Abstract
Background
Hepatic encephalopathy is considered to be mainly caused by increased ammonia metabolism of the brain. If this hypothesis is true, cerebral glucose utilisation, which is considered to represent brain function, should be closely related to cerebral ammonia metabolism. The aim of the present study was to analyse whether cerebral ammonia and glucose metabolism in cirrhotic patients with early grades of hepatic encephalopathy are as closely related as could be expected from current hypotheses on hepatic encephalopathy.
Methods
13N‐ammonia and 18F‐fluorodesoxyglucose positron emission tomography, magnetic resonance imaging and magnetic resonance spectroscopy (MRS) were performed in 21 cirrhotic patients with grade 0–1 hepatic encephalopathy. Quantitative values of cerebral ammonia uptake and retention rate and glucose utilisation were derived for several regions of interest and were correlated with the MRS data of the basal ganglia, white matter and frontal cortex.
Results
A significant correlation between plasma ammonia levels and cerebral ammonia metabolism, respectively, and MRS alterations could be shown only for white matter. In contrast, MRS alterations in all three regions studied were significantly correlated with the glucose utilisation of several brain regions. Cerebral ammonia and glucose metabolism were not correlated.
Conclusion
Increase of cerebral ammonia metabolism is an important but not exclusive causal factor for the development of hepatic encephalopathy.
Keywords: liver cirrhosis, early hepatic encephalopathy, magnetic resonance spectroscopy, positron emission tomography
Cerebral ammonia metabolism is considered to play a key role in the pathophysiology of hepatic encephalopathy in acute and chronic liver failure.1,2 According to current hypotheses, hyperammonemia caused by liver dysfunction leads to an increased brain supply of ammonia. There the removal of ammonia totally depends on its astrocytic metabolism into glutamine.3 With an increasing ammonia load the astrocytic content of glutamine also increases, leading to cell swelling if the accumulation of glutamine cannot be counterbalanced by release of the osmolyte myo‐inositol.4 Cell swelling is considered to be one of the major mechanisms in the development of hepatic encephalopathy. A second mechanism is the modulatory effect of ammonia on inhibitory and excitatory neurotransmission.1,5
Although there is a role for ammonia in the pathogenesis of hepatic encephalopathy, without doubt the relationship between plasma ammonia levels and the severity of cerebral dysfunction is controversial, and it has been suggested that the toxic effects of ammonia are mediated by alteration of its metabolism rather than by itself.3,5
Modern brain imaging methods allow the study of different steps of cerebral ammonia metabolism and its functional consequences in humans in vivo. Cerebral ammonia uptake and metabolism, for example, can be studied using 13N‐ammonia positron emission tomography (PET), whereas the glutamine content of the brain can be evaluated by magnetic resonance spectroscopy (MRS). 18F‐fluorodesoxyglucose (FDG)‐PET measures cerebral glucose utilisation, which is dedicated to the maintenance of ion gradients after depolarisation on principle, and can be considered to represent brain function.
The aim of the present study was to look for interdependencies between regional cerebral ammonia metabolism and glucose utilisation in patients with early grades of hepatic encephalopathy. As an increased cerebral supply with and metabolism of ammonia is considered a key factor in the pathogenesis of hepatic encephalopathy,5 a significant interdependence between the different aspects of cerebral ammonia metabolism (plasma ammonia levels, ammonia uptake and retention rate, metabolic rate of ammonia and glutamate/glutamine signal intensities) and neuronal function as represented by the FDG‐PET data was expected.
Parts of the results have been presented in abstract form.6
Patients and methods
Twenty‐one patients with biopsy‐confirmed liver cirrhosis (16 men, five women) aged 32–74 (50.4 ± 11.8) years underwent magnetic resonance imaging (MRI) and 1H MRS of the basal ganglia, white matter and frontal gray matter. In 17 patients (12 men, five women, aged 49.1 ± 12.2 years) 13N‐ammonia PET of the brain was also performed. Eighteen of the patients (13 men, five women, aged 49.3 ± 11.9 years) underwent FDG‐PET of the brain.
Fourteen patients suffered from posthepatic cirrhosis, two from primary biliary cirrhosis, one from secondary biliary cirrhosis, two from cryptogenic cirrhosis, one from haemochromatosis, and one from alcoholic cirrhosis.
