Abstract
Background and aims
Crohn's disease is a life‐long form of inflammatory bowel disease (IBD) mediated by mucosal immune abnormalities. Understanding of the pathogenesis is limited because it is based on data from adults with chronic Crohn's disease. We investigated mucosal T‐cell immunoregulatory events in children with early Crohn's disease.
Methods
Mucosal biopsies and T‐cell clones were derived from children experiencing the first attack of Crohn's disease, children with long‐standing Crohn's disease, infectious colitis, and children without gut inflammation.
Results
As in acute infectious colitis, interleukin (IL) 12 induced T cells from early Crohn's disease to acquire a strongly polarised T helper (Th) type 1 response characterised by high IFN‐γ production and IL12Rβ2 chain expression. Th1 polarisation was not induced in clones from late Crohn's disease. Mucosal levels of IL12p40 and IL12Rβ2 messenger RNA were significantly higher in children with early than late Crohn's disease. These results demonstrate that susceptibility to IL12‐mediated modulation is strongly dependent on the stage of Crohn's disease.
Conclusions
At the onset of Crohn's disease mucosal T cells appear to mount a typical Th1 response that resembles an acute infectious process, and is lost with progression to late Crohn's disease. This suggests that mucosal T‐cell immunoregulation varies with the course of human IBD. Patients with the initial manifestations of IBD may represent an ideal population in which immunomodulation may have optimal therapeutic efficacy.
Both forms of inflammatory bowel disease (IBD), Crohn's disease and ulcerative colitis are life‐long conditions whose initial clinical manifestations appear in the first decades of life. The incidence of IBD is increasing worldwide, and recent epidemiological data show that the diagnosis of IBD is increasingly more frequent in children as the age of onset is decreasing,1,2 with an equal incidence among all ethnic groups.3 Investigation of IBD has largely relied on studies of adult patients with established disease or animal models of acute IBD,4 making it difficult to reconcile data from humans with chronic disease with data from animals with acute disease. Pathogenic events may vary during the course of chronic gut inflammation,5 but claims that new‐onset and long‐standing IBD may be different, or that IBD in children is distinct from IBD in adults are so far unsubstantiated.6 The investigation of human IBD at the earliest possible time, as in children with the first clinical manifestations of Crohn's disease, could obviate the many confounding variables associated with chronicity. In this population early mechanisms of gut inflammation could be examined before masking or modification by disease evolution or therapy. In addition, unique immunological reactivities associated with specific types of inflammation may be uncovered, as demonstrated in children with Crohn's disease.7
In addition to the type of triggering agents and the intrinsic properties of the affected tissue, the long‐term outcome of an inflammatory response is strongly influenced by the local cytokine milieu. This milieu changes with time, and different mediators are involved in the inductive versus the effector phase of an immune response, which eventually acquires distinctive T helper (Th) type 1, Th2, Th17 or alternative profiles.8,9 On the basis of this paradigm, evidence indicates that Crohn's disease is a Th1/Th17‐like condition,10,11 whereas ulcerative colitis appears to represent an atypical Th2 condition.12 These conclusions are largely based on T‐cell cytokine profiles from adults with long‐standing disease and numerous animal models of IBD.13 There is some evidence that cytokine levels may vary with the evolution of human IBD,5 but whether T cells produce quantitatively or qualitatively different cytokine profiles in the early compared with the late stages of IBD is unclear. More importantly, no information is available on cytokines produced by mucosal T cells at the time of disease onset. In addition to the type and quantity of antigen, and how antigen‐presenting cells handle the antigen(s), the outcome of an inflammatory process also depends on the cytokine make‐up at the beginning of such a process. Therefore, it seems essential to define as early as possible in the course of Crohn's disease the response of mucosal T cells to the immunoregulatory cytokines that condition T helper cell differentiation.14 This could reveal whether the cytokine profiles found in late Crohn's disease reflect a fixed response set in motion at the initiation of disease, or represent an evolutionary response to the progression of IBD. This fundamental question cannot be answered using T cells from resected tissues because children with early Crohn's disease seldom undergo an operation. Therefore, with the caveat that the chosen approach may not be entirely representative of the intestinal immune response because other compartments such as the mesenteric lymph nodes are not sampled, we studied T cells derived from colonoscopic mucosal biopsies from a unique population of children with the very first attack of Crohn's disease. The production of interleukin (IL) 2, IFN‐γ, IL4, and IL10, as well as IL12 receptor (IL12R) β1 and β2 chain expression were measured in T‐cell clones exposed to the immunomodulatory effects of IL12 and IL4. In addition, total levels of IL12 and IL12Rβ2 chain messenger RNA were measured in the biopsies. The results show that differences in cytokine production and susceptibility to immune modulation occur exclusively in early Crohn's disease. IL12 conditioning of mucosal T cells from children with early Crohn's disease induces high levels of IFN‐γ similar to those produced by IL12‐stimulated T cells from children with acute infectious colitis. In contrast, mucosal T cells of children with late Crohn's disease fail to upregulate IFN‐γ production in response to IL12. In addition, the expression level of the IL12Rβ2 chain is significantly higher in T cells from children with early Crohn's disease and infectious colitis than children with late IBD, and tissue IL12 and IL12Rβ2 chain mRNA were also higher in early than late Crohn's disease. Therefore, at the beginning of IBD, mucosal T cells mount a Th1 response that resembles an acute infectious process, and is lost with progression to late disease.
