Abstract
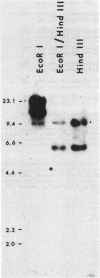
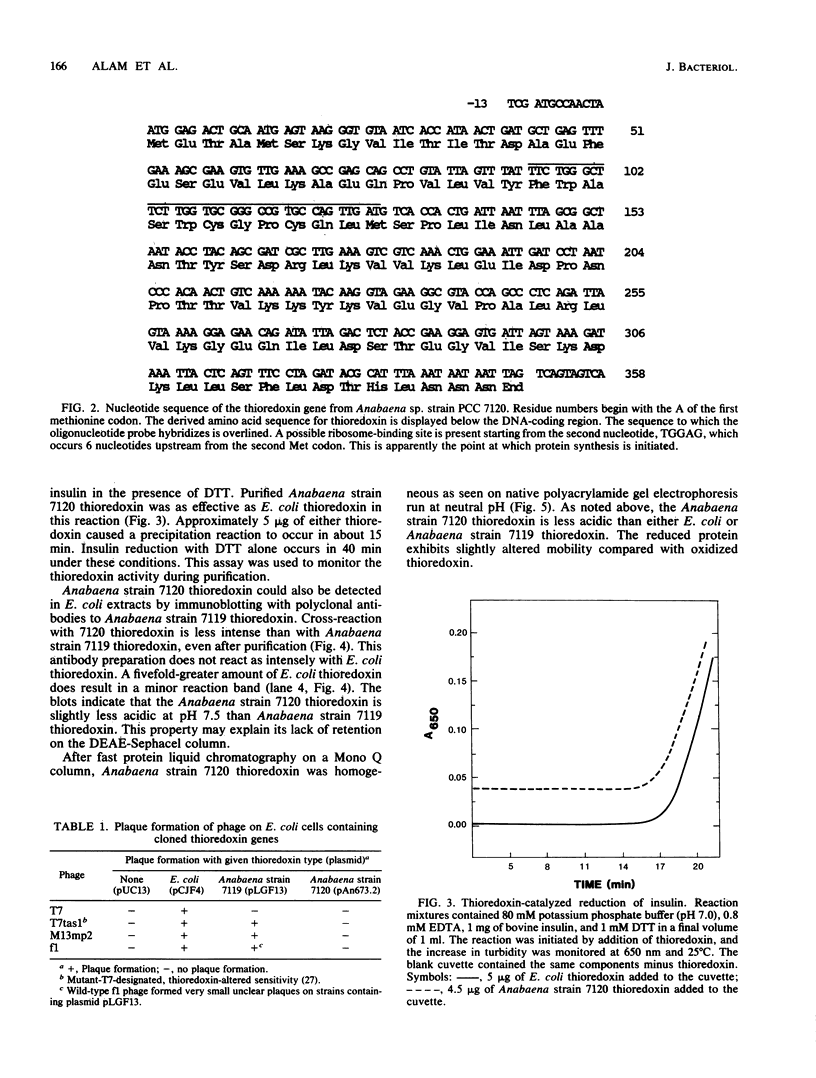
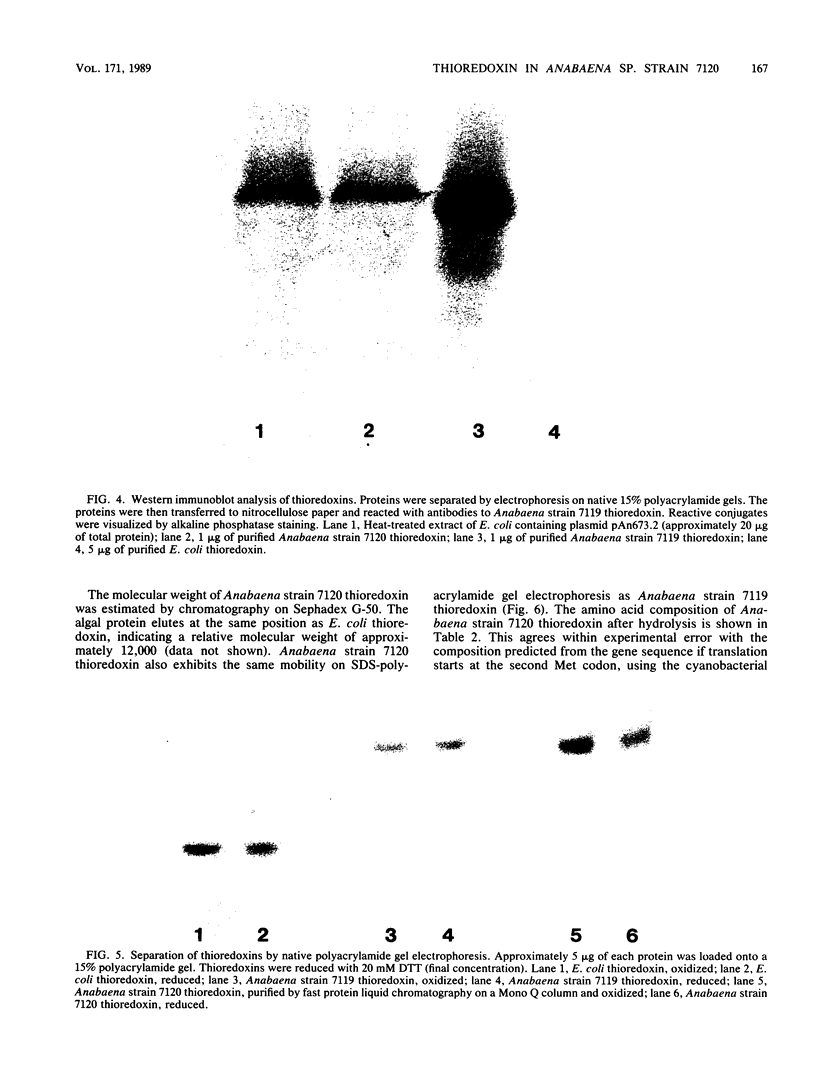
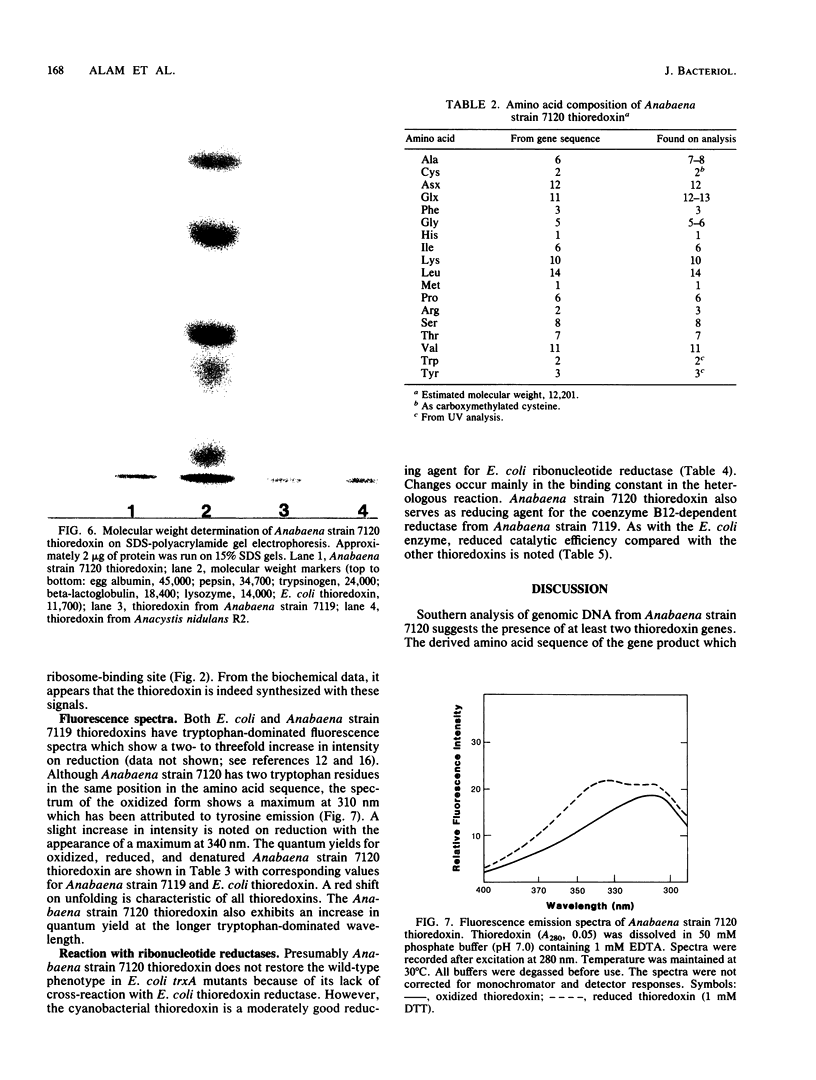
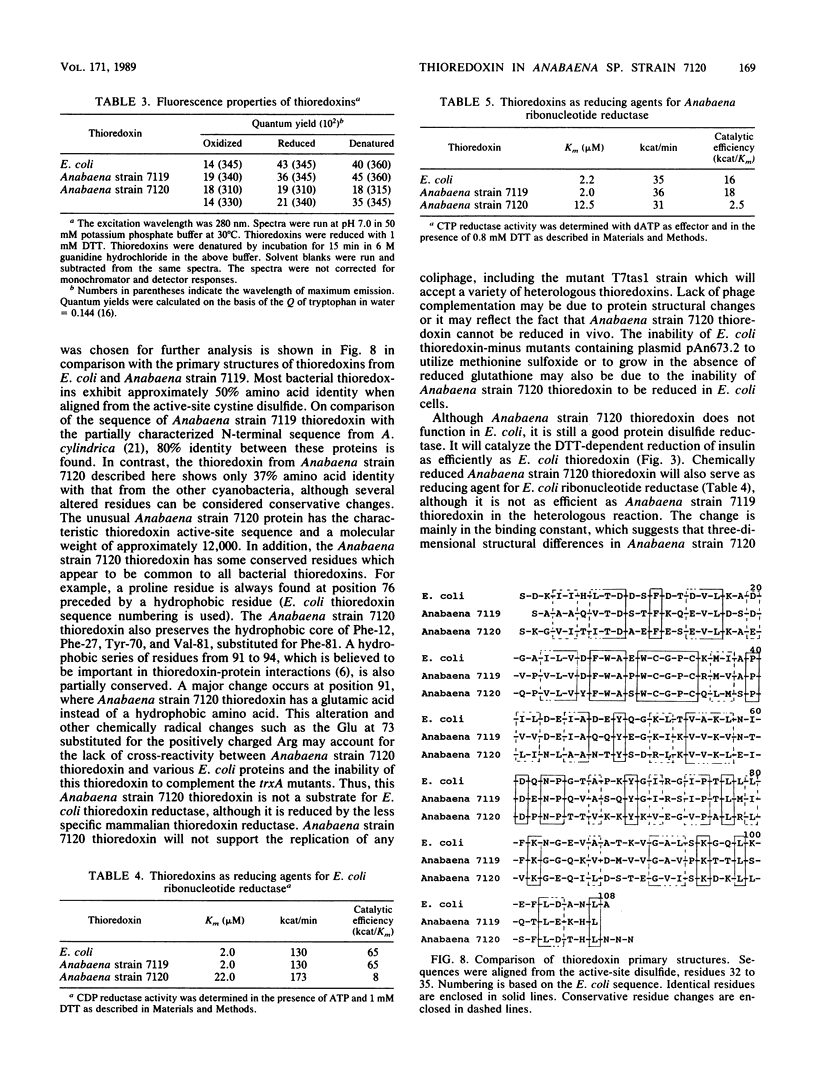
Two sequences with homology to a thioredoxin oligonucleotide probe were detected by Southern blot analysis of Anabaena sp. strain PCC 7120 genomic DNA. One of the sequences was shown to code for a protein with 37% amino acid identity to thioredoxins from Escherichia coli and Anabaena sp. strain PCC 7119. This is in contrast to the usual 50% homology observed among most procaryotic thioredoxins. One gene was identified in a library and was subcloned into a pUC vector and used to transform E. coli strains lacking functional thioredoxin. The Anabaena strain 7120 thioredoxin gene did not complement the trxA mutation in E. coli. Transformed cells were not able to use methionine sulfoxide as a methionine source or support replication of T7 bacteriophage or the filamentous viruses M13 and f1. Sequence analysis of a 720-base-pair TaqI fragment indicated an open reading frame of 115 amino acids. The Anabaena strain 7120 thioredoxin gene was expressed in E. coli, and the protein was purified by assaying for protein disulfide reductase activity, using insulin as a substrate. The Anabaena strain 7120 thioredoxin exhibited the properties of a conventional thioredoxin. It is a small heat-stable redox protein and an efficient protein disulfide reductase. It is not a substrate for E. coli thioredoxin reductase. Chemically reduced Anabaena strain 7120 thioredoxin was able to serve as reducing agent for both E. coli and Anabaena strain 7119 ribonucleotide reductases, although with less efficiency than the homologous counterparts. The Anabaena strain 7120 thioredoxin cross-reacted with polyclonal antibodies to Anabaena strain 7119 thioredoxin. However, this unusual thioredoxin was not detected in extracts of Anabaena strain 7120, and its physiological function is unknown.