Abstract
Objective
S100A12 is a pro‐inflammatory protein that is secreted by granulocytes. S100A12 serum levels increase during inflammatory bowel disease (IBD). We performed the first study analysing faecal S100A12 in adults with signs of intestinal inflammation.
Methods
Faecal S100A12 was determined by ELISA in faecal specimens of 171 consecutive patients and 24 healthy controls. Patients either suffered from infectious gastroenteritis confirmed by stool analysis (65 bacterial, 23 viral) or underwent endoscopic and histological investigation (32 with Crohn's disease, 27 with ulcerative colitis, and 24 with irritable bowel syndrome; IBS). Intestinal S100A12 expression was analysed in biopsies obtained from all patients. Faecal calprotectin was used as an additional non‐invasive surrogate marker.
Results
Faecal S100A12 was significantly higher in patients with active IBD (2.45 ± 1.15 mg/kg) compared with healthy controls (0.006 ± 0.03 mg/kg; p<0.001) or patients with IBS (0.05 ± 0.11 mg/kg; p<0.001). Faecal S100A12 distinguished active IBD from healthy controls with a sensitivity of 86% and a specificity of 100%. We also found excellent sensitivity of 86% and specificity of 96% for distinguishing IBD from IBS. Faecal S100A12 was also elevated in bacterial enteritis but not in viral gastroenteritis. Faecal S100A12 correlated better with intestinal inflammation than faecal calprotectin or other biomarkers.
Conclusions
Faecal S100A12 is a novel non‐invasive marker distinguishing IBD from IBS or healthy individuals with a high sensitivity and specificity. Furthermore, S100A12 reflects inflammatory activity of chronic IBD. As a marker for neutrophil activation, faecal S100A12 may significantly improve our arsenal of non‐invasive biomarkers of intestinal inflammation.
The etiology of inflammatory bowel disease (IBD) consisting of ulcerative colitis and Crohn's disease involves complex interactions among susceptibility genes, the environment, and the immune system. These interactions lead to a cascade of events that involve the activation of neutrophils, production of proinflammatory mediators, and tissue damage.1 As intestinal symptoms are a frequent cause of referrals to gastroenterologists, it is crucial to differentiate between non‐inflammatory irritable bowel syndrome (IBS) and IBD. To date, there is a lack of biological markers to determine intestinal inflammation.2,3 Therefore, invasive procedures are required to confirm the diagnosis of IBD. Furthermore, the natural history of chronic IBD is characterised by an unpredictable variation in the degree of inflammation. Biological markers are needed to confirm remission, detect early relapses or local reactivation, and to monitor anti‐inflammatory therapies reliably. Whereas serum markers of inflammation are still not very helpful,3,4,5 assays that determine intestinal inflammation by detecting neutrophil‐derived products in stool show great potential.6
An important mechanism in the initiation and perturbation of inflammation in IBD is the activation of innate immune mechanisms.7,8 Among the factors released by infiltrating neutrophils are proteins of the S100 family.9,10 One example is calprotectin, which is detectable in the serum and stool during intestinal inflammation.11 Calprotectin was initially described as a protein of 36 kDa, but was later characterised as a complex of two distinct S100 proteins, S100A8 and S100A9.12,13,14 In recent years, calprotectin has been proposed as a faecal marker of gut inflammation reflecting the degree of phagocyte activation.6,15,16,17,18,19,20 Unfortunately, variation in faecal calprotectin assays still impedes the routine use of this marker as a sole parameter in clinical practice. The observed variation may be caused by the broad expression pattern of calprotectin, which is found in granulocytes as well as monocytes and is also inducible in epithelial cells.21,22 In this context, the elevation in lactose intolerance is notable.16,17,23
S100A12 is more restricted to granulocytes. It is secreted by activated neutrophils and is abundant in the intestinal mucosa of patients with IBD.9,24 Overexpression at the site of inflammation and correlation with disease activity in a variety of inflammatory disorders underscore the role of this granulocytic protein as a proinflammatory molecule.25 The binding of S100A12 to the receptor for advanced glycation endproducts (RAGE) leads to the long‐term activation of nuclear factor kappa B, which promotes inflammation.26 In mouse models of colitis, blocking the interaction of S100A12 with RAGE has been proved to attenuate inflammation. Data on murine models of colitis as well as human IBD point to an important role for S100A12 during the pathogenesis of these disorders.9,26,27
In a previous study, we demonstrated that S100A12 is overexpressed during chronic active IBD and serves as a useful serum marker for disease activity in patients with IBD.9 De Jong et al.28 recently reported that S100A12 can be detected in the stool of children with Crohn's disease. The aim of our present study was thus to analyse S100A12 in stool samples as well as its expression in the intestinal tissue of patients with confirmed IBD or IBS and in the stool of a normal control group. We correlated faecal S100A12 levels with endoscopic and histological findings in the same patients and investigated the diagnostic accuracy of S100A12 to detect intestinal inflammation.
Methods
Patients with IBD or IBS
We included 83 consecutive patients with an established diagnosis of IBD or symptoms suggesting gastrointestinal inflammation. The study was designed as a prospective trial in which data collection was planned before the measurements of diagnostic accuracy were performed. The patients were included between May 2004 and May 2006 at the University of Duisburg, Essen, Germany. Thirty‐two patients had Crohn's disease, 27 were identified to have ulcerative colitis, and 24 patients were diagnosed with IBS (table 1). All patients underwent endoscopic examination, and biopsies were taken for histological investigations at all colonoscopies. Each patient gave signed informed consent. The study protocol was approved by the ethics committee of the medical faculty of the University of Duisburg, Essen, Germany. All patients with IBS fulfilled the ROME II criteria.29 Symptoms suggesting gastrointestinal inflammation in IBS included straining during bowel movement, the passage of mucus, weight loss, and fatigue.
