Short abstract
Probiotics organise gut microflora for better regulation of the HPA axis not only in the early years but also during adulthood
It is suggested that daily environmental and emotional stressful life events contribute to the development and reactivation of intestinal inflammation in chronic inflammatory bowel disease (IBD), to the clinical manifestations of irritable bowel syndrome (IBS) and to the development of food allergies by sensitisation of intestinal tissue to oral antigens through an increase of transepithelial permeability and luminal antigen uptake.1,2,3 In animal models of IBD, stress increases the severity of colitis and lowers the threshold for reactivation of mucosal inflammation.4 Stressful stimuli are known to affect gastrointestinal functions such as gut motility and secretion, and to increase paracelullar permeability. Defective epithelial barrier function, which can be measured as increased intestinal permeability, has been implicated in IBS and in IBD, in which it can predict relapse during clinical remission.5,6 In animal models, both acute (partial restraint stress) and chronic (neonatal) stress enhance luminal bacterial adherence and internalisation,7 increase bacterial translocation8 and activate immune reactions within the gut resulting from alterations in gut paracellular permeability.9 Recently, it was also shown that acute stress‐induced hypersensitivity to distension results from an alteration of colonic paracellular permeability.10
Probiotics correcting gut microbiota and gut permeability disturbances induced by stress
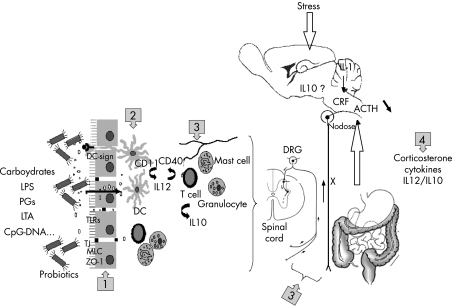
Figure 1 (points 1 and 2) illustrates the following reactions.
Figure 1 Proposed pathways for the correction by probiotics of abnormalities of colonic flora induced by stress. Published reports provide evidence for a potential use of probiotics in gastrointestinal disorders such as inflammatory bowel disease, inflammatory bowel syndrome and food allergies. Stressful life events contribute to the development of these diseases accompanied with gastrointestinal function alterations. Probiotics can correct gut disturbances induced by stress by targeting several sites of action, resulting in a long gut–brain neuroimmune reflex pathway. (1) Locally, at the epithelium site, probiotics have numerous properties—namely, a trophic effect on intestinal villosities, normalisation of gut microbiota, prevention of adhesion of luminal bacteria, and reinforcement of the barrier function by enhancing barrier integrity. (2) At the intestinal mucosal level, probiotics by secretion of soluble factors or directly through activation of specific receptors, such as Toll‐like receptors (TLRs), mannose receptors or dendritic cell‐specific intercellular adhesion molecule 3‐grabbing nonintegrin (DC‐SIGN) from dendritic cells (DCs) can stimulate other immune cells like mast cells, T lymphocytes, normalising the ratio of pro‐ versus anti‐inflammatory cytokines. (3) Through a cytokine neurohumoral route, probiotics may indirectly stimulate afferent nerve fibres—that is, vagus afferents, resulting in (4) a reduction in the levels of systemic corticosterone and adrenocorticotrophic hormone (ACTH).
Numerous abnormalities of the gut flora have been described in patients with IBD and IBS. The presence of bacterial overgrowth in some patients with IBS and the improvement of their symptoms by oral antibiotics illustrate an enteric microbiota involvement in the genesis of the disease.11,12 Recent data support a potential role of probiotics for in alleviating IBS symptoms and suggest that the effects are strain‐dependent. For example, a dietary administration of Lactobacillus plantarum 299 V has been shown to improve abdominal pain and to normalise stool frequency in constipated patients with IBS,13 whereas L rhamnosus GG did not improve pain but had a beneficial effect in patients with IBS who predominantly had diarrhoea.14 In IBDs the probiotic mixture VSL#3 and the non‐pathogenic Escherichia coli Nissle 1917 help to induce remission and to prevent relapse of ulcerative colitis, respectively.15,16
Additionally, accumulated data potentially support a distinct role for probiotics in alleviating stress‐mediated alterations of gut functions, particularly by improving the intestinal barrier through the reduction of altered gut paracellular permeability.17,18,19 However, the mechanism by which probiotics reduce epithelial dysfunction induced by stress remains to be elucidated.