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASAHI T., BANDURSKI R. S., WILSON L. G. Yeast sulfate-reducing system. II. Enzymatic reduction of protein disulfide. J Biol Chem. 1961 Jun;236:1830–1835. [PubMed] [Google Scholar]
- Alam J., Whitaker R. A., Krogmann D. W., Curtis S. E. Isolation and sequence of the gene for ferredoxin I from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1986 Dec;168(3):1265–1271. doi: 10.1128/jb.168.3.1265-1271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Brennan T., Anderson L. E. Thioredoxin-like Activity of Thylakoid Membranes: THIOREDOXIN CATALYZING THE REDUCTIVE INACTIVATION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE OCCURS IN BOTH SOLUBLE AND MEMBRANE-BOUND FORM. Plant Physiol. 1980 Oct;66(4):605–608. doi: 10.1104/pp.66.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Clancey C. J., Gilbert H. F. Thiol/disulfide exchange in the thioredoxin-catalyzed reductive activation of spinach chloroplast fructose-1,6-bisphosphatase. Kinetics and thermodynamics. J Biol Chem. 1987 Oct 5;262(28):13545–13549. [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason F. K., Frick T. D. Adenosylcobalamin-dependent ribonucleotide reductase from the blue-green alga, Anabaena sp. Purification and partial characterization. J Biol Chem. 1980 Aug 25;255(16):7728–7733. [PubMed] [Google Scholar]
- Gleason F. K., Holmgren A. Isolation and characterization of thioredoxin from the cyanobacterium, Anabaena sp. J Biol Chem. 1981 Aug 25;256(16):8306–8309. [PubMed] [Google Scholar]