Applying the criteria of Pugh et al.,7 the grade of liver dysfunction was scored Child A in four, Child B in nine and Child C in eight patients (13N‐ammonia PET: Child A: 4, Child B: 7, Child C: 6; FDG‐PET: Child A: 4, Child B: 8, Child C: 6). The grading of hepatic encephalopathy was done with regard to the results of a comprehensive neurological examination and the results of the Present State Examination (PSE) Syndrome test, a battery of five neuropsychological tests, which has been recommended for the assessment of minimal hepatic encephalopathy.8,9 Those patients who presented with normal neurological examination and normal PSE‐Syndrome test results were graded hepatic encephalopathy 0, those with normal results in the physical examination but pathological PSE‐Syndrome test results as minimal hepatic encephalopathy, and those with psychomotor slowing but no disorientation as grade I hepatic encephalopathy. Nine patients had grade 0 hepatic encephalopathy, six minimal hepatic encephalopathy and six grade I hepatic encephalopathy (13N‐ammonia PET: hepatic encephalopathy 0: 7, minimal hepatic encephalopathy: 6, hepatic encephalopathy I: 4; 18FDG‐PET: hepatic encephalopathy 0: 8, minimal hepatic encephalopathy: 6, hepatic encephalopathy I: 4). In addition to the PSE‐Syndrome test, the cancelling d test10 was performed. Clinical and psychometric examination, MRI, MRS and PET studies were performed within 24 hours. All patients gave their written informed consent. The study was, a priori, approved by the local ethics committee. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Magnetic resonance imaging/MRS
MRI and MRS were performed using a 1.5 T scanner (GE Medical Systems, Milwaukee, Wisconsin, USA). Transverse and coronal T1‐weighted images (TR 500 ms/TE 15 ms/slice thickness 5 mm/slice gap 1.5 mm) were obtained. These images were used for the co‐registration with PET studies and for the definition of regions of interest (ROI) for the MRS study. The ROI, approximately 8 cm3 in size, were placed within the basal ganglia, the parietal white matter and the frontal gray matter (fig 1). Within these ROI localised proton spectra were achieved using the STEAM/VOSY technique (TR 3000 ms/TE 18 ms/TM 14 ms/128 averages) using the PROBE/SV packet (GE Medical Systems). For quantification of the metabolite ratios (N‐acetyl‐aspartate; NAA, glutamate/glutamine, myo‐inositol, choline) relative to creatine, the spectra were transferred to a workstation for calculation of the peak area integrals.
Figure 1 Location of the magnetic resonance spectroscopy voxels used in this study.
Positron emission tomography
All patients were investigated using a Siemens 951/31 ECAT tomograph in standard head position. 13N‐ammonia PET and FDG‐PET were performed consecutively. Thirty‐one slices (plane separation 3.4 mm) were obtained simultaneously. The axial and transaxial resolution of the reconstructed image (filtered backprojection, Hann filter, cut‐off frequency 0.4, 128 × 128 matrix, pixel size 1.9 mm) was approximately 8 mm full‐width half maximum. Attenuation correction was performed with a measured transmission scan (10 minutes). After a slow bolus intravenous injection of 740 MBq 13N‐ammonia, the data were recorded dynamically (12 × 10 s, 5 × 30 s, 2 × 120 s, 1 × 900 s frames; = 23.5 minutes). Arterial blood samples were taken simultaneously. After reconstruction (including correction of the PET data for decay and attenuation) parametric images were created. Like Keiding et al.11 we applied an irreversible two tissue compartment model with three transport rate constants for kinetic analysis (the term “irreversible” refers to the fact that the tracer is, at least partly, trapped in tissue, namely the second compartment of the model).12 The model was originally developed for the quantification of ammonia studies of the heart 13 and is comparable to the model used to study cerebral glucose utilisation by FDG‐PET. It is in accord with the findings of Lockwood and colleagues14,15 on ammonia metabolism in humans. For the generation of parametric images we used a modified linearised approach16 with automatic correction of input function delay and dispersion.17 In addition, correction for ammonia metabolism was performed in accordance with the data of Rosenspire et al.18 Images were generated for the rate constants K1, K2, K3, Km and the fractional blood volume.19 For details of the method see Ahl et al.12
The cerebral metabolic rate for ammonia (CMRA) was not calculated in this study as arterial plasma ammonia levels were not available for all patients. According to recent data of Ong et al.,20 however, there is a close correlation between venous and arterial plasma ammonia levels with a correlation coefficient of 0.86639 (p<0.0001). Ong et al.20 were kind enough to provide us with the linear regression equation that describes the regression between venous and arterial plasma ammonia levels in a group of 53 patients with grades 0 and 1 hepatic encephalopathy: arterial plasma ammonia level 18.74 + 0.892 × venous plasma ammonia level. On the basis of this equation we estimated the arterial plasma ammonia levels in our patients using their venous ammonia levels and used these data for an estimation of the metabolic rate for ammonia (estimated CMRA).
Sixty minutes after the injection of 13N‐ammonia, 370 MBq FDG were administered intravenously after a second transmission scan had been performed. The tissue activity concentration was measured dynamically over a period of 50 minutes (6 × 20 s, 3 × 60 s, 2 × 150 s, 2 × 300 s, 3 × 600 s frames; … 50 minutes). To obtain the tracer input function, 20 arterialised blood samples of 1 ml each were taken according to the dynamic measurement protocol at midframe time. Quantification of glucose metabolism was performed using the method of Patlak et al.19
For both glucose and ammonia metabolism, ROI were defined after co‐registration of the PET and MRI studies of each patient. ROI were defined on the respective MRI studies using a stereotactical anatomical atlas.21 Defined ROI were the thalamus, caudate nucleus, lenticular nucleus, hippocampus, cerebellum, frontomedial and frontolateral cortex, motor cortex, sensory cortex, parieto‐occipital cortex and parietal white matter. Absolute values for K1 and Km for ammonia PET and the cerebral metabolic rate of glucose were extracted as a maximum of the defined ROI. In addition, each regional value was individually normalised to global data.
Statistical analysis
For each parameter the closed test procedure was used to compare the different patient groups. Thereby, the global null hypothesis was tested using analysis of variance, and pairwise comparison was based on the t‐test. Dependencies between MRI, MRS and PET parameters were analysed by pairwise Pearson correlations. Multiplicity within each MRS and MRI parameter with respect to the PET parameters was controlled for by the Bonferroni–Holm procedure. Furthermore, for each PET parameter as a dependent variable a stepwise multiple regression analysis with backward elimination was performed on MRI and MRS parameters by applying an exclusion limit of p>0.05. The results are described as standardised regression coefficients and the proportion of explained variance, β and R2. All tests were performed two‐sided on the level α = 0.05.