Adherence to intestinal epithelial cells is the first step for colonisation and penetration of pathogenic bacteria. Adding yeast Saccharomyces boulardii to food increases villous length, indicating the trophic effects of this probiotic on the intestinal architecture in pigs. Several lactobacilli adhere to mucosal surfaces inhibiting attachment of pathogenic bacteria, and enhancing secretion of mucins.20 Zareie et al in a recent study showed that probiotic treatment prevents adhesion of luminal bacteria to the surface of the intestinal epithelium.7 These different properties may counterbalance the stress‐induced opening of tight junctions (TJ) by subsequently limiting the paracellular entry of pathogens. TJ are dynamic structures, which represent the major barrier that regulates paracellular permeability. It was recently established that several pathogenic bacteria modulate intestinal permeability by altering TJ.21 For example, live probiotic L farciminis prevents acute stress‐induced colonic paracellular permeability, and sensitivity increased through an action on colonic epithelial barrier resulting from inhibition of myosin light chain phosphorylation and cytoskeleton contraction.22
Increased intestinal or colonic permeability, or both, is described in Crohn's disease, gluten‐sensitive enteropathy, acute gastroenteritis and alcoholism.23,24,25,26 These situations lead to an excessive antigen load, inducing lamina propria immunocyte activation and bacterial translocation. Acute stress in mice and neonatal stress in rats induce long‐term bacterial translocation into mesenteric lymph nodes, spleen and liver.27,28,29 For neonatal stress these alterations occur concomitantly with an increase of gut paracellular permeability, increased number of colonic mast cells and increased colonic mucosal expression of mRNA encoding interleukin (IL)1β, IL2, IL4 IL10 and interferon γ (IFNγ).
Immune changes occurring in the intestine of patients with IBS and in patients with IBD are well documented. It was illustrated that a probiotic, B infantis 35624, alleviates symptoms in IBS associated with normalisation of the proinflammatory cytokine ratio IL12/IL10.28 Moreover, it has been shown that a mixture of probiotics VSL#3 reduced production of proinflammatory cytokines, such as IFNγ, responsible for the increase in intestinal permeability.29 Interestingly, in animals with experimental colitis induced by transfer of CD45Rhigh T cells into SCID mice, Dalmasso et al showed that daily feeds of S boulardii both prevented and improved colitis associated with the decrease of mucosal NF‐κB activity and proinflammatory cytokine (tumour necrosis factor α, IL1β, IFNγ and IL6) expression.30 Consequently, probiotics can reinforce barrier function by secretion of soluble factors that enhance barrier integrity and by regulation of TJ.31 All these observations suggest that the beneficial effect of probiotics in gut disorders provoked by stress may be mediated by interfering in a secondary step on a neuroimmune regulation consecutive to a primary digestive epithelial barrier impairment.
Probiotics correcting dysfunction of the hypothalamic‐pituitary‐adrenal (HPA) axis induced by stress
Figure 1 (points 2 and 3) illustrates the following reactions.
The enteric and central nervous systems are linked bidirectionally by the sympathetic and parasympathetic pathways forming the brain–gut axis. Acute stress induces distinct motor effects in the upper and lower gastrointestinal tract. For example, stress increases colonic motility, circulating corticosterone levels and release of corticotrophin‐releasing factor (CRF) at the paraventricular nucleus and locus coeruleus. These effects are blocked by CRF antagonists and reproduced by intracerebroventricular administration of CRF.32 In this issue of Gut, Gareau et al add an interesting effect to the existing knowledge of probiotic activity (see page 1522).33 These authors illustrates that probiotics can ameliorate the enhanced HPA axis activity induced by maternal separation stress in rats.