Methods
Patient population
The patient population comprised children referred to the Division of Pediatric Gastroenterology and Nutrition at Rainbow Babies and Children's Hospital, Cleveland, Ohio, and the Children's Hospital of Wisconsin, Milwaukee, Wisconsin, for the evaluation of known or suspected IBD, or other gastrointestinal symptoms. Both are tertiary academic referral centers with essentially identical patient populations with regard to race, ethnicity and social strata. Patients were selected on the basis of their need for colonoscopy, as a result of one or more of the following symptoms: abdominal pain, diarrhea, rectal bleeding, weight loss, growth failure, anemia, hypoproteinemia, or fever of unknown origin. The patient population evaluated at Rainbow Babies and Children's Hospital was utilised to study cytokine production and modulation by mucosal T‐cell clones, and that evaluated at the Children's Hospital of Wisconsin was utilised to measure levels of IL12Rβ2 mRNA in whole mucosal biopsies. The study was approved by the Institutional Review Boards of the University Hospitals of Cleveland and of the Children's Hospital of Wisconsin, and mucosal biopsies were obtained after receiving informed consent from the parents and the patients.
Clinical groups and diagnostic criteria
Clinical groups were defined on the basis of stringent clinical, radiological, endoscopic, microbiological and histopathological criteria, as previously reported.7,15 Normal was defined by the absence of any radiological and endoscopic abnormalities, and the presence of a histologically normal mucosa. Crohn's disease was defined based on the presence of unequivocal radiological evidence of small bowel abnormalities, endoscopic evidence of discontinuous inflammation in the small or large bowel, histological detection of granulomas, or their combination. The mere presence of non‐specific chronic inflammation in either the small or large bowel extending beyond the muscularis mucosae excluded patients from the Crohn's disease group. Early and late Crohn's disease were defined based on the duration of disease according to the following criteria: Early: first attack of Crohn's disease, as defined above, in a child with no previous history of any gastrointestinal symptoms; Late: Crohn's disease in a child with at least five years history from the time of initial diagnosis and with persistent clinical activity. Infectious colitis was defined as acute mucosal inflammation without any previous history of gastrointestinal symptoms and with stool cultures negative for a defined bacterial pathogen. Eosinophilic/allergic colitis was defined by evidence of massive eosinophil infiltration of the colon in the absence of IBD and other known causes of eosinophilia (e.g. drug reactions or parasitic infections).16
The patient population evaluated at Rainbow Babies and Children's Hospital included 18 patients (10 male and eight female) ranging from four to 18 years old. On the basis of stringent clinical, radiological, endoscopic, microbiological and histopathological criteria,8,14 12 of them were classified as normal (n = 3) or Crohn's disease (n = 9); in addition, four had infectious colitis (two Clostridium difficile, one Campylobacter, and one Aeromonas) and two eosinophilic colitis. The early IBD group included five children with Crohn's disease with duration of symptoms from zero to six months before diagnosis; these children were studied at the same time that the diagnosis of Crohn's disease was made. The late Crohn's disease group included four children with five to eight years of established disease before the time of study. The population evaluated at the Children's Hospital of Wisconsin included 25 patients (14 male and 11 female) ranging from six to 17 years old. They included 15 children with early Crohn's disease, with duration of symptoms from zero to six months before diagnosis; six with late Crohn's disease, with five to 10 years of established disease before the time of study; and four normal control children.
According to the above criteria,7,15 all children had moderate to severe disease activity based on physician global assessment,17 endoscopic18 and histological scores,15 with no significant differences between the patients recruited at the two hospitals. None of the children with early Crohn's disease and children in the normal control group were exposed to any medication before the endoscopic retrieval of biopsies. By definition, children with late Crohn's disease had the disease for a minimum of five years, and the details of their treatment are outlined in table 1.