Results
The key findings of our study relate to the correlation between different parameters of cerebral ammonia metabolism and cerebral glucose metabolism in cirrhotic patients with mild grades of hepatic encephalopathy. As cerebral MRS alterations, especially the increase in the glutamate–glutamine/creatine signal intensity ratio and the decrease in the myo‐inositol/creatine ratio, are considered to represent the metabolic endpoint of increased ammonia metabolism in cirrhotic patients we used the MRS results as a reference parameter to detect possible interrelationships between cerebral ammonia and glucose metabolism.
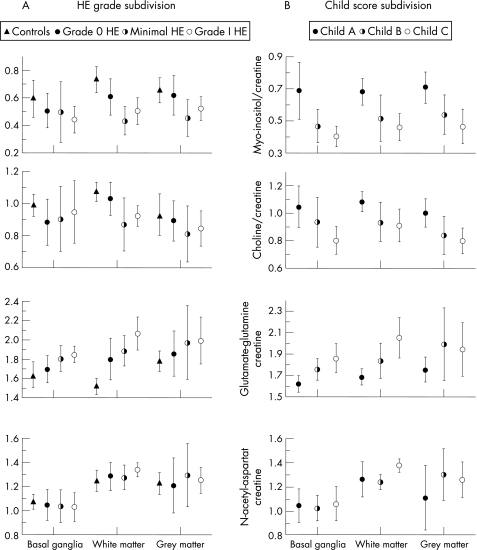
The MRS alterations found in our patient group resembled those published by other groups in the past (fig 2, table 1),4,21,22,23,24,25,26 whereas the NAA/creatine ratio remained constant, the myo‐inositol/creatine and choline/creatine signal ratio decreased and the glutamate–glutamine/creatine ratio increased with increasing grade of encephalopathy (table 1). However, except for the myo‐inositol/creatine and the choline/creatine ratio in the white matter the differences between the three patient groups (hepatic encephalopathy 0, minimal hepatic encephalopathy, hepatic encephalopathy I) were not significant.
Figure 2 Magnetic resonance spectroscopy results of the patients subdivided with regard to their grade of hepatic encephalopathy (HE; on the left) and with regard to their grade of liver dysfunction. The metabolite ratios of normal controls are added to this figure to give an impression of the extent of the metabolic alterations in the different patient groups compared with normal controls.
Table 1 Magnetic resonance spectroscopy data of the patient group subdivided according to the grade of encephalopathy and the grade of liver dysfunction, respectively.
| Myo‐inositol/creatine | Choline/creatine | Glutamate–glutamine/creatine | NAA/creatine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BG | WM | GM | BG | WM | GM | BG | WM | GM | BG | WM | GM | |
| HE 0 | 0.51 ± 0.13 | 0.61 ± 0.13 | 0.62 ± 0.14 | 0.89 ± 0.14 | 1.03 ± 0.10 | 0.89 ± 0.13 | 1.70 ± 0.14 | 1.80 ± 0.22 | 1.86 ± 0.23 | 1.05 ± 0.13 | 1.29 ± 0.12 | 1.21 ± 0.23 |
| Minimal HE | 0.50 ± 0.22 | 0.43 ± 0.10 | 0.45 ± 0.13 | 0.90 ± 0.20 | 0.87 ± 0.16 | 0.81 ± 0.17 | 1.81 ± 0.14 | 1.89 ± 0.16 | 1.97 ± 0.39 | 1.04 ± 0.14 | 1.28 ± 0.10 | 1.30 ± 0.26 |
| HE I | 0.44 ± 0.10 | 0.50 ± 0.10 | 0.52 ± 0.09 | 0.95 ± 0.20 | 0.92 ± 0.06 | 0.85 ± 0.11 | 1.85 ± 0.09 | 2.07 ± 0.17 | 2.00 ± 0.25 | 1.03 ± 0.12 | 1.34 ± 0.06 | 1.25 ± 0.11 |
| ANOVA | p = 0.71 | p = 0.032 | p = 0.064 | p = 0.791 | p = 0.046 | p = 0.529 | p = 0.070 | p = 0.075 | p = 0.598 | p = 0.965 | p = 0.535 | p = 0.746 |
| R2 0.037 | R2 0.333 | R2 0.264 | R2 0.026 | R2 0.304 | R2 0.068 | R2 0.256 | R2 0.262 | R2 0.055 | R2 0.004 | R2 0.071 | R2 0.032 | |
| Child A | 0.69 ± 0.18 | 0.68 ± 0.08 | 0.71 ± 0.10 | 1.05 ± 0.15 | 1.09 ± 0.07 | 1.00 ± 0.10 | 1.62 ± 0.08 | 1.67 ± 0.08 | 1.76 ± 0.12 | 1.04 ± 0.14 | 1.26 ± 0.15 | 1.11 ± 0.27 |
| Child B | 0.47 ± 0.11 | 0.52 ± 0.14 | 0.54 ± 0.12 | 0.94 ± 0.18 | 0.94 ± 0.14 | 0.84 ± 0.14 | 1.76 ± 0.10 | 1.84 ± 0.08 | 1.99 ± 0.34 | 1.02 ± 0.10 | 1.24 ± 0.06 | 1.30 ± 0.22 |
| Child C | 0.41 ± 0.06 | 0.46 ± 0.08 | 0.47 ± 0.11 | 0.81 ± 0.10 | 0.91 ± 0.12 | 0.80 ± 0.09 | 1.87 ± 0.14 | 2.05 ± 0.19 | 1.95 ± 0.25 | 1.06 ± 0.14 | 1.38 ± 0.06 | 1.26 ± 0.15 |
| ANOVA | p = 0.002 | p = 0.016 | p = 0.009 | p = 0.041 | p = 0.081 | p = 0.032 | p = 0.008 | p = 0.005 | p = 0.377 | p = 0.808 | p = 0.009 | p = 0.305 |
| R2 0.512 | R2 0.384 | R2 0.411 | R2 0.299 | R2 0.256 | R2 0.318 | R2 0.416 | R2 0.465 | R2 0.103 | R2 0.023 | R2 0.423 | R2 0.124 | |
ANOVA, Analysis of variance; BG, basal ganglia; GM, gray matter (frontal cortex); HE, hepatic encephalopathy; NAA, N‐acetyl‐aspartate; WM, white matter.