The enteric nervous system (ENS) influences all gastrointestinal functions (secretion, motility and sensitivity). Nowadays, only a few investigations demonstrate the influence of probiotics on the neurochemical modulation of the ENS. For example, Kamm et al, have shown that S boulardii significantly decrease the number of calbindin‐positive neurones in pigs.34 Barreau et al described the normalisation of greater enteric cholinergic changes observed in maternal separated pups previously treated by a mixture of Lrhamnosus and L helveticus. According to their own and other previous studies, illustrating that early traumatic experience modifies CRF and corticosterone level‐induced neuroimmunoendocrine dysfunctions in alterations of gut mucosal barrier,35 these observations added the important observation that treatment with L rhamnosus and L helveticus significantly reduced the enhanced level of circulating corticosterone levels in MS pups to control values; this suggests that probiotics normalise the activity of the HPA axis.
Enhanced HPA axis activity has been shown in patients with IBS with a history of childhood abuse, demonstrated by higher basal cortisol levels than in control subjects.36 So far, there is little information about HPA axis modulation related to the composition of the gut microflora. Sudo et al have shown that commensal microbiota are environmental factors that regulate the development of the HPA stress response.37 Indeed, increased responses to restraint stress‐associated HPA axis perturbation have been observed in germ‐free mice under normal intestinal bacterial colonisation. These observations were reversed by a pretreatment with Bifidobaterium animalis, illustrated by a reduction of corticosterone and adrenocorticotrophic hormone levels.
However, the mechanism by which probiotics can regulate the gut–brain axis response remains unknown. In their paper, Gareau et al, focused their hypothesis on the involvement of enterochromaffin cells in the probiotic HPA axis modulating effect, since enterochromaffin cells are affected by enteric flora and can release neuroendocrine mediators, which can activate afferent nerves that project to central structures connection to the HPA axis. However as mentioned before, a cytokine neurohumoral route pathway can be advanced. It has now been clearly shown that several cytokines, like IL1 centrally released after an acute stress session, increase the secretory activity of the HPA axis.38 Also, stress‐altering gut paracellular permeability integrity induces bacterial translocation from the gut to the liver, to mesenteric lymph nodes and to the spleen.8,9 In this context it is reasonable to suppose that endotoxins as well as components of the bacterial cell wall—that is, peptidoglycan, stimulate immune cells within the gut and, in turn, directly or in relay via a nervous pathway promote the release of cytokines.
Probiotics role in the gut–brain axis
Figure 1 (points 1–4) illustrates the following reactions.
Rescigno et al have described a new mechanism for bacterial uptake of the gut lumen by extending dendrites between intestinal epithelial cells.39 Dendritic cells (DCs) can discriminate between pathogenic compounds through the expression of various pattern‐recognition receptors. Among these receptors are the families of the Toll‐like receptors and C‐type leptins such as dendritic cell‐specific intercellular adhesion molecule 3‐grabbing nonintegrin (DC‐SIGN) and mannose receptor, which recognise carbohydrate structures on pathogens and self‐glycoproteins.
Recently, Smits et al have shown that selective probiotic bacteria that is, L reuteri and L casei, induce IL10 production by T regulatory cells by modulating DC function through DC‐SIGN binding. In another way, transfers of selected probiotic‐treated DCs confer protection against 2,4,6‐trinitrobenzene sulphonic acid‐induced colitis in mice.40 These probiotics specifically stimulate DC regulatory functions requiring MyD88, TLR2 and NOD2‐dependent signalling pathways and the induction of CD4+ CD25+ regulatory cells in an IL10 independent pathway.41 Taken together these observations illustrate a specific strain mechanism of action of probiotics on DCs regulatory functions by targeting different pattern‐recognition receptor pathways and, in consequence, the release or not of IL10. IL10 and its receptor are expressed in pituitary and hypothalamic tissues and IL10 can regulate gene expression in cells of HPA axis origin.42 Moreover, wild‐type mice produce less corticosterone than IL10 knockout mice during immune and physiological stress, suggesting that IL10 may be an important endogenous regulator of HPA axis activity.