Table 1 Characteristics of the study population.
| Crohn's disease | Ulcerative colitis | IBS | Bacterial enteritis | Viral enteritis | Controls | |
|---|---|---|---|---|---|---|
| Patients, n | 32 | 27 | 24 | 65 | 23 | 24 |
| Sex, female/male | 21/11 | 11/16 | 21/3 | 33/32 | 10/13 | 10/14 |
| Age, median (range) | 34 (19–62) | 46 (22–71) | 46 (16–70) | 31 (1–93) | 32 (1–83) | 35 (17–43) |
| Disease activity, n | ||||||
| Inactive | 6 | 3 | – | – | – | – |
| Active | 26 | 24 | – | – | – | – |
| Localisation, n | ||||||
| Pancolitis | – | 7 | – | – | – | ‐ |
| Leftside colitis | – | 12 | – | – | – | – |
| Terminal ileum | 12 | – | – | – | – | – |
| Caecum | 2 | – | – | – | – | ‐ |
| Colon | 9 | – | – | – | – | – |
| Other | 4 | 5 | – | – | – | – |
| Parameters, mean (SEM) | ||||||
| CRP, mg/dl | 2.7 (0.63) | 1.2 (0.23) | 0.3 (0.06) | – | – | – |
| CDAI/CAI | 109 (9.5) | 6 (0.6) | – | – | – | |
| Histology inflammation score | 1.5 (0.2) | 1.6 (0.2) | 0 | – | – | – |
| WBC count, 1000/μl | 8.9 (0.8) | 7.1 (0.4) | 6.4 (0.3) | – | – | – |
| ESR, mm/h | 28.8 (4.2) | 25.6 (2.9) | 14.6 (1.9) | – | – | – |
| Medication, n | ||||||
| 5‐ASA | 11 | 14 | – | – | – | – |
| Steroids | – | – | – | – | ||
| – Systemic | 7 | 8 | ||||
| – Local | 4 | 1 | ||||
| Azathioprine | 1 | 3 | – | – | – | – |
CDAI/CAI, Crohn's disease activity index/colitis activity index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; 5‐ASA, 5‐aminosalicylic acid; IBS, irritable bowel syndrome; WBC, white blood cell.
Patients with infectious gastroenteritis
We included 88 patients who suffered from infectious gastroenteritis confirmed by microbiological stool examination, including cultures for enteropathogenic bacteria and the detection of enteropathogenic viruses according to standard procedures. Only one isolate per patient was included (table 1). Of these, 65 had proof of bacterial origin (18 Escherichia coli, 10 Clostridium difficile, 15 Campylobacter group, 17 Salmonella group, five Yersinia enterocolitica) and in 23 a virus was detected (10 Norwalk‐like virus, 13 rotavirus).
Determination of disease activity
To define inflammatory activity in the gut, macroscopic signs of inflammation were recorded during colonoscopy by the experienced gastroenterologist using the simple endoscopic score for Crohn's disease or a similar index for ulcerative colitis.30,31 In addition, biopsies were taken from multiple sites. Biopsy sections were encoded and analysed semiquantitatively on a four‐point scale by two independent observers who were blinded to the diagnosis and clinical data (0, no inflammation; 1, mild signs of inflammation; 2, moderate inflammation; 3, severe inflammation). As a further inclusion criterion, the histology score had to be in accordance with endoscopic signs of inflammation. This histology inflammation score served as the reference standard to determine the absence of inflammation or active IBD in our study cohort. In addition, clinical disease activity in Crohn's disease was documented by using the Crohn's disease activity index (CDAI),32 and for ulcerative colitis by using the colitis activity index (CAI) according to Rachmilewitz33 and using the criteria of Truelove and Witts.34 Stool samples were collected from all patients. Levels of C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count, thrombocytes, haemoglobin and haematocrit were determined in all patients. IBD patients were considered inactive if there were no endoscopic and histological signs of inflammation (histology inflammation score < 1) and normal disease activity scores (CDAI < 150; CAI < 6). In addition, extraintestinal disease and small bowel involvement were excluded in Crohn's disease patients by clinical examination and contrast enhanced magnetic resonance imaging using the Sellink technique.35
Healthy controls
The control group contained 24 healthy people without any signs of inflammation or intestinal symptoms. They supplied a single stool sample after giving informed consent. Demographic data are summarised in table 1.
Stool analyses
The whole stool specimens were collected by the patients 72 h before endoscopy using a disposable plastic bucket‐type device that avoids toilet water artefact and simplifies laboratory sampling. In patients with acute gastroenteritis, stool was collected in regular sterile sample containers, and sent to the laboratory within 24 h. Microbiological analyses comprised classic culture methods. Stool specimens were frozen to −30°C immediately after collection and sent to the laboratory (L+S AG, Bad Bocklet, Grossenbrach, Germany) by mail. Approximately 100 mg of the stool samples were immediately suspended in extraction buffer at 1 : 50 dilution for homogenisation as described and extensively validated previously.6,28 After homogenisation and centrifugation the supernatants were stored at −80°C until analysis for both calprotectin and S100A12. The analyses for these markers were performed using aliquots from identical samples. Concentrations of S100A12 were determined by a double sandwich ELISA system established in our laboratory as described previously.9 The inter and intraassay coefficients of variation were 12.1% (n = 35) and 4.8% (n = 10), respectively. Faecal calprotectin was determined by a commercially available ELISA (Calpro AS, Oslo, Norway) following the manufacturer's instructions. The upper value of the normal range is 50 mg/kg. The readers of the laboratory assay were blinded to the diagnosis and the results of our reference standard, i.e. the inflammation score.