Table 1 Medications in use at the time of mucosal biopsies in children with late inflammatory bowel disease.
| Diagnosis | Type of medication | |||
|---|---|---|---|---|
| 5‐ASA* | Prednisone* | Immunomodulators* | Biologicals** | |
| Crohn's disease | No | No | Yes | No |
| Crohn's disease | Yes | No | Yes | No |
| Crohn's disease | No | No | Yes | No |
| Crohn's disease | No | Yes | Yes | No |
| Crohn's disease | No | No | Yes | No |
| Crohn's disease | No | No | Yes | No |
| Crohn's disease | No | Yes | No | No |
| Crohn's disease | No | No | Yes | No |
| Crohn's disease | No | No | Yes | Yes |
| Crohn's disease | No | Yes | No | Yes |
5‐ASA, 5‐Aminosalicylic acid.
*Dose: 5‐ASA: 50–70 mg/kg per day; corticosteroids: 1–2 mg/kg per day; azathioprine: 2–3 mg/kg per day; methotrexate: 15–25 mg/week. Duration: 5‐ASA, azathioprine and methotrexate were used intermittently from the time of diagnosis, and prednisone was used for 1–2 months before biopsy.
**Infliximab: infusion of 5 mg/kg every 8 weeks
Mucosal T‐cell cloning
Mucosal T‐cell clones were derived from endoscopic biopsies obtained from colonic mucosa using the same size biopsy forceps in all patients. Biopsies were rinsed and incubated in Hanks balanced salt solution with 2.5% penicillin–streptomycin–fungizone mixture for four to five hours at room temperature to prevent bacterial contamination. Each of four biopsies was cut into three pieces, yielding 12 fragments. Each fragment was put into a 24‐cluster plate well (Costar, Cambridge, Massachusetts, USA) containing 1 ml RPMI 1640, 20% fetal bovine serum (Biowhittaker, Walkersville, Maryland, USA), penicillin–streptomycin–fungizone–gentamycin mixture and 20 units/ml of recombinant human IL2 (kindly provided by Chiron, Emeryville, California, USA). Cell growth was invariably observed by the end of the first week of culture with IL2, but no growth was observed in the absence of IL2 or exposure to IL4 or IL12 alone. T‐cell cloning was performed as previously reported with some modifications.19 Five to seven day‐old T‐cell cultures were suspended at 104/ml, and serially diluted to 0.3 cells/well of a 96 round‐bottom microtiter plate. Each well received 5 × 104 irradiated (5000 rads) allogeneic feeder cells (pooled fresh peripheral blood mononuclear cells (PBMC) from multiple normal donors), 20 units/ml of recombinant IL2 in 100 μl, without any additional stimuli. After three to four days 50% of the volume was removed from each well, replaced by fresh medium containing 40 units/ml IL2, and this was repeated twice weekly. Feeder cells were added every week, and wells were examined weekly for growth. Growing cells were transferred to larger vessels while feeding continued using fresh medium and IL2. The clonal efficiency (percentage of wells giving rise to a clone compared with all wells seeded) of control cells was 15% (10 patients) and that of Crohn's disease was 20% (19 patients), regardless of early versus late disease and the type or presence or absence of medications. The number of cells per clone was 1.1 × 106 for control clones and 1.5 × 106 for Crohn's disease clones (60 clones in each group), and these values were not affected by the stage of disease or use of medications.
Vβ chain analysis, Vβ polymerase chain reaction (PCR) product sequencing, and complementary determining region 3 (CDR3)
For each T‐cell clone inflammatory bowel disease analysed, 50 ng total RNA were converted to complementary DNA using Superscript II (GIBCO Life Technologies, Gaithersburg, Maryland, USA) and Cβ‐specific primers.20 Each cDNA sample was subsequently diluted in a PCR cocktail and equally divided into 24 reactions for PCR analysis (2 ng RNA/Vβ reaction). Amplifications of 25 cycles of this quantity template of total RNA isolated from PBMC have revealed Vβ‐specific bands for essentially all Vβs included in the assay (unpublished observations). The Vβ‐specific oligos used in the PCR assays have been described previously20 with the exception of the oligos for the pseudogenes Vβ10 and Vβ19. A fluorescent (FAM)‐labeled (Applied Biosystems Inc., Foster City, California, USA) Cβ antisense oligo was used for all reactions to detect the Vβ‐specific PCR products. A Cβ sense oligo reaction was included for each sample as a template control. After 25–30 cycles of PCR amplification (94°C, 55°C, 72°C for one minute each), 3.2 μl of the 50 μl PCR reaction was electrophoresed through a 5% polyacrylamide gel. PCR fragment analysis was performed with an ABI 373 DNA sequencer and Gene Scan software (both from Applied Biosystems Inc.).