A comparison of the MRS results after partitioning of the patient group according to the Child score (table 1) showed a significant decrease of the myo‐inositol/creatine signal ratio with an increasing grade of liver dysfunction for all regions studied. The choline/creatine signal intensity significantly decreased with increasing Child grade in the basal ganglia and the gray matter, whereas the level of significance was failed in the white matter. A significant increase in the glutamate–glutamine/creatine ratio with an increasing Child score could be observed in the basal ganglia and white matter, but not in the frontal cortex. The NAA/creatine ratio remained constant with the increasing Child score in the basal ganglia and gray matter, but was significantly increased, especially in Child C compared with Child B patients, in the white matter.27,28,29,30,31
Overall, MRS differences between the groups were more pronounced when the patients were divided into Child classes than into hepatic encephalopathy classes (table 1).
MRS versus psychometric measures
A regression analysis considering the PSE‐Syndrome test results and the MRS data showed a significant negative correlation between the tests' composite score (the so‐called psychometric hepatic encephalopathy score) and the glutamate–glutamine/creatine ratio of the basal ganglia (p = 0.038, r = −0.462). With regard to the single tests of the battery, a significant negative correlation could be observed between the score of the digit symbol test and the glutamate–glutamine/creatine ratio of the basal ganglia (p = 0.02, r = −0.503). The cancelling d test results were positively correlated with the myo‐inositol/creatine and choline/creatine ratio of the white matter (myo‐inositol/creatine p = 0.007, r = 0.617; choline/creatine p = 0.016, r = 0.554) and the frontal cortex (myo‐inositol/creatine p = 0.02, r = 0.526; choline/creatine p = 0.03, r = 0.502).
MRS versus ammonia metabolism
A correlation analysis between venous plasma ammonia levels and MRS data (n = 17) revealed significant results only for the white matter. With increasing plasma ammonia levels the glutamate–glutamine/creatine ratio (p = 0.03, r = 0.535) increased, whereas the myo‐inositol/creatine ratio (p = 0.02, r = −0.557) and the choline/creatine ratio (p = 0.02, r = −0.568) decreased.
A significant correlation was also found between the initial ammonia uptake rate (K1) in the caudate nucleus and the myo‐inositol/creatine and choline/creatine ratio in the basal ganglia voxel (myo‐inositol/creatine p = 0.042, r = −0.498; choline/creatine p = 0.023, r = −0.548).
There was no correlation between the ammonia retention rate (Km) in any region and the MRS results.
Considering the estimated metabolic rate of ammonia we found a significant positive correlation between the eCMRA and the myo‐inositol/creatine (p = 0.0102, r = 0.660) and the choline/creatine (p = 0.0015, r = 0.763) ratio for the white matter, but not for any other region studied.
MRS versus cerebral glucose utilisation
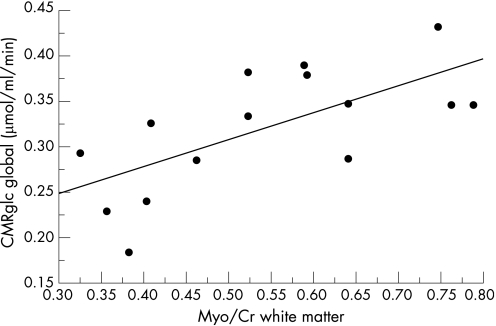
The cerebral glucose utilisation showed significant correlations with the MRS results, both globally as well as with regard to several ROI (table 2). Using a stepwise multiple regression analysis that included the global cerebral glucose utilisation rate (CMRglc) as a dependent and the relative signal intensities of the four metabolites NAA, choline, myo‐inositol and glutamate–glutamine in the three regions studied as independent variables, we found a significant correlation between the MRS data and the CMRglc (p = 0.0058) that was predominantly based on a correlation between CMRglc and the myo‐inositol/creatine signal ratio in the white matter voxel (fig. 3).