Immunohistochemical analyses
S100A12 expression was detected on paraffin‐embedded bowel specimens by staining with rabbit antihuman S100A12, as described previously.9 To identify neutrophils in the sections, murine antihuman Crohn's disease15 or alternatively antihuman elastase antibodies (DAKO, Glostrup, Denmark) were employed. The co‐expression of S100A12 and neutrophil markers was analysed on serial sections. As control antibodies we used monoclonal murine antihuman IgM and polyclonal rabbit antihuman IgG (Pierce, Rockford, Illinois, USA) with irrelevant specificity. Secondary antibodies and substrates for colour reaction were used as described before.36 Sections were analysed using a Zeiss Axioskop connected to an Axiocam camera supplied with software Axiovision 3.0 for Windows (Zeiss, Goettingen, Germany).
Sections were analysed semiquantitatively by two independent observers who were blinded to the diagnosis and clinical data. The analysis included all areas of the sections at a magnification of 200‐fold. A global score was given for each parameter, using a semiquantitative four‐point scale (0, lowest level of expression, no positive cells; 3, highest level of expression, all cells positive in all areas).
Statistical analyses
All statistical analyses were performed using SPSS software package version 12 (SPSS Inc., Chicago, Illinois, USA). Differences for parameters found to be significant in analysis of variance followed by Dunnett's post‐hoc analysis were further compared group‐to‐group using the Mann–Whitney U‐test. Receiver operating curves were plotted to determine the accuracy of S100A12 measurements as a diagnostic test.37,38 The correlation of different markers and scores with disease activity was analysed using Spearman's rho‐test. All tests of significance were two‐tailed. A p value of less than 0.05 was considered to be significant. Data are presented as median ±95% CI except when otherwise stated. There were no missing test results, and no indeterminates or outliers were excluded.
Results
Stool analyses
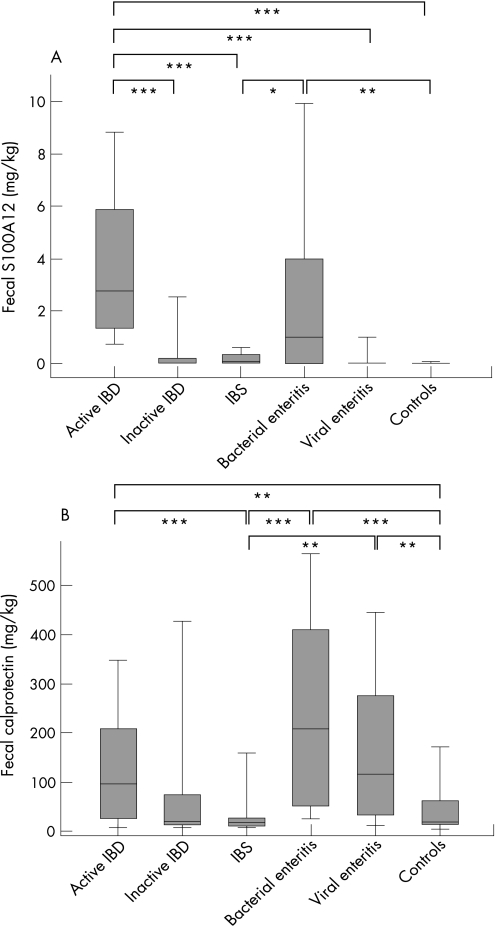
Faecal S100A12 levels from patients with active IBD were significantly elevated (median 2.45 ± 1.15 mg/kg) compared with healthy controls (0.006 ± 0.03 mg/kg; p<0.001) or patients with IBS (0.05 ± 0.11 mg/kg; p<0.001). The levels from IBS patients did not differ significantly from those found in healthy controls (p = 0.171). There was also no significant difference for S100A12 levels between Crohn's disease (1.84 ± 1.84 mg/kg) and ulcerative colitis (2.67 ± 1.38 mg/kg; p = 0.41) patients. Significantly higher levels were found in patients during active disease in Crohn's disease as well as ulcerative colitis (histology inflammation score ⩾1) compared with patients with no evidence of inflammation (fig 1A). Faecal S100A12 levels were 0.005 ± 1.03 mg/kg in patients with inactive IBD (p<0.001). There were no significant age or gender differences for faecal S100A12 in our cohort (not shown). Faecal S100A12 was also significantly elevated in bacterial enteritis (1.0 ± 1.21 mg/kg) but not in patients suffering from viral gastroenteritis (0.01 ± 0.31 mg/kg). Within these groups, the S100A12 results revealed no pathogen‐dependent variations.
Figure 1 S100A12 and calprotectin in faeces. Faecal S100A12 (A) and faecal calprotectin (B) were determined in stool samples of patients with active inflammatory bowel disease (IBD; n = 50), inactive IBD (n = 9), irritable bowel syndrome (IBS; n = 24), bacterial enteritis (n = 65), viral gastroenteritis (n = 23) and healthy controls (n = 24). Box plots indicate the median as well as the 25th and 75th percentile. Error bars indicate 10th and 90th percentiles. Fecal S100A12 was significantly higher in active IBD compared with all other groups except bacterial enteritis. Fecal calprotectin was higher in active IBD compared with IBS and healthy controls, but differences between patients with active and inactive IBD did not reach statistical significance. In addition, faecal calprotectin was significantly elevated both in bacterial enteritis and viral gastroenteritis (***p<0.001; **p<0.01; *p<0.05).