In their article Gareau et al suggest that probiotics can have an HPA modulating effect on stressors that appear only during the early years—that is, maternal separation stress. However, we carried out investigations on adult rats submitted to an acute restraint stress session (2 hours), and obtained similar results—that is, lower corticosterone levels in animals pretreated with the L farciminis strain, and also able to release IL10 (unpublished data). Consequently, these observations suggest that organisation of gut microflora for better regulation of the HPA axis depends not only on the early years but also the adult years.
Therefore, studies should follow the new finding of Gareau et al taking into account the involvement of a complex long neuroimmune reflex pathway starting at the intestinal level in order to elucidate the beneficial effect of probiotics in stressful situations.
Footnotes
Conflict of interest: None.
References
- 1.Mayer E A. The neurobiology of stress and gastrointestinal disease. Gut 200047861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins S M. Stress and the gastrointestinal tract IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol 2001280G315–G318. [DOI] [PubMed] [Google Scholar]
- 3.Yang P C, Jury J, Soderholm J D.et al Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol 20061683–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gue M, Bonbonne C, Fioramonti J.et al Stress‐induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol 1997272G84–G91. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop S P, Hebden J, Campbell E.et al Abnormal intestinal permeability in subgroups of diarrhea‐predominant irritable bowel syndromes. Am J Gastroenterol 20061011288–1294. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt J, Vogelsang H, Hubl W.et al Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 19933411437–1439. [DOI] [PubMed] [Google Scholar]
- 7.Zareie M, Johnson‐Henry K, Jury J.et al Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006551553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaude J, Salvador‐Cartier C, Fioramonti J.et al Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut 200655655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gareau F, Ferrier L, Fioramonti J.et al Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 200453501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ait‐Belgnaoui A, Bradesi S, Fioramonti J.et al Acute stress‐induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 2005113141–147. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary C, Quigley E M. Bowel bacterial overgrowth, celiac disease, and IBS: what are the real associations? Am J Gastroenterol 200398720–722. [DOI] [PubMed] [Google Scholar]
- 12.Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol 200398839–843. [DOI] [PubMed] [Google Scholar]
- 13.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double‐blind, randomized study on the efficacy of Lactobacillus plantarum 299 V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 2001131143–1147. [DOI] [PubMed] [Google Scholar]
- 14.O'Sullivan M A, O'Morain C A. Bacterial supplementation in the irritable bowel syndrome. A randomised double‐blind placebo‐controlled crossover study. Dig Liver Dis 200032294–301. [DOI] [PubMed] [Google Scholar]
- 15.Gionchetti P, Rizzello F, Helwig U.et al Prophylaxis of pouchitis onset with probiotic therapy: a double‐blind, placebo‐controlled trial. Gastroenterology 20031241202–1209. [DOI] [PubMed] [Google Scholar]
- 16.Bibiloni R, Fedorak R N, Tannock G W.et al VSL#3 probiotic‐mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 20051001539–1546. [DOI] [PubMed] [Google Scholar]
- 17.Cucchiara S, Falconieri P, Di Nardo G.et al New therapeutic approach in the management of intestinal disease: probiotics in intestinal disease in paediatric age. Dig Liver Dis 200234S44–S47. [DOI] [PubMed] [Google Scholar]
- 18.Resta‐Lenert S, Barrett K E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 200352988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eutamene H, Lamine F, Chabo C.et al Synergy between Lactobacillus paracasei and its bacterial products to counteract stress‐induced gut permeability and sensitivity increase in rats. J Nutr 20071371–7. [DOI] [PubMed] [Google Scholar]