Table 2 The table shows the correlation coefficients (r) between magnetic resonance spectroscopy data and glucose utilisation of several brain regions.
| PET glucose utilisation | MRS results | Multiple regression after backward elimination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Basal ganglia | White matter | Gray matter | ||||||||
| Glx/Cr | Myo/Cr | Cho/Cr | Glx/Cr | Myo/Cr | Cho/Cr | Glx/Cr | Myo/Cr | Cho/Cr | ||
| Motor cortex | ||||||||||
| r | −0.716 | 0.787 | 0.560 | −0.832 | 0.826 | 0.737 | −0.283 | 0.807 | 0.530 | |
| p | 0.0012* | 0.0002* | 0.0193 | 0.0001* | <0.0001* | 0.0011* | 0.2715 | <0.0001* | 0.0285 | p<0.0001 |
| β | 0.424 | −0.535 | 0.251 | R2 0.963 | ||||||
| Sensory cortex | ||||||||||
| r | −0.017 | 0.285 | −0.083 | −0.069 | 0.123 | 0.047 | −0.369 | 0.329 | −0.215 | n.s. |
| p | 0.9494 | 0.2668 | 0.7511 | 0.7984 | 0.6512 | 0.8629 | 0.1451 | 0.1969 | 0.4078 | |
| Parieto‐occipital cortex r | ||||||||||
| p | −0.076 | 0.013 | 0.165 | 0.023 | 0.006 | −0.026 | 0.226 | 0.265 | −0.075 | n.s. |
| 0.7721 | 0.9605 | 0.5276 | 0.9340 | 0.9828 | 0.9237 | 0.3841 | 0.3047 | 0.7758 | ||
| Frontolateral cortex | ||||||||||
| r | −0.086 | −0.466 | −0.380 | 0.235 | −0.531 | −0.606 | 0.379 | −0.639 | −0.344 | |
| p | 0.7427 | 0.0594 | 0.1327 | 0.3818 | 0.0342 | 0.0128 | 0.1335 | 0.0058 | 0.1768 | p = 0.0032 |
| β | −0.689 | R2 0.475 | ||||||||
| Frontomedial cortex | ||||||||||
| r | 0.182 | −0.564 | −0.641 | 0.252 | −0.408 | −0.424 | 0.528 | −0.422 | −0.424 | |
| p | 0.4853 | 0.0183 | 0.0056 | 0.3465 | 0.1169 | 0.1015 | 0.0294 | 0.0915 | 0.0899 | p = 0.0089 |
| β | −0.506 | 0.409 | R2 0.516 | |||||||
| Hippocampus | ||||||||||
| r | 0.730 | −0.425 | −0.326 | 0.503 | −0.499 | −0.474 | 0.005 | −0.405 | −0.250 | |
| p | 0.0009* | 0.0890 | 0.2098 | 0.0557 | 0.0490 | 0.0635 | 0.9838 | 0.1072 | 0.3335 | p = 0.0024 |
| β | R2 0.494 | |||||||||
| Cerebellum | ||||||||||
| r | 0.621 | −0.148 | −0.150 | 0.454 | −0.558 | −0.421 | 0.164 | −0.594 | 0.147 | |
| p | 0.0177 | = 0.6138 | 0.6080 | 0.1195 | 0.0474 | 0.1521 | 0.5746 | 0.0251 | 0.6148 | p = 0.0026 |
| β | −0.994 | 0.628 | R2 0.696 | |||||||
| Caudate nucleus r | ||||||||||
| p | −0.106 | 0.157 | 0.121 | −0.056 | 0.164 | 0.334 | 0.031 | 0.087 | −0.217 | n.s. |
| 0.6847 | 0.5478 | 0.6447 | 0.8370 | 0.5440 | 0.2064 | 0.9071 | 0.7406 | 0.4022 | ||
| Lenticular nucleus | ||||||||||
| r | −0.547 | 0.391 | 0.387 | −0.336 | 0.376 | 0.514 | 0.059 | 0.284 | 0.154 | |
| p | 0.0230 | 0.1208 | 0.1250 | 0.2036 | 0.1510 | 0.0417 | 0.8212 | 0.2686 | 0.5543 | p = 0.0104 |
| β | −0.620 | R2 0.385 | ||||||||
| Thalamus | ||||||||||
| r | 0.575 | −0.378 | −0.420 | 0.341 | −0.602 | −0.330 | 0.036 | −0.641 | −0.328 | |
| p | 0.0157 | 0.1352 | 0.0932 | 0.1956 | 0.0136 | 0.2113 | 0.8914 | 0.0056 | 0.1984 | p = 0.0076 |
| β | −0.640 | R2 0.409 | ||||||||
Cho, Choline; Cr, creatine; Glx, glutamate–glutamine; MRS, magnetic resonance spectroscopy; Myo, myo‐inositol; PET, positron emission tomography. *p Values that remain significant after Bonferroni–Holm correction. Added are the results of a stepwise multiple regression analysis R2, significance of variables after backward elimination and the standardised regression coefficient β.
Figure 3 The global cerebral metabolisation rate of glucose decreases with increasing magnetic spectroscopy alterations. This figure shows the regression line between the myo‐inositol/creatine (Myo/Cr) ratio in the white matter and the global cerebral glucose utilisation rate (CMRglc).
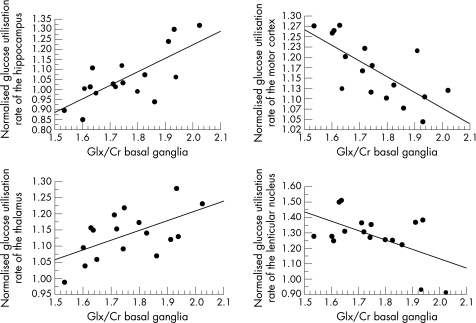
Considering the normalised regional glucose utilisation rates, we found that the thalamic glucose utilisation (R = 0.575; p = 0.0157) and the glucose utilisation in the hippocampus (R = 0.730; p = 0.0009) increased with an increasing glutamate–glutamine/creatine ratio in the basal ganglia voxel, whereas the glucose utilisation in the lenticular nucleus (R = −0.547; p = 0.0230) and the motor cortex (R = −0.716; p = 0.0012) decreased (fig 4). In addition, we found a significant increase in the glucose utilisation rate of the frontomedial cortex with decreasing myo‐inositol/creatine (R = −0.564; p = 0.0183) and choline/creatine (R = −0.641; p = 0.0056) ratios in the basal ganglia.
