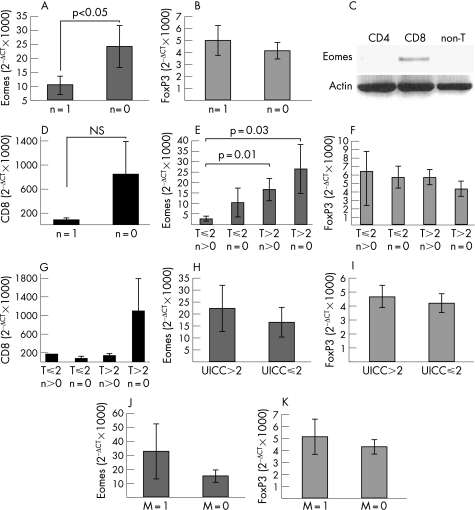
Figure 1 Expression of eomesodermin in CRC. The expression of eomesodermin was analysed in cryosections of tumour and non‐tumour colon tissue by in situ hybridisation (A). Probes of 88 different colorectal tumours were analysed for the expression of eomesodermin by RT‐PCR. According to the results of RT‐PCR, tumours were separated into Eomes negative or Eomes positive tumours and compared with clinical data. Numbers of tumours, fulfilling the respective criteria, are indicated (B).
To explore a possible relation between intratumoral expression of eomesodermin and tumour prognosis, we analysed eomesodermin mRNA expression in CRC by semi‐quantitative PCR. Eomesodermin expression was analysed using cDNA probes of 88 different colorectal tumours by RT‐PCR (fig 1B). Based on the presence or absence of PCR bands, tumours were stratified into eomesodermin negative and eomesodermin positive tissues and the results were compared with clinical data. Interestingly, a significant (p<0.02) inverse correlation between expression of eomesodermin in colorectal tumours and the presence of lymph node metastases could be observed (fig 1B). In contrast, there was no significant correlation between expression of eomesodermin and venous or lymph vessel invasion of tumour cells (fig 1B).
To confirm the above findings, quantitative analysis of eomesodermin expression was performed in probes of 51 different colorectal tumours by real time PCR. Patients included in this experiment are characterised in table 1.
Table 1 Clinical data of patients who were included in the experiments in figure 4, are summarised here.
| Group | No of patients | Average age* | Sex | Tumour localisation | M (SD) | UICC |
|---|---|---|---|---|---|---|
| T⩽2 N>0 | 4 | 71.4 (5.4) | 4× female | 1× caecum | 0.25 (0.5) | 3.25 (0.5) |
| 1× colon descendens | ||||||
| 2× rectum | ||||||
| T⩽2 N = 0 | 8 | 66.4 (11.9) | 2× female | 1× caecum | 0.13 (0.4) | 1.4 (0.7) |
| 6× male | 2× sigma | |||||
| 5× rectum | ||||||
| T>2 N>0 | 16 | 62.8 (8.2) | 4× female | 3× caecum | 0.4 (0.5) | 3.4 (0.5) |
| 12× male | 1× colon ascendens | |||||
| 1× colon transversum | ||||||
| 4× sigma | ||||||
| 7× rectum | ||||||
| T>2 N = 0 | 23 | 68.1 (9.9) | 12× female | 2× caecum | 0.1 (0.3) | 2.2 (0.6) |
| 11× male | 3× colon ascendens | |||||
| 3× colon transversum | ||||||
| 1× colon descendens | ||||||
| 6× sigma | ||||||
| 8× rectum |
*Mean (SD) is indicated for average age, M (in accordance to TNM classification) and UICC (International Union against Cancer).
Consistent with the previous experiments, a significantly (p<0.05) higher expression of eomesodermin was found in colorectal tumours without detectable lymph node metastases (N = 0 according to the TNM classification) compared to tumours with lymph node metastases (N = 1) (fig 2A). In contrast, no correlation between the presence of lymph node metastases and the expression of FoxP3, the master transcription factor of regulatory T cells was noted (fig 2B).11,12 Since it is known from the murine system, that eomesodermin is expressed mainly in CD8 T cells,6 this inverse correlation between eomesodermin levels and the presence of lymph node metastases might be explained by an increased number of CD8 T cells in colorectal tumours without metastases. However, although eomesodermin expression was mainly detectable in CD8 T cells within human colon tissue (fig 2C), no significant correlation between the expression of CD8alpha in colorectal tumours and the absence of lymph node metastases could be shown (fig 2D), suggesting that eomesodermin is potentially involved in controlling the activation of CD8 T cells in colorectal cancer. These findings strengthen the hypothesis that increased numbers of eomesodermin expressing cytolytic CD8 T cells within the tumour lesions are associated with an increased ability of the immune system to prevent the formation of lymph node metastasis.