- Gleason F. K., Whittaker M. M., Holmgren A., Jörnvall H. The primary structure of thioredoxin from the filamentous cyanobacterium Anabaena sp. 7119. J Biol Chem. 1985 Aug 15;260(17):9567–9573. [PubMed] [Google Scholar]
- Holmgren A., Reichard P. Thioredoxin 2: cleavage with cyanogen bromide. Eur J Biochem. 1967 Sep;2(2):187–196. doi: 10.1111/j.1432-1033.1967.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Tryptophan fluorescence study of conformational transitions of the oxidized and reduced form of thioredoxin. J Biol Chem. 1972 Apr 10;247(7):1992–1998. [PubMed] [Google Scholar]
- Huber H. E., Russel M., Model P., Richardson C. C. Interaction of mutant thioredoxins of Escherichia coli with the gene 5 protein of phage T7. The redox capacity of thioredoxin is not required for stimulation of DNA polymerase activity. J Biol Chem. 1986 Nov 15;261(32):15006–15012. [PubMed] [Google Scholar]
- Ip S. M., Rowell P., Aitken A., Stewart W. D. Purification and characterization of thioredoxin from the N2-fixing cyanobacterium Anabaena cylindrica. Eur J Biochem. 1984 Jun 15;141(3):497–504. doi: 10.1111/j.1432-1033.1984.tb08220.x. [DOI] [PubMed] [Google Scholar]
- Kallis G. B., Holmgren A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J Biol Chem. 1980 Nov 10;255(21):10261–10265. [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Levine R. L., Federici M. M. Quantitation of aromatic residues in proteins: model compounds for second-derivative spectroscopy. Biochemistry. 1982 May 25;21(11):2600–2606. doi: 10.1021/bi00540a004. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Fuchs J. A., McFarlan S. C., Hogenkamp H. P. Cloning, expression, and nucleotide sequence of a gene encoding a second thioredoxin from Corynebacterium nephridii. J Biol Chem. 1987 Sep 5;262(25):12114–12119. [PubMed] [Google Scholar]