Figure 4 With increasing magnetic resonance spectroscopy (MRS) alterations in the brain of cirrhotic patients we found alterations in the cerebral glucose utilisation rate that were oppositely directed. Whereas the glucose utilisation rate of the hippocampus and thalamus increased with increasing MRS alterations, it decreased in the motor cortex and lenticular nucleus, for example. Glx/Cr, Glutamate–glutamine/creatine ratio.##
The glucose utilisation of the motor cortex also decreased with decreasing myo‐inositol/creatine and choline/creatine ratios and with increasing glutamate–glutamine/creatine intensity in the white matter, and with decreasing myo‐inositol/creatine and choline/creatine ratios in the gray matter voxel.
In the frontomedial cortex the glucose utilisation increased with the increasing glutamate–glutamine/creatine ratio in the gray matter, whereas it increased in the frontolateral cortex with decreasing myo‐inositol/creatine ratios in the white and gray matter voxels and with decreasing choline/creatine ratios in the white matter voxel.
The myo‐inositol/creatine ratio in the white matter was also significantly negatively correlated with the glucose utilisation of the hippocampus, cerebellum and thalamus. That of the gray matter was significantly negatively correlated with the glucose utilisation of the cerebellum and the thalamus.
After α‐correction, the number of regions according to Bonferroni–Holm significance could be assumed for the following correlations: (1) myo‐inositol/creatine ratio in all regions studied versus the glucose utilisation of the motor cortex; (2) glutamate–glutamine/creatine ratio of the basal ganglia and the white matter voxel versus glucose utilisation rate of the motor cortex; (3) glutamate–glutamine/creatine ratio of the basal ganglia versus glucose utilisation rate of the hippocampus; and (4) choline/creatine ratio of the white matter versus glucose utilisation of the motor cortex (table 2). There is thus a significant correlation between the MRS changes in the brain in cirrhotic individuals and the glucose utilisation especially of the motor cortex. The glucose utilisation in this area decreased with an increasing glutamate–glutamine/creatine ratio in different regions of the brain and also with decreasing myo‐inositol/creatine and choline/creatine ratios.
A stepwise multiple regression analysis confirmed the findings of the linear correlation analysis. Again we found significant correlations between MRS alterations and the glucose utilisation of several brain regions, with an increase in the glucose utilisation in the thalamus, cerebellum, frontomedial and frontolateral cortex and hippocampus, as well as a decrease in glucose utilisation in the motor cortex and lenticular nucleus (table 2). There was no hint that the MRS alterations in one of the three regions studied were more related to the glucose utilisation of selected brain regions than the other.
Cerebral ammonia metabolism versus cerebral glucose utilisation
There was no correlation between the 13N‐ammonia and FDG‐PET data.
Discussion
Cerebral MRS alterations in cirrhotic individuals are considered to represent alterations of astrocytic metabolism; as a result of an increase in brain ammonia levels the astrocytic glutamine content increases, followed by a decrease in myo‐inositol, an astrocytic osmolyte that counterbalances the osmotic consequence of an increased glutamine synthesis.4 A close correlation between plasma ammonia levels, cerebral ammonia metabolism and MRS alterations could thus be expected. If ammonia detoxification to glutamine is the key factor in the pathogenesis of hepatic encephalopathy, then the parameters of ammonia metabolism should also be correlated with cerebral glucose utilisation.
Actually, the present study showed a clear correlation between plasma ammonia levels and the estimated cerebral metabolic rate of ammonia and MRS alterations in the white matter. This fits with a recent observation of Balata et al.,32 who were able to show that even acute induced hyperammonemia leads to the characteristic MRS alterations in cirrhotic patients. We did not, however, find a significant correlation between plasma ammonia levels or the estimated cerebral metabolic rate of ammonia and the MRS data in the basal ganglia and gray matter. In addition, we were not able to show a close correlation between the actual cerebral ammonia uptake and retention rates and MRS alterations. Only the ammonia uptake rate in the caudate nucleus was significantly correlated with the myo‐inositol/creatine and choline/creatine alterations in the basal ganglia. In none of the regions studied, however, was a correlation between the ammonia retention rate and the MRS results observed. These results suggest diverse conclusions: (1) the MRS changes seem not to be much related to the actual amount of ammonia that is metabolised by the brain in cirrhotic individuals; or (2) the correlation analysis failed to show a significant interdependence because of the remarkable variances of both MRS results and 13N‐ammonia PET results, which could be overpowered only in the white matter voxel, a region that, at least in MRS, is less prone to artefacts than the basal ganglia or frontal cortex.
In contrast to 13N‐ammonia PET results, the FDG‐PET results were closely correlated with the patients' MRS data. The data can be summarised as follows: with increasing MRS alterations, the glucose utilisation of the lenticular nucleus and several cortical regions, especially the motor cortex, decreased whereas the glucose utilisation of the frontomedial and frontolateral cortex, thalamus, hippocampus and cerebellum increased or remained unchanged. When dealing with normalised glucose utilisation rates it has to be taken into consideration that in subjects with MRS abnormalities global glucose utilisation decreases. In these patients a decrease in the normalised utilisation rate within a special region can be interpreted as a real decrease, whereas an increase in the normalised rate may reflect either a real increase or only stability of regional glucose utilisation.