Figure 2 Quantification of eomesodermin and FoxP3 expression in CRC. Expression of eomesodermin, FoxP3 and CD8alpha was analysed in probes of 51 (Eomes), 89 (FoxP3) or 69 (CD8alpha) colorectal tumours by real time PCR. HPRT was used as reference gene. Calculation of relative amount of eomesodermin: relative amount of target gene = 2−ΔCT with ΔCT = CTEomes− CTHPRT. Results were set in relation to clinical data of patients. Correlation between eomesodermin, FoxP3 or CD8alpha and the presence of lymph node metastasis (N in accordance with TNM classification) (A, B, D) and tumour size (T in accordance with TNM classification) (E, F, G) were analysed. Furthermore, the correlation between eomesodermin or FoxP3 expression and tumour prognosis (UICC classification) (H, I) or the presence of distant metastasis (M in accordance with TNM classification) (J, K) was analysed. Significance of correlation is indicated by p values (in accordance with t test). In addition, the expression of eomesodermin in lamina propria CD4, CD8 and non‐T cells, isolated from a human colon specimen, was analysed by RT‐PCR (C).
In contrast to FoxP3 and CD8alpha, further stratification showed lowest eomesodermin expression levels in smaller tumours (T⩽2) with lymph node metastases compared to larger tumours with or without lymph node metastases suggesting that eomesodermin expression may prevent early lymph node metastases (fig 2E–G). However, no significant correlation was found between intratumoral expression of FoxP3 or eomesodermin and the tumour stadium (UICC classification) or the presence of other metastases (M0 vs M1 tumours) (fig 2H–K). Thus, in contrast to FoxP3, eomesodermin expression shows a significant inverse correlation with lymph node metastasis but not for haematogenic metastasis of CRC.
IL4 and TGF‐β synergistically induce the expression of eomesodermin in human CD8 T lymphocytes
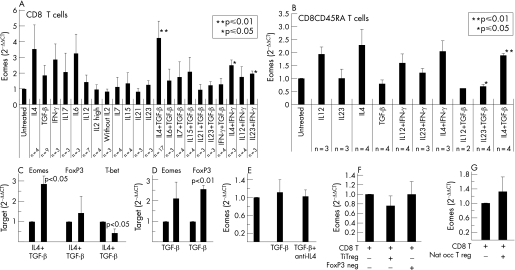
As eomesodermin is specifically expressed in T lymphocytes, the above data were consistent with a model in which high expression of eomesodermin in tumour infiltrating CD8 T cells prevents the formation of lymph node metastasis in CRC. However, in contrast to the murine system, no data on the regulation and role of this transcription factor in human T cells are available. We therefore focused on the regulated expression and function of eomesodermin in human CD8 T cells. Accordingly, we aimed at the identification of intracellular or intercellular mediators that control the expression of eomesodermin in human T cells using quantitative PCR. A broad panel of different cytokines was tested for the capacity to regulate the expression of eomesodermin in human CD8 T cells. Interestingly, simultaneous stimulation of human CD8 T lymphocytes with IL4 and TGF‐β resulted in the strongest and most significant induction of eomesodermin expression, whereas IL4 alone led to a remarkable but not significant induction of eomesodermin levels and TGF‐β alone showed only small and not significant influences on eomesodermin expression (fig 3A). Obviously, the TH2 cytokine IL4 is a key player within the regulation of eomesodermin levels in human CD8 T cells, but it is strongly dependent on TGF‐β mediated support. None of the other proinflammatory cytokines such as IFN‐γ, IL6, IL12, IL23, IL21, IL7, IL15 and IL17 nor a combination of TGF‐β with these cytokines were able to influence the expression of eomesodermin significantly (fig 3A). The modest but significant induction of eomesodermin after a combined treatment witl IL4 and IFN‐γ or IL23 and IFN‐γ might be a hint for the involvement of the Th1 cytokine IFN‐γ in the induction of eomesodermin expression, but these effects were less pronounced than the effects mediated by combined IL4 and TGF‐β treatment. Similarly to total CD8 T cells, combined IL4 and TGF‐β treatment induced a significant upregulation of eomesodermin expression in naive human CD4 CD45RA T cells (fig 3B). In contrast, none of the other cytokines tested was able to significantly induce eomesodermin expression in naive CD8 T cells (fig 3B). The fact that IL4 plus TGF‐β mediated effects in naive CD8 T cells were significant but less pronounced than in complete CD8 T cells (duplication versus quadruplication of eomesodermin levels) suggests that eomesodermin levels in memory T cells are also induced by IL4 and TGF‐β mediated signalling.