- Lim C. J., Geraghty D., Fuchs J. A. Cloning and nucleotide sequence of the trxA gene of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):311–316. doi: 10.1128/jb.163.1.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. J., Gleason F. K., Fuchs J. A. Cloning, expression, and characterization of the Anabaena thioredoxin gene in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1258–1264. doi: 10.1128/jb.168.3.1258-1264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. J., Gleason F. K., Jacobson B. A., Fuchs J. A. Characterization of Escherichia coli-Anabaena sp. hybrid thioredoxins. Biochemistry. 1988 Mar 8;27(5):1401–1408. doi: 10.1021/bi00405a002. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Haller B., Fuchs J. A. Thioredoxin is the bacterial protein encoded by fip that is required for filamentous bacteriophage f1 assembly. J Bacteriol. 1985 Feb;161(2):799–802. doi: 10.1128/jb.161.2.799-802.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication invitro. Bacteriophage T7 DNA polymerase: an an emzyme composed of phage- and host-specific subunits. J Biol Chem. 1975 Jul 25;250(14):5515–5522. [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M. L., Schiff J. A. Sulfate-reducing pathway in Escherichia coli involving bound intermediates. J Bacteriol. 1976 Mar;125(3):923–933. doi: 10.1128/jb.125.3.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Whittaker M. M., Gleason F. K. Isolation and characterization of thioredoxin f from the filamentous cyanobacterium, Anabaena sp. 7119. J Biol Chem. 1984 Nov 25;259(22):14088–14093. [PubMed] [Google Scholar]