Correlation with disease activity
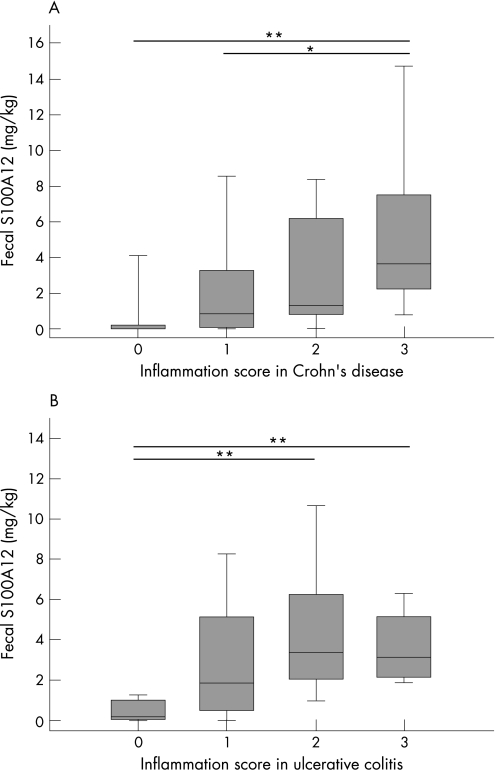
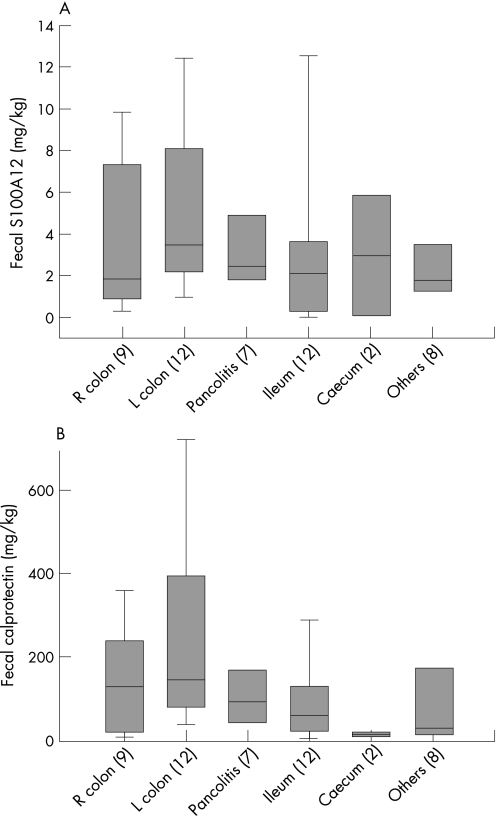
Levels of faecal S100A12 correlated with the histology inflammation score (r = 0.440; p<0.01), ESR (r = 0.377; p<0.01), CRP (r = 0.396; p<0.01), thrombocytes (r = 0.418; p<0.01), white blood cell count (r = 0.287; p<0.05), haemoglobin (r = −0.512; p<0.001) and haematocrit (r = −0.460; p = 0.001) in IBD patients. Levels of faecal S100A12 in ulcerative colitis correlated with the CAI (r = 0.415, p = 0.039) and the inflammation score (r = 0.440, p = 0.025). There was no correlation between the CDAI and faecal S100A12 in patients with Crohn's disease (r = 0.296, p = 0.100), but we also found no significant correlation between the inflammation score and the CDAI (r = 0.189, p = 0.317). The correlation between the inflammation score and faecal S100A12 in Crohn's disease (r = 0.451, p = 0.010) was similar to the ulcerative colitis group and was significant (fig 2). Table 2 summarises correlations of faecal S100A12 with different parameters in Crohn's disease and ulcerative colitis. The test for faecal S100A12 performs equally well regardless of disease location (fig 3).
Figure 2 Correlation of faecal S100A12 with intestinal inflammation. A histology inflammation score was calculated by combining endoscopic and histological information in Crohn's disease (A) and ulcerative colitis (B). Faecal S100A12 results were lower in patients without proof of inflammation (score 0: Crohn's disease n = 6, ulcerative colitis n = 3) than in patients with signs of inflammation (mild inflammation, score 1: Crohn's disease n = 11, ulcerative colitis n = 5; moderate inflammation, score 2: Crohn's disease n = 8, ulcerative colitis n = 14; severe inflammation, score 3: Crohn's disease n = 8, ulcerative colitis n = 6). Box plots indicate the median as well as the 25th and 75th percentile. Error bars indicate the 10th and 90th percentile (**p<0.01; *p<0.05).
Table 2 Correlation of fecal biomarkers with other parameters in inflammatory bowel disease.
| Fecal calprotectin | Clinical score | Inflammatory score* | CRP | ESR | Haematocrit | WBC count | Thrombocytes | |
|---|---|---|---|---|---|---|---|---|
| Crohn's disease | CDAI | |||||||
| Fecal S100A12 | 0.324 | 0.296 | 0.451 | 0.563 | 0.533 | −0.615 | 0.311 | 0.433 |
| p = 0.08 | p = 0.1 | p = 0.01 | p<0.01 | p<0.01 | p<0.001 | p = 0.08 | p = 0.01 | |
| Fecal calprotectin | 0.065 | 0.412 | 0.307 | 0.188 | −0.531 | −0.112 | 0.243 | |
| p = 0.73 | p<0.05 | p = 0.09 | p = 0.31 | p<0.01 | p = 0.55 | p = 0.19 | ||
| Ulcerative colitis | CAI | |||||||
| Fecal S100A12 | 0.531 | 0.415 | 0.440 | 0.096 | −0.129 | −0.013 | −0.016 | 0.206 |
| p<0.01 | p<0.05 | p<0.05 | p = 0.66 | p = 0.54 | p = 0.95 | p = 0.94 | p = 0.32 | |
| Fecal calprotectin | 0.334 | 0.311 | 0.057 | 0.120 | −0.019 | 0.156 | 0.435 | |
| p = 0.11 | p = 0.14 | p = 0.80 | p = 0.58 | p = 0.93 | p = 0.47 | p = 0.08 |
CAI, colitis activity index; CDAI, Crohn's disease activity index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
Significant correlations highlighted in bold.
*Histology inflammation score.