Figure 3 Regulators of eomesodermin expression in human CD8 T cells. Human CD8 T cells or naive CD8CD45RA T cells were stimulated by irradiated APCs. IL2 (200 U/ml) in combination with the indicated recombinant cytokines was added to the culture medium: IL4 (10 ng/ml; R&D), TGF‐β1 (10 ng/ml; R&D); IFN‐γ (200 U/ml; R&D), IL17 (100 ng/ml; R&D), IL6 (100 ng/ml; R&D), IL12 (1000 U/ml), IL7 (240 U/ml), IL15 (200 U/ml), IL21 (50 ng/ml), IL23 (10 ng/ml) or large amounts of IL2 (500 U/ml). Cells were harvested after 72 hours and expression of eomesodermin was analysed by real time PCR. HPRT was used as reference gene and untreated CD8 T cells were used as reference probe. Calculation of relative amount of eomesodermin: relative amount of target gene = 2−ΔΔCT with ΔCT = CTEomes− CTreference gene and ΔΔCT = ΔCTn − ΔCTreference probe. n indicates the number of performed experiments (A, B). In the same way, expression of FoxP3 and T‐bet were analysed 72 hours after addition of TGF‐β1 plus IL4 to the culture medium (C). Expression of FoxP3 was analysed in human CD8 T cells 72 hours after addition of TGF‐β1 to culture medium (D). Furthermore, expression of eomesodermin was analysed in human CD8 T cells 72 hours after addition of TGF‐β1 or of TGF‐β plus a neutralising anti‐IL4 antibody to the culture medium in three different experiments (E). Induced CD4CD25FoxP3 positive regulatory cells (Ti‐Treg cells), CD4CD25FoxP3 negative cells or naturally occurring regulatory T cells (Nat occ T reg) were cultured together with freshly isolated human CD8 T lymphocytes in 1:1‐ ratio. Cells were stimulated with irradiated APCs and recombinant IL2 was added. Cells were analysed at day 3 of co‐culture. RT‐PCR was performed and analysed as described above. CD8alpha was used as reference gene (F, G).
In further studies, we determined the effects of TGF‐β plus IL4 and TGF‐β alone on the expression of T‐bet and FoxP3 in addition to eomesodermin (figs 3C, D). Interestingly, TGF‐β stimulation alone significantly induced FoxP3 rather than eomesodermin expression. Furthermore, expression of T‐bet was significantly suppressed by TGF‐β plus IL4, whereas no changes in FoxP3 expression occurred and eomesodermin expression was upregulated (fig 3C). To clarify whether the amount of endogenous IL4 within our experimental setting was relevant for the modest induction of eomesodermin by TGF‐β treatment alone, endogenous IL4 under these stimulation conditions was blocked by a neutralising anti‐IL4 antibody. However, no significant effects were observed (fig 3E).
Taken together, in contrast to the T‐box transcription factor T‐bet, a well defined activator of human cytolytic T cells (CTLs),13 the expression of eomesodermin was strongly enhanced by synergistic effects of the TH2/β cytokines IL4 and TGF‐β, which are known to be produced by regulatory T cells and by the tumour cells themselves.14,15
As TGF‐β was capable of inducing eomesodermin levels together with IL4, we next assessed whether regulatory T cells might be involved in the induction of eomesodermin. To address this question, primary human CD8 T cells were co‐cultured together with TGF‐β induced CD4CD25FoxP3 regulatory cells (Ti‐Treg) or with naturally occuring regulatory CD4CD25 T cells for 3 days. Co‐culture of CD8 T cells with Ti‐Treg cells (fig 3F) as well as with naturally occurring regulatory T cells (fig 3G) did not result in any induction of eomesodermin expression in human CD8 T cells. These data suggested that regulatory T cells are not directly responsible for the regulation of eomesodermin expression in tumour infiltrating CD8 T cells.