- 20.Mack D R, Michail S, Wei S.et al Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 1999276G941–G950. [DOI] [PubMed] [Google Scholar]
- 21.Fasano A, Nataro J P. Intestinal epithelial tight junctions as targets for enteric bacteria‐derived toxins. Adv Drug Deliv Rev 200456795–807. [DOI] [PubMed] [Google Scholar]
- 22.Ait‐Belgnaoui A, Han W, Lamine F.et al Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 2006551090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soderholm J D, Peterson K H, Olaison G.et al Epithelial permeability to proteins in the noninflamed ileum of Crohn's disease? Gastroenterology 199911765–72. [DOI] [PubMed] [Google Scholar]
- 24.Cobden I, Rothwell J, Axon A T. Intestinal permeability and screening tests for coeliac disease. Gut 198021512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuckerman M J, Watts M T, Bhatt B D.et al Intestinal permeability to [51Cr]EDTA in infectious diarrhea. Dig Dis Sci 1993381651–1657. [DOI] [PubMed] [Google Scholar]
- 26.Bjarnason I, Peters T J, Wise R J. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 19841179–182. [DOI] [PubMed] [Google Scholar]
- 27.Ferrier L, Mazelin L, Cenac N.et al Stress‐induced disruption of colonic epithelial barrier: role of interferon‐gamma and myosin light chain kinase in mice. Gastroenterology 2003125795–804. [DOI] [PubMed] [Google Scholar]
- 28.O'Mahony L, McCarthy J, Kelly P.et al Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005128541–551. [DOI] [PubMed] [Google Scholar]
- 29.Madsen K, Cornish A, Soper P.et al Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001121580–591. [DOI] [PubMed] [Google Scholar]
- 30.Dalmasso G, Cottrez F, Imbert V.et al Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology 20061311812–1825. [DOI] [PubMed] [Google Scholar]
- 31.Lammers K M, Helwig U, Swennen E.et al Effect of probiotic strains on interleukin 8 production by HT29/19A cells. Am J Gastroenterol 2002971182–1186. [DOI] [PubMed] [Google Scholar]
- 32.Gue M, Junien J L, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin‐releasing factor. Gastroenterology 1991100964–970. [DOI] [PubMed] [Google Scholar]
- 33.Gareau M G, Jury J, MacQueen G.et al Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007561522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamm K, Hoppe S, Breves G.et al Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil 20041653–60. [DOI] [PubMed] [Google Scholar]
- 35.Barreau F, Cartier C, Leveque M.et al Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin‐releasing factor and nerve growth factor interplay. J Physiol 2007580347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinan T G, Quigley E M, Ahmed S M.et al Hypothalamic‐pituitary‐gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 2006130304–311. [DOI] [PubMed] [Google Scholar]
- 37.Sudo N, Chida Y, Aiba Y.et al Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. J Physiol 2004558263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eutamene H, Theodorou V, Fioramonti J.et al Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin‐1 and corticotropin‐releasing factor release in rats. J Physiol 2003553959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rescigno M, Urbano M, Valzasina B.et al Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 20012361–367. [DOI] [PubMed] [Google Scholar]
- 40.Smits H H, Engering A, van der Kleij D.et al Selective probiotic bacteria induce IL‐10‐producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell‐specific intercellular adhesion molecule 3‐grabbing nonintegrin. J Allergy Clin Immunol 20051151260–1267. [DOI] [PubMed] [Google Scholar]
- 41.Foligne B, Zoumpopoulou G, Dewulf J.et al A key role of dendritic cells in probiotic functionality. PLoS ONE 20072e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu H, Rady P L, Juelich T.et al Interleukin‐10 regulated gene expression in cells of hypothalamic‐pituitary‐adrenal axis origin. Cell Mol Neurobiol 200727161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]