Figure 3 Correlation of faecal S100A12 and faecal calprotectin with disease location. In patients with active inflammatory bowel disease (IBD), the test for faecal S100A12 performs equally well regardless of disease location. The analysis of variance revealed no significant differences, although there was a trend towards higher levels of distal inflammation compared with more proximal inflammation. Faecal S100A12 seems to be a more robust test in this regard than faecal calprotectin (L, left; R, right; Others, proctitis and isolated sigma inflammation).
Sensitivity and specificity
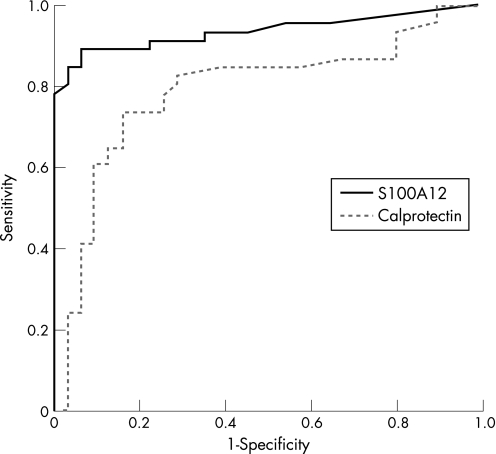
We performed receiver operating curve analyses to determine cutoff points for faecal S100A12 measurements. A cutoff point of 0.8 mg/kg was used to distinguish active IBD from healthy controls and IBS. Comparing ulcerative colitis and Crohn's disease in differentiation to healthy controls we could determine a specificity of 100% for both but a lower sensitivity in Crohn's disease (81%) than in ulcerative colitis (91%). In differentiating IBS from active inflammation in IBD we observed a sensitivity of 86% and a specificity of 96% (fig 4). Details are summarised in table 3.
Figure 4 Receiver operating curves. Receiver operating curve analyses were performed to analyse the trade‐off between the sensitivity and specificity of faecal S100A12 and faecal calprotectin in differentiating patients with active inflammatory bowel disease (IBD) from those with irritable bowel syndrome (IBS). Both biomarkers were found to distinguish between IBD and IBS, with a better result for faecal S100A12 (area under the curve 0.936; 95% CI 0.879 to 0.993) than for faecal calprotectin (area under the curve 0.788; 95% CI 0.679 to 0.896).
Table 3 Diagnostic accuracy in distinguishing active inflammatory bowel disease from irritable bowel syndrome.
| Sensitivity % | Specificity % | LR+ | LR− | PPV | NPV | OR | |
|---|---|---|---|---|---|---|---|
| Fecal S100A12 | 86 | 96 | 21.5 | 6.9 | 0.98 | 0.76 | 13.5 |
| Fecal calprotectin | 63 | 86 | 4.5 | 2.3 | 0.90 | 0.51 | 9.9 |
| CRP | 58 | 79 | 2.8 | 1.9 | 0.76 | 0.53 | 3.2 |
| ESR | 56 | 75 | 2.24 | 1.7 | 0.74 | 0.42 | 2.9 |
| CDAI | 27 | 100* | – | – | 1 | 0.24 | – |
| CAI | 63 | 100† | – | – | 1 | 0.38 | – |
CAI, Colitis activity index; CDAI, Crohn's disease activity index; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value.
*CDAI < 150 in inactive Crohn's disease (n = 6), †CAI ⩽ 5 in inactive ulcerative colitis (n = 3).
Other markers of inflammation
Between active IBD and IBS, there were significant differences for CRP (2.3 versus 0.5 mg/dl; p<0.001) and ESR (29 versus 15 mm/h; p = 0.003), whereas white blood cell count, thrombocytes, haemoglobin, and haematocrit showed no differences. These markers did not differentiate between active and inactive IBD. Faecal calprotectin was higher in active IBD (median 97 ± 45 mg/kg) compared with inactive IBD (19 ± 158 mg/kg), IBS (18 ± 36 mg/kg) and healthy controls (19 ± 25 mg/kg; fig 1B). In addition, faecal calprotectin was significantly elevated both in bacterial enteritis (209 ± 61 mg/kg) and viral gastroenteritis (107 ± 80 mg/kg). The accuracy of other markers is summarised in table 3. Test accuracy summarised by sensitivity, specificity, likelihood ratio, positive predictive value, negative predictive value, and odds ratio was found to be superior for faecal S100A12 compared with any other marker.
Immunohistochemical results
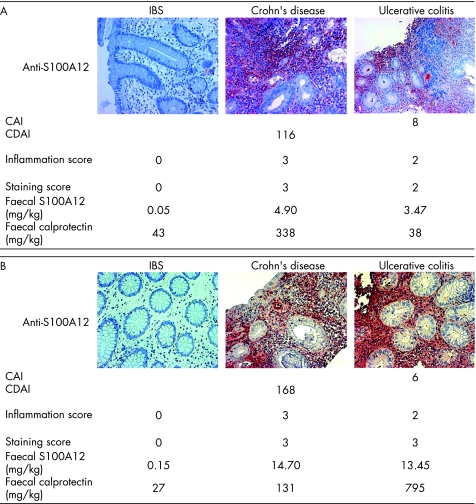
Staining of tissue from patients with IBD showed a specific distribution of S100A12 expression by infiltrating cells in inflamed areas of the gut. Patients with ulcerative colitis had distorted crypt architecture. Crypts were reduced in number; edema and focal haemorrhage were observed. Epithelial cells were S100A12 negative in all cases, whereas profound subepithelial expression of S100A12 in granulocytes was found in IBD. In gut biopsies with a high degree of inflammation, S100A12 was present in an extracellular distribution surrounding S100A12‐positive cells, reflecting secretion of the protein. Particularly around granulomatous lesions or in crypt abscesses, large numbers of S100A12‐positive cells as well as the release of S100A12 into the extracellular space were present (fig 5). In contrast, there was no S100A12 expression in tissue samples from patients with IBS and only few S100A12‐positive cells in samples of IBD patients with no evidence of inflammation in endoscopic or histological examination. The pattern of CD15 or elastase as markers of neutrophils in the tissue was similar to the distribution of S100A12‐positive cells. Coexpression of S100A12 with neutrophil markers was detected in serial sections as already described before (not shown).9 The staining of S100A12 in the samples of patients with IBD correlated with the histology inflammation score (r = 0.331; p<0.05) and with faecal levels of S100A12 (r = 0.306; p = 0.05). We found no significant correlation between the clinical activity scores CAI or CDAI and our staining for S100A12.