Eomesodermin regulates cytolytic capacity of human CD8 T lymphocytes
Although a key role of eomesodermin in the regulation of cytolytic activity of murine CD8 T lymphocytes has been recently described,6 its function in the human immune system remains to be elucidated. To investigate eomesodermin mediated effects in human CTLs, eomesodermin expression was specifically modulated in primary human CD8 T cells. Whereas overexpression of eomesodermin by transfection with a cDNA expression vector up‐regulated the expression of perforin, the expression of granzyme‐B remained unaffected (fig 4A). Furthermore, siRNA mediated silencing of eomesodermin expression in these cells led to suppression of the expression of the cytolytic gene perforin as well as of interferon‐γ (IFN‐γ) (fig 4B, C). In contrast to eomesodermin regulated expression of granzyme‐B in murine CTLs,6 however, granzyme‐B expression in human CD8 T cells was not affected by specific silencing of eomesodermin expression (fig 4B).
Figure 4 Human CD8 T lymphocytes were isolated from blood and transiently transfected with Eomes/pcDNA3.1 vector or control vector (pmaxGFP vector). The next day cells were stimulated with anti‐CD3, anti‐CD28 and recombinant IL2. 10 hours post‐stimulation, cells were harvested and analysed for the expression of eomesodermin, perforin, granzyme‐B and actin by RT‐PCR (A) siRNA mediated silencing of eomesodermin in human CD8 T lymphocytes. Human CD8 T lymphocytes were isolated and transiently transfected with Eomes siRNA or control siRNA. Next day cells were stimulated with anti‐CD3, anti‐CD28 and recombinant IL2. 10 hours post‐stimulation, cells were harvested and analysed for the expression of eomesodermin, perforin, granzyme‐B, IFN‐γ and actin by RT‐PCR (B). Supernatants of these cells were analysed for IFN‐γ concentrations by ELISA. IFN‐γ production of cells, transfected with control siRNA, was defined as 100%. The mean (SEM) of three independent experiments are shown (C). Primary human CD8 T lymphocytes stimulated with irradiated AK‐EBVB cells (10 000 rad) at day 0 followed by addition of recombinant IL2 at day 3. At day 7 cells were restimulated with AK‐EBVB cells and transient transfections of stimulated T lymphocytes with eomesodermin siRNA or control siRNA was performed at day 9. At day 10 responder T cells were analysed for cytotoxicity against AK‐EBVB cells at effector to target ratio of 90:1 in the 51chromium release assay. Data from three independent experiments are shown (D).
In subsequent functional studies, we next determined whether the eomesodermin mediated regulation of perforin expression would result in an altered cytolytic activity of human CD8 T lymphocytes. Accordingly, 51chromium release assays were performed in primary T cells exposed to eomesodermin specific siRNA or control siRNA. The cytolytic capacity of eomesodermin siRNA treated human CD8 T cells was reproducibly reduced in three independent experiments as compared to CD8 T cells transfected with control siRNA (fig 4D) suggesting that eomesodermin has a key regulatory function for cytolytic activity of human CD8 T lymphocytes.
Discussion
Tumour infiltrating lymphocytes have an important prognostic role in patients with CRC,4,16,17,18 although the regulation of their functional activity is still poorly understood. Here, we have identified an important regulatory role of the T‐box transcription factor eomesodermin in controlling the cytolytic activity of human CD8 effector T cells and found an inverse correlation between the expression of eomesodermin in CRC and the formation of lymph node metastasis in patients with CRC. Our data are consistent with a model in which eomesodermin expression in tumour infiltrating T cells regulates cytolytic functions of CD8 T cells mainly via the induction of the cytolytic gene perforin. These data provide novel insights in control mechanisms governing the functional activity of human CD8 T lymphocytes via T‐box transcription factors in cancer.
CD8 T cells produce IFN‐γ when activated by intracellular pathogens. In spite of some conflicting results,19 previous studies in mice showed that the T‐box transcription factor T‐bet controls IFN‐γ production of CD8 effector T cells.6,13 Furthermore, it has been recently reported that eomesodermin, a paralogue of T‐bet, plays an important role in IFN‐γ production and cytolytic effector mechanisms of CD8 T cells in mice.20 Ectopic expression of eomesodermin in murine Th2 cells was sufficient to induce IFN‐γ production even in the absence of T‐bet. In contrast to the murine system, little is known about the role of eomesodermin in human T cells. Accordingly, we have addressed the functional role and regulated expression of this T‐box transcription factor.