The alterations in cerebral glucose utilisation in cirrhotic individuals observed in this study fit well with those described in former studies,33,34,35 except for two findings: the reduction in glucose metabolism within the motor cortex, a region that has not been studied in detail before, and the finding of stable or increased glucose utilisation rates within the frontolateral cortex. The latter could be because the ROI defined in the present study differs from the frontal regions where glucose utilisation was shown to be decreased in hepatic encephalopathy by SPM analysis. In conclusion, we found a correlation between plasma ammonia levels and the estimated cerebral metabolic rate of ammonia and the extent of the characteristic MRS changes in the brain of cirrhotic individuals, especially for the white matter voxels. We did not find any correlation between the parameters of cerebral ammonia metabolism and global or regional cerebral glucose utilisation as measured by PET. We were, however, able to show a strong correlation between alterations in the glutamate/glutamine, choline and myo‐inositol content of the brain (related to creatine) and cerebral glucose utilisation.
How can the difference between ammonia and glucose metabolism be explained in their relationship to MRS data? Again methodological restrictions have to be considered. Correlation analysis can not be used to prove or exclude a causal relationship between different parts of brain metabolism, on principle. In addition, MRS and PET results show a remarkable variance, which might obscure the presence and extent of interdependencies between cerebral ammonia and glucose metabolism. It may also be assumed, however, that actual cerebral ammonia metabolism, as represented by the eCMRA for example, is just one influencing variable with regard to both the concentration of the metabolites measured by MRS and cerebral glucose utilisation in cirrhotic individuals with hepatic encephalopathy. In particular, the concentration of the osmolytes myo‐inositol and choline is regulated by many factors beyond the intracellular glutamine concentration.36 It was the myo‐inositol/creatine ratio that was especially significantly correlated with cerebral glucose utilisation. Our data may thus be considered as an additional indication that cerebral dysfunction in cirrhotic patients depends on many more metabolic alterations than just ammonia detoxification via the synthesis of glutamine.5,36
Acknowledgements
The authors appreciate the continuous helpful support of this study provided by Professor Dr M P Manns, Director and Chairman, Department of Gastroenterology, Hepatology and Endocrinology, Medizinische Hochschule Hannover, and his co‐workers. The authors also wish to extend many thanks to Dr Janus Ong and his co‐workers who provided details of their ammonia data. They also wish to thank Dr Elinor Switzer for English proofreading of the manuscript.
Abbreviations
CMRA - cerebral metabolic rate for ammonia
CMRglc - cerebral glucose utilisation rate
FDG - 18F‐fluorodesoxyglucose
MRI - magnetic resonance imaging
MRS - magnetic resonance spectroscopy
NAA - N‐acetyl‐aspartate
PET - positron emission tomography
PSE - Present State Examination
ROI - region of interest
Footnotes
Conflict of interest: None declared.
References
- 1.Butterworth R F. Pathogenesis of hepatic encephalopathy: new insights from neuroimaging and molecular studies. J Hepatol 200339278–285. [DOI] [PubMed] [Google Scholar]
- 2.Shawcross J, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci 2005622295–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose C, Felipo V. Limited capacity for ammonia removal by brain in chronic liver failure: potential role of nitric oxide. Met Brain Dis 200520275–283. [DOI] [PubMed] [Google Scholar]
- 4.Häussinger D, Laubenberger J, vom Dahl S.et al Proton magnetic resonance spectroscopy studies on human brain myo‐inositol in hypo‐osmolarity and hepatic encephalopathy. Gastroenterology 19941071475–1480. [DOI] [PubMed] [Google Scholar]
- 5.Zwingmann C, Butterworth R. An update on the role of brain glutamine synthesis and its relation to cell‐specific energy metabolism in the hyperammonemic brain: further studies using NMR spectroscopy. Neurochem Int 20054719–30. [DOI] [PubMed] [Google Scholar]
- 6.Fischer‐Wasels D, Burchert W, Köstler H.et al Comparison between MRI, MRS, 18‐FDG‐ and 13‐NH3‐PET data in cirrhotics with minimal hepatic encephalopathy (HE). J Hepatol 200032(Suppl.2)64 [Google Scholar]
- 7.Pugh R N H, Murray‐Lyon I M, Dawson J L.et al Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 197360646–649. [DOI] [PubMed] [Google Scholar]
- 8.Schomerus H, Weissenborn K, Hamster W.et al PSE‐Syndrom‐Test. Psychodiagnostisches Verfahren zur quantitativen Erfassung der (minimalen) portosystemischen Enzephalopathie. Frankfurt: Swets Test Services 1999
- 9.Ferenci P, Lockwood A H, Mullen K.et al Hepatic encephalopathy – definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congress of Gastroenterology, Vienna 1998. Hepatology 200235716–721. [DOI] [PubMed] [Google Scholar]
- 10.Brickenkamp R. Test d2. Aufmerksamkeits Belastungs Test. Göttingen, Toronto, Zürich: Verlag für Psychologie J. Hogrefe 1981