Figure 5 Immunohistochemical S100A12 staining and correlation with disease activity. S100A12 was stained in biopsies obtained at colonoscopy. The staining was compared with the disease activity index, faecal S100A12, and other markers (faecal calprotectin shown as example). Image sets (A,B) represent individual examples of patients with irritable bowel syndrome (IBS), Crohn's disease, and ulcerative colitis. Local S100A12 tissue expression was found to correlate with the histology inflammation score and with faecal levels of S100A12, whereas there was only a weak correlation with the clinical activity scores the colitis activity index (CAI) or the Crohn's disease activity index (CDAI) when analysing the whole cohort.
Discussion
Faecal biomarkers of intestinal inflammation are attractive in the clinical routine as they provide a non‐invasive examination that can help screening patients with mild symptoms such as abdominal pain and diarrhoea. Furthermore, the measurement of such biomarkers can be repeated to follow up inflammation or to detect subclinical activity early.18 As markers of neutrophil‐derived proteins, lysozyme, myeloperoxidase, human neutrophil lipocalin or polymorphonuclear neutrophil elastase are less promising than lactoferrin.39,40,41,42,43 As these proteins are expressed by infiltrating granulocytes, levels in feces are significantly higher in IBD than in IBS, but an overlap of levels in active disease compared with inactive IBD has been observed.44,45
The most extensively investigated faecal marker is the complex of S100A8/S100A9 termed ‘calprotectin'. Previous studies have shown that calprotectin correlates well with disease activity in IBD and can be a useful biomarker for distinguishing IBD and IBS.6,11,19,20,46 The reported sensitivity varies between 63% and 95%, whereas specificity has been reported to be between 79% and 93%.19,47,48,49,50,51 See supplementary table 4 for the diagnostic accuracy of faecal calprotectin in the differentiation of IBD versus IBS reported in other studies (supplementary table 4 can be viewed on the Gut website at http://gut.bmj.com/supplemental). Our results for faecal calprotectin are therefore within the range found in previous studies (summarised in Konikoff and Denson46). The observed variation could be caused by varying presentation of epitopes on the calprotectin complex under different conditions.52 On the other hand, the broad expression pattern of calprotectin, which is inducible in epithelial cells under certain circumstances, may be influencing the results.21,22 In this regard, it is remarkable that faecal S100A12 was not elevated in viral gastroenteritis as was faecal calprotectin. Elevated levels of faecal calprotectin have also been reported in one previous study including bacterial enteritis and rotavirus infections.53 In contrast, faecal S100A12 was not elevated in viral gastroenteritis, which was also reported for lactoferrin, indicating that these markers are more specific for neutrophils than calprotectin and that neutrophil activation is less involved in viral gastroenteritis than in bacterial infections.54
In contrast to calprotectin, S100A12 is exclusively expressed in relevant amounts by granulocytes, which play an important role in the pathogenesis of IBD. S100A12 is secreted by activated neutrophils and promotes inflammation through the activation of RAGE.26 As neutrophil influx into the intestinal mucosa is closely linked to IBD activity, especially during early steps of inflammation, neutrophil‐derived S100A12 in tissue and exudates strongly correlates with inflammatory activity.9,55 Excretion of the protein into the gut lumen could reflect the number of neutrophils infiltrating the mucosa as well as their activation status. The measurement of S100A12 in feces is not strictly disease specific, but is probably specific for neutrophil activity during bowel inflammation. It was recently reported that S100A12 is detectable in stool samples, where it is evenly distributed and, similar to calprotectin, is stable for 7 days.28 Faecal S100A12 was increased in paediatric Crohn's disease patients in whom disease activity was only determined by a clinical score and not by invasive procedures such as colonoscopy. This approach, however, is critical taking into account the observed inaccuracy of clinical indices. The CAI and the CDAI poorly correlate with the invasive assessment of inflammation by endoscopy and histology (which was our gold standard) and depend on interobserver variations.56,57 Not surprisingly, these clinical scores have been found to be of little use in the monitoring of disease activity in a number of studies, including ours.5
Our analysis is the first study to show a strong correlation between endoscopically and histologically confirmed intestinal inflammation and faecal levels of S100A12 both in Crohn's disease and ulcerative colitis. We provide evidence that faecal S100A12 discriminates between IBD and IBS with high accuracy. Furthermore, faecal S100A12 differed significantly between active and inactive IBD. As an important feature, the test for faecal S100A12 performs equally well regardless of disease location. The results for S100A12 improved when we included only patients with ulcerative colitis (sensitivity 91%, specificity 96%). These findings are analogous to calprotectin, which is also more accurate in ulcerative colitis.51 The results are comparable to those reported previously in children with Crohn's disease, although the concentrations determined with our ELISA were lower.28 It appears rather unlikely, however, that the concentrations of S100A12 in stool reach the levels of calprotectin, because the latter protein complex is far more abundant.22 Our results also confirm the fact that neither faecal marker of intestinal inflammation is completely specific for IBD, but rather for intestinal inflammation in general, because the levels of these phagocyte‐derived proteins are also elevated during infectious enteritis.54 In this respect, S100A12 as a specific marker of neutrophil activation may, however, also be very useful in monitoring disease activity.