In contrast to the murine system where IFN‐γ induces and IL4 represses eomesodermin expression,21,22 both cytokines had no significant effect on eomesodermin levels in human CD8 T cells. Interestingly, we found that the expression of T‐bet and eomesodermin was differentially regulated in human CD8 T cells by TGF‐β plus IL4 stimulation rather than by IL4 stimulation alone. In fact, the expression of eomesodermin in human CD8 T cells was significantly enhanced by the synergistic effects of TGF‐β and the TH2 cytokine IL4, whereas expression of T‐bet was markedly reduced by the same cytokines.
Although TGF‐β is a well known anti‐inflammatory cytokine by suppressing T‐bet and GATA‐3 expression,23,24,25 some pro‐inflammatory functions of TGF‐β have been described recently.26 It was shown that de novo generation of IL17 producing T cells from naive CD4 T cells was strongly induced by TGF‐β. However, TGF‐β required the presence of another cytokine, IL6, to augment the IL17 production of T cells.26 As we found that the ability of the TH2 cytokine IL4 to induce eomesodermin expression in human CD8 T cells is strongly dependent on TGF‐β, the presence or absence of other proinflammatory cytokines seems to be of great importance for the function of TGF‐β. In any case, eomesodermin expression is induced by the same factors that suppress the expression of its paralogue T‐bet in human CD8 T cells. Moreover, eomesodermin expression is induced by prototypical TH2 and TH3 cytokines that are abundantly present in CRC tissue.27 It is tempting to speculate that the induction of eomesodermin levels under these stimulatory conditions may compensate for reduced T‐bet levels in these tumours.
Expression of the cytolytic gene perforin and the pro‐inflammatory cytokine IFN‐γ in human CD8 T cells were controlled by eomesodermin. In contrast to the murine system,6 the expression of granzyme‐B in human CTLs was not affected by silencing or augmenting eomesodermin expression. This could be due to differences between the murine and human systems with regard to eomesodermin function. However, eomesodermin dependent induction of granzyme‐B has only been shown in murine TH2 cells using retroviral overexpression.6 As these cells are characterised by the absence of the TH1 specific transcription factor T‐bet, it is possible that eomesodermin may have different function in T cells in which T‐bet is lacking. In any case, our data showed a reduced killing capacity of human CD8 T cells in which the expression of eomesodermin was silenced suggesting an important role of eomesodermin in controlling the cytolytic activity of human T cells.
Our data identify an important role of eomesodermin in the development of lymph node metastasis in CRC. PCR data showed that high expression of eomesodermin in colorectal tumours was associated with significantly reduced lymph node metastases. As this finding was particularly evident in smaller tumours, our data are consistent with the concept that eomesodermin controls early lymph node metastasis probably because of its regulation of the cytolytic activation of human CD8 T cells. However, further experiments are required to exactly delineate the functional role of eomesodermin in colon carcinogenesis and lymph node metastases. Interestingly, however, a recent study has also described a regulatory role of the T‐box transcription factor T‐bet in colorectal tumours.28 A significantly increased expression of T‐bet in colorectal tumours of patients without detectable early metastatic invasion and relapses compared to tumours from patients with detectable metastatic invasion and clinical relapses could be shown.28 Both findings, the increased expression of T‐bet28 as well as enhanced eomesodermin levels in tumours without lymph node metastasis clearly underline the importance of activated cytolytic CD8 T cells for the immunological control of tumour spread in CRC.
Abbreviations
APCs - antigen presenting cells
CRC - colorectal cancer
CTLs - cytolytic T cells
IL - interleukin
IFN‐γ - interferon‐γ
RT‐PCR - reverse transcription polymerase chain reaction
siRNA - small interfering RNA
TGF‐β - transforming growth factor β
UICC - International Union against Cancer
Footnotes
Competing interest: None.