- 11.Keiding S, Sorensen M, Bender D.et al Brain metabolism of 13N‐ammonia during acute hepatic encephalopathy in cirrhosis measured by postron emission tomography. Hepatology 20064342–50. [DOI] [PubMed] [Google Scholar]
- 12.Ahl B, Weissenborn K, van den Hoff J.et al Regional differences in cerebral blood flow and cerebral ammonia metabolism in cirrhotics. Hepatology 20044073–79. [DOI] [PubMed] [Google Scholar]
- 13.Hutchins G D, Schwaiger M, Rosenspire K C.et al Noninvasive quantification of regional blood flow in the human heart using N‐13‐ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol 1990151032–1042. [DOI] [PubMed] [Google Scholar]
- 14.Lockwood A H, Bolomey L, Napoleon F. Blood–brain barrier to ammonia in humans. J Cereb Blood Flow Metabol 19844516–522. [DOI] [PubMed] [Google Scholar]
- 15.Lockwood A H, Yap E W H, Wong W ‐ H. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab 199111337–341. [DOI] [PubMed] [Google Scholar]
- 16.Blomqvist G. On the construction of functional maps in positron emission tomography. J Cereb Blood Flow Metab 19844629–632. [DOI] [PubMed] [Google Scholar]
- 17.van den Hoff J, Burchert W, Müller‐Schauenburg W.et al Accurate local blood flow measurements with dynamic PET: fast determination of input function delay and dispersion by multilinear minimization. J Nucl Med 1993341770–1772. [PubMed] [Google Scholar]
- 18.Rosenspire K C, Schwaiger M, Manger T J.et al Metabolic fate of [13N] ammonia in human and canine blood. J Nucl Med 199031163–167. [PubMed] [Google Scholar]
- 19.Patlak C, Blasberg R G, Fenstermacher J D. Graphical evaluation of blood‐to‐brain transfer constants from multiple‐time uptake data. J Cereb Blood Flow Metabol 198331–7. [DOI] [PubMed] [Google Scholar]
- 20.Ong J P, Aggarwal A, Krieger D.et al Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 2003114188–193. [DOI] [PubMed] [Google Scholar]
- 21.Talairach J, Tournoux P. Co‐planar stereotaxic atlas of the human brain, 2nd ed. Stuttgart: Thieme Verlag 1988
- 22.Chamuleau R, Bosman D K, Bovee W.et al What the clinician can learn from MR glutamine/glutamate assays. NMR Biomed 19914103–108. [DOI] [PubMed] [Google Scholar]
- 23.Kreis R, Farrow N, Ross B D. Localized 1H NMR spectroscopy in patients with chronic hepatic encephalopathy: analysis of changes in cerebral glutamine, choline and inositols. NMR Biomed 19914109–116. [DOI] [PubMed] [Google Scholar]
- 24.Geissler A, Lock G, Fründ R.et al Cerebral abnormalities in patients with cirrhosis detected by proton magnetic resonance spectroscopy and magnetic resonance imaging. Hepatology 19972548–54. [DOI] [PubMed] [Google Scholar]
- 25.Köstler H. Proton magnetic resonance spectroscopy in portal‐systemic encephalopathy. Metab Brain Dis 199813291–301. [DOI] [PubMed] [Google Scholar]
- 26.Lee J H, Seo D W, Lee Y S.et al Proton magnetic resonance spectroscopy (1H‐MRS) findings in the brain in patients with liver cirrhosis reflect the hepatic functional reserve. Am J Gastroenterol 1999942206–2213. [DOI] [PubMed] [Google Scholar]
- 27.Cordoba J, Sanpedro F, Alonso J.et al 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metabol Brain Dis 200217415–429. [DOI] [PubMed] [Google Scholar]
- 28.Taylor‐Robinson S D, Sargentoni J, Marcus C D.et al Regional variations in cerebral proton spectroscopy in patients with chronic hepatic encephalopathy. Metab Brain Dis 19949347–359. [DOI] [PubMed] [Google Scholar]
- 29.Pujol J, Kulisevsky J, Moreno A.et al Neurospectroscopic alterations and globus pallidus hyperintensity as related magnetic resonance markers of reversible hepatic encephalopathy. Neurology 1996471526–1530. [DOI] [PubMed] [Google Scholar]
- 30.Spahr L, Vingerhoets F, Lazeyras F.et al Magnetic resonance imaging and proton spectroscopic alterations correlate with parkinsonian signs in patients with cirrhosis. Gastroenterology 2000119774–781. [DOI] [PubMed] [Google Scholar]
- 31.Morgan M Y. Cerebral magnetic resonance imaging in patients with chronic liver disease. Metab Brain Dis 199813273–290. [DOI] [PubMed] [Google Scholar]
- 32.Balata S, Olde Damink S W M, Ferguson, Marshall I, et al Induced hyperammmonemia alters neuropsychology, brain mr spectroscopy and magnetization transfer in cirrhosis. Hepatology 200337931–939. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood A H, Yap E W H, Rhoades H M.et al Altered cerebral blood flow and glucose metabolism in patients with liver disease and minimal encephalopathy. J Cereb Blood Flow Metab 199111331–336. [DOI] [PubMed] [Google Scholar]
- 34.Lockwood A H, Murphy B W, Donnelly K Z.et al Positron‐emission tomographic localization of abnormalities of brain metabolism in patients with minimal hepatic encephalopathy. Hepatology 1993181061–1068. [PubMed] [Google Scholar]
- 35.Lockwood A H, Weissenborn K, Bokemeyer M.et al Correlations between cerebral glucose metabolism and neuropsychological test performance in non‐alcoholic cirrhotics. Metab Brain Dis 20021729–40. [DOI] [PubMed] [Google Scholar]
- 36.Häussinger D. Low grade cerebral edema in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006431187–1190. [DOI] [PubMed] [Google Scholar]