It was our aim to include only well‐characterised IBD patients, in whom endoscopic investigation with the collection of bowel biopsies was performed, allowing the use of a histology inflammation score as a gold standard. Furthermore, immunohistochemical staining of tissue sections confirmed S100A12 expression in the gut of each patient. We could thereby confirm that infiltrating neutrophils are the main source of faecal S100A12. Although the number of individuals included in this study is rather small as a result of the study design, our data prove that faecal S100A12 strongly correlates with intestinal tissue inflammation. Longitudinal studies will be performed in the future to determine the value of faecal S100A12 in the monitoring of inflammation and the prediction of the disease course.
In conclusion, clinical indices are too indistinct to reflect inflammatory activity in chronic IBD and cannot provide precise information about the patient status. In contrast, most procedures used to confirm inflammation in the gut are too invasive or expensive for routine use, or they require radiation exposure. Whereas most serological biomarkers are of limited use, faecal markers of neutrophil activity can indicate intestinal inflammation. Once bacterial enteritis is ruled out, faecal S100A12 may be an excellent non‐invasive marker of disease activity of IBD superior to other biomarkers including faecal calprotectin, which is also derived from monocytes and potentially from epithelial cells, making it less specific for infiltrating neutrophils. Faecal S100A12 correlates with inflammation and can distinguish chronic IBD from non‐organic disease including IBS, with high sensitivity and specificity. This neutrophil‐derived protein can significantly improve our arsenal of non‐invasive biomarkers of intestinal inflammation.
Supplementary table 4 can be viewed on the Gut website at http://gut.bmj.com/supplemental
Copyright © 2007 BMJ Publishing Group & British Society of Gastroenterology
Supplementary Material
Acknowledgements
The authors would like to thank R Gross, T P Kaiser and W Treder for providing a part of the stool samples and M Saers for excellent technical assistance.
Abbreviations
IBD - inflammatory bowel disease
IBS - irritable bowel syndrome
CAI - colitis activity index
CDAI - Crohn's disease activity index
CRP - C‐reactive protein
ESR - erythrocyte sedimentation rate
RAGE - receptor for advanced glycation endproducts
Footnotes
Funding: This work was supported by grants from the Broad Medical Research Program (IBD‐0076) and the Interdisciplinary Clinical Research Center at the University of Muenster (Foe2/005/06).
Competing interests: None.
Supplementary table 4 can be viewed on the Gut website at http://gut.bmj.com/supplemental
References
- 1.Hanauer S B. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1)S3–S9. [DOI] [PubMed]
- 2.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 200655426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niederau C, Backmerhoff F, Schumacher B. Inflammatory mediators and acute phase proteins in patients with Crohn's disease and ulcerative colitis. Hepatogastroenterology 19974490–107. [PubMed] [Google Scholar]
- 4.Nielsen O H, Vainer B, Madsen S M.et al Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol 200095359–367. [DOI] [PubMed] [Google Scholar]
- 5.Brignola C, Lanfranchi G A, Campieri M.et al Importance of laboratory parameters in the evaluation of Crohn's disease activity. J Clin Gastroenterol 19868245–248. [DOI] [PubMed] [Google Scholar]
- 6.Langhorst J, Elsenbruch S, Mueller T.et al Comparison of 4 neutrophil‐derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis 2005111085–1091. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P, Haraldsen G, Rugtveit J. Immunopathology of human inflammatory bowel disease. Springer Semin Immunopathol 199718555–589. [DOI] [PubMed] [Google Scholar]
- 8.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 20033521–533. [DOI] [PubMed] [Google Scholar]
- 9.Foell D, Kucharzik T, Kraft M.et al Neutrophil derived human S100A12 (EN‐RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 200352847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foell D, Wittkowski H, Vogl T.et al S100 proteins expressed in phagocytes: a novel group of damage‐associated molecular pattern molecules. J Leukoc Biol 20078128–37. [DOI] [PubMed] [Google Scholar]
- 11.Fagerhol M K. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet 20003561783–1784. [DOI] [PubMed] [Google Scholar]
- 12.Hunter M J, Chazin W J. High level expression and dimer characterization of the S100 EF‐hand proteins, migration inhibitory factor‐related proteins 8 and 14. J Biol Chem 199827312427–12435. [DOI] [PubMed] [Google Scholar]
- 13.Roth J, Goebeler M, Sorg C. S100A8 and S100A9 in inflammatory diseases. Lancet 20013571041. [DOI] [PubMed] [Google Scholar]
- 14.Roth J, Vogl T, Sorg C.et al Phagocyte‐specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 200324155–158. [DOI] [PubMed] [Google Scholar]
- 15.Lugering N, Stoll R, Kucharzik T.et al Immunohistochemical distribution and serum levels of the Ca(2+)‐binding proteins MRP8, MRP14 and their heterodimeric form MRP8/14 in Crohn's disease. Digestion 199556406–414. [DOI] [PubMed] [Google Scholar]
- 16.Olafsdottir E, Aksnes L, Fluge G.et al Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr 20029145–50. [DOI] [PubMed] [Google Scholar]
- 17.Nissen A C, van Gils C E, Menheere P P.et al Faecal calprotectin in healthy term and preterm infants. J Pediatr Gastroenterol Nutr 200438107–108. [DOI] [PubMed] [Google Scholar]
- 18.Pardi D S, Sandborn W J. Predicting relapse in patients with inflammatory bowel disease: what is the role of biomarkers? Gut 200554321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tibble J A, Sigthorsson G, Bridger S.et al Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 200011915–22. [DOI] [PubMed] [Google Scholar]
- 20.Tibble J A, Sigthorsson G, Foster R.et al Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002123450–460. [DOI] [PubMed] [Google Scholar]