References
- 1.Weitz J, Koch M, Debus J.et al Colorectal cancer. Lancet 2005365153–165. [DOI] [PubMed] [Google Scholar]
- 2.Dalerba P, Maccalli C, Casati C.et al Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol 20034633–57. [DOI] [PubMed] [Google Scholar]
- 3.Titu L V, Monson J R, Greenman J. The role of CD8(+) T cells in immune responses to colorectal cancer. Cancer Immunol Immunother 200251235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez‐Cabo F.et al Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Sience 20063131960–1964. [DOI] [PubMed] [Google Scholar]
- 5.Showell C, Binder O, Conlon F L. T‐box genes in early embryogenesis. Dev Dyn 2004229201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce E L, Mullen A C, Martins G A.et al Control of effector CD8+ T cell function by the transcription factor β. Science 20033021041–1043. [DOI] [PubMed] [Google Scholar]
- 7.Tayade C, Fang Y, Black G P.et al Differential transcription of Eomes and T‐bet during maturation of mouse uterine natural killer cells. J Leukoc Biol 2005781347–1355. [DOI] [PubMed] [Google Scholar]
- 8.Ryan K, Garrett N, Mitchell A.et al Eomesodermin, a key early gene in Xenopus mesoderm differentiation. Cell 199687989–1000. [DOI] [PubMed] [Google Scholar]
- 9.Fantini M C, Becker C, Tubbe I.et al Transforming growth factor β induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut 200655671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dörrschuck A, Schmidt A, Schnürer E.et al CD8+ cytotoxic T lymphocytes isolated from allogeneic healthy donors recognize HLA class Ia/Ib‐associated renal carcinoma antigens with ubiquitous or restricted tissue expression. Blood 20041042591–2599. [DOI] [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 20032991057–1061. [PubMed] [Google Scholar]
- 12.Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol 20044408–414. [DOI] [PubMed] [Google Scholar]
- 13.Glimcher L H, Townsend M J, Sullivan B M.et al Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol 20044900–911. [DOI] [PubMed] [Google Scholar]
- 14.Derynck R, Akhurst R J, Balmain A. TGF‐β signaling in tumor suppression and cancer progression. Nat Genet 200129117–129. [DOI] [PubMed] [Google Scholar]
- 15.Derynck R, Jarrett J A, Chen E Y.et al Human transforming growth factor‐beta complementary DNA sequence and expression in normal and transformed cells. Nature 1985316701–705. [DOI] [PubMed] [Google Scholar]
- 16.Naito Y, Saito K, Shiiba K.et al CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998583491–3494. [PubMed] [Google Scholar]
- 17.Prall F, Duhrkop T, Weirich V.et al Prognostic role of CD8+ tumor‐infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol 200435808–816. [DOI] [PubMed] [Google Scholar]
- 18.Waldner M, Schimanski C C, Neurath M F. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol 2006127233–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo S J, Sullivan B M, Stemmann C.et al Distinct effects of T‐bet in TH1 lineage commitment and IFN‐gamma production in CD4 and CD8 T cells. Science 2002295338–342. [DOI] [PubMed] [Google Scholar]
- 20.Intlekofer A M, Takemoto N, Wherry E J.et al Effector and memory CD8+ T cell fate coupled by T‐bet and eomesodermin. Nat Immunol 200561236–1244. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Lund R, Aittokallio T.et al Identification of novel IL‐4/Stat6‐regulated genes in T lymphocytes. J Immunol 20031713627–3635. [DOI] [PubMed] [Google Scholar]
- 22.Suto A, Wurster A L, Reiner S L.et al IL‐21 inhibits IFN‐gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol 20061773721–3727. [DOI] [PubMed] [Google Scholar]
- 23.Bright J J, Kerr L D, Sriram S. TGF‐β inhibits IL‐2‐induced tyrosine phosphorylation and activation of Jak‐1 and Stat 5 in T lymphocytes. J Immunol 1997159175–183. [PubMed] [Google Scholar]
- 24.Ahmadzadeh M, Eosenberg S A. TGF‐β1 attenuates the acquisition and expression of effector function by tumor antigen‐specific human memory CD8 T cells. J Immunol 20051745215–5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelik L, Fields P E, Flavell R A. TGF‐β inhibits Th Type 2 developement through inhibition of GATA‐3 expression. J Immunol 20001654773–4777. [DOI] [PubMed] [Google Scholar]
- 26.Veldhoen M, Hocking R J, Atkins C J.et al TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL‐17‐producing T cells. Immunity 200624179–189. [DOI] [PubMed] [Google Scholar]
- 27.Baier P K, Wolff‐Vorbeck G, Eggstein S.et al Cytokine expression in colon carcinoma. Anticancer Res 2005252135–2139. [PubMed] [Google Scholar]
- 28.Pagès F, Berger A, Camus M.et al Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 20053532654–2666. [DOI] [PubMed] [Google Scholar]