- 21.Frosch M, Metze D, Foell D.et al Early activation of cutaneous vessels and epithelial cells is characteristic of acute systemic onset juvenile idiopathic arthritis. Exp Dermatol 200514259–265. [DOI] [PubMed] [Google Scholar]
- 22.Foell D, Frosch M, Sorg C.et al Phagocyte‐specific calcium‐binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta 200434437–51. [DOI] [PubMed] [Google Scholar]
- 23.Campeotto F, Butel M J, Kalach N.et al High faecal calprotectin concentrations in newborn infants. Arch Dis Child Fetal Neonatal Ed 200489F353–F355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogl T, Propper C, Hartmann M.et al S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem 199927425291–25296. [DOI] [PubMed] [Google Scholar]
- 25.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum 2004503762–3771. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann M A, Drury S, Fu C.et al RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 199997889–901. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt A M, Yan S D, Yan S F.et al The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001108949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong N S, Leach S T, Day A S. Faecal S100A12: a novel noninvasive marker in children with Crohn's disease. Inflamm Bowel Dis 200612566–572. [DOI] [PubMed] [Google Scholar]
- 29.Thompson W G, Longstreth G F, Drossman D A.et al Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2)II43–II47. [DOI] [PMC free article] [PubMed]
- 30.Schroeder K W, Tremaine W J, Illstrup D M. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately acitve ulcerative colitis. A randomized study. N Engl J Med 19873171625–1629. [DOI] [PubMed] [Google Scholar]
- 31.Daperno M, D'Haens G, Van Assche G.et al Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES‐CD. Gastrointest Endosc 200460505–512. [DOI] [PubMed] [Google Scholar]
- 32.Best W R, Becktel J M, Singleton J W.et al Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 197670439–444. [PubMed] [Google Scholar]
- 33.Rachmilewitz D. Coated mesalazine (5‐aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 198929882–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truelove S C, Witts L J. Cortisone in ulcerative colitis. Final report on a therapeutic trial. BMJ 195521041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajaj W, Lauenstein T C, Langhorst J.et al Small bowel hydro‐MR imaging for optimized ileocecal distension in Crohn's disease: should an additional rectal enema filling be performed? J Magn Reson Imaging 20052292–100. [DOI] [PubMed] [Google Scholar]
- 36.Foell D, Hernandez‐Rodriguez J, Sanchez M.et al Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol 2004204311–316. [DOI] [PubMed] [Google Scholar]
- 37.American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum 200247429–433. [DOI] [PubMed] [Google Scholar]
- 38.Zweig M H, Campbell G. Receiver‐operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 199339561–577. [PubMed] [Google Scholar]
- 39.Peterson C G, Eklund E, Taha Y.et al A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol 2002971755–1762. [DOI] [PubMed] [Google Scholar]
- 40.Klass H J, Neale G. Serum and faecal lysozyme in inflammatory bowel disease. Gut 197819233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med J 19984569–73. [DOI] [PubMed] [Google Scholar]
- 42.Adeyemi E O, Hodgson H J. Faecal elastase reflects disease activity in active ulcerative colitis. Scand J Gastroenterol 199227139–142. [DOI] [PubMed] [Google Scholar]
- 43.van der Sluys Veer A, Biemond I, Verspaget H W.et al Faecal parameters in the assessment of activity in inflammatory bowel disease. Scand J Gastroenterol Suppl 1999230106–110. [DOI] [PubMed] [Google Scholar]
- 44.Kane S V, Sandborn W J, Rufo P A.et al Faecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol 2003981309–1314. [DOI] [PubMed] [Google Scholar]
- 45.Sugi K, Saitoh O, Hirata I.et al Faecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil‐derived proteins. Am J Gastroenterol 199691927–934. [PubMed] [Google Scholar]
- 46.Konikoff M R, Denson L A. Role of faecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis 200612524–534. [DOI] [PubMed] [Google Scholar]
- 47.Limburg P J, Ahlquist D A, Sandborn W J.et al Faecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol 2000952831–2837. [DOI] [PubMed] [Google Scholar]
- 48.Carroccio A, Iacono G, Cottone M.et al Diagnostic accuracy of faecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem 200349861–867. [DOI] [PubMed] [Google Scholar]
- 49.Thjodleifsson B, Sigthorsson G, Cariglia N.et al Subclinical intestinal inflammation: an inherited abnormality in Crohn's disease relatives? Gastroenterology 20031241728–1737. [DOI] [PubMed] [Google Scholar]
- 50.Costa F, Mumolo M G, Bellini M.et al Role of faecal calprotectin as non‐invasive marker of intestinal inflammation. Dig Liver Dis 200335642–647. [DOI] [PubMed] [Google Scholar]
- 51.Costa F, Mumolo M G, Ceccarelli L.et al Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut 200554364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foell D, Roth J. S100 proteins in monitoring inflammation: the importance of a gold standard and a validated methodology. J Immunol. 2005;175:3459; author reply 3459–60. [DOI] [PubMed]
- 53.Berni Canani R, Rapacciuolo L, Romano M T.et al Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig Liver Dis 200436467–470. [DOI] [PubMed] [Google Scholar]
- 54.Huicho L, Campos M, Rivera J.et al Faecal screening tests in the approach to acute infectious diarrhea: a scientific overview. Pediatr Infect Dis J 199615486–494. [DOI] [PubMed] [Google Scholar]
- 55.Carlson M, Raab Y, Seveus L.et al Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis. Gut 200250501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomes P, du Boulay C, Smith C L.et al Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut 19862792–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischbach W, Becker W. Clinical relevance of activity parameters in Crohn's disease estimated by the faecal excretion of 111In‐labeled granulocytes. Digestion 199150149–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.