Randomly selected Vβ3‐positive clones were further analysed by sequence analysis. Two microliters of the Vβ3 PCR products were re‐amplified with non‐fluorescent labeled oligos and the PCR product gel purified and ligated into the SmaI site of PUC18. Several colonies for each sample were picked and miniprep DNA sequenced by the ABI cycle sequencing method using the ABI 373 sequencer. Productive (in‐frame) rearrangements for all clones were observed (i.e. an open reading frame from the Vβ3 segment through the Dβ–Jβ and Cβ junctions) and the predicted amino acid sequences deduced from the nucleotide sequence.
For CDR3 display, equal amounts of cDNA were amplified by PCR for 32 cycles in a series of 10 μl reactions that contained a consensus TCRB constant region (TCRBC) antisense primer (GCCTTTTCCCTGTGGGAG at 5 pmol), and TCRB V1‐24 region family‐specific primers (5 pmol).21 The labeled PCR products were resolved on a 6% polyacrylamide gel to create a CDR3 display.
Measurements of cytokine‐level
Supernatants were assayed using an ELISA developed with pairs of commercially available monoclonal antibodies. ELISA plates (Dynatech, Chantilly, Virginia, USA) were coated with 100 μl human IL2 (4 μg/ml; R&D, Minneapolis, Minnesota, USA), IL4 (2.5 μg/ml; PharMingen, San Diego, California, USA), IL10 (1 μg/ml; PharMingen) and IFN‐γ antibody (2 μg/ml; Endogen, Cambridge, Massachusetts, USA) in phosphate‐buffered saline (PBS) and incubated at 4°C. After an overnight incubation, wells were blocked by adding 250 μl 3% bovine serum albumin in PBS for one hour at 37°C. After every step wells were washed four times in PBS containing a 0.05% Tween solution. Experimental samples and standards were prepared in 1% bovine serum albumin and 100 μl samples were placed in individual wells and incubated at 4°C overnight. The following biotin‐labeled detection antibodies were added and incubated at room temperature for two hours: 25 ng/ml anti‐human IL2 (R&D), 2.5 μg/ml anti‐human IL4 (PharMingen), 1 μg/ml anti‐human IL10 (PharMingen) and 0.5 μg/ml anti‐human IFN‐γ (Endogen). Peroxidase‐conjugated streptavidin (Zymed, San Francisco, California, USA) was added to each well and incubated for 30 minutes at room temperature. ABTS‐peroxidase (Kerkegaard and Perry Laboratories, Gaithersburg, Maryland, USA) was used for detection, and after 20 minutes the absorbance at 405 nm was read in an ELISA reader (Biotek Instruments, Inc., Winooski, Vermont, USA).
IL12 and IL4 modulation of cytokine production by mucosal T‐cell clones
T‐cell clones were derived as detailed above, but in addition to IL2 parallel clones were generated in the presence of IL2 plus IL12, and IL2 plus IL4 from the beginning of the cultures. To do so, biopsies from the same site of the same patient were divided into three separate cultures, and each one was exposed to IL2 (20 U/ml), IL2 plus IL12 (1 ng/ml, kindly provided by Hoffmann‐La Roche, Inc., Nutley, New Jersey, USA), and IL2 plus IL4 (5 ng/ml, kindly provided by Monsanto, St Louis, Missouri, USA). These culture conditions were maintained throughout the entire cloning procedure. Control cultures were carried out with IL12 and IL4 alone, but no growth was observed. Six‐week‐old T‐cell clones were washed and stimulated by a combination of 1 μg/ml anti‐CD3 antibody (Orthodiagnostics, Raritan, New Jersey, USA) and 40 ng/ml phorbol ester (Sigma, St Louis, Missouri, USA). After 48 hours supernatants were harvested and stored at −70°C.
Assessment of IL12R mRNA expression levels
The expression level of IL12Rβ1 and β2 chain by mucosal T‐cell clones was assessed by semiquantitative reverse transcriptase (RT)–PCR using glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) as an internal standard. Primers were designed using the MacVector program (Oxford Molecular Group, Oxford, UK) and synthesised by Bio‐synthesis, Inc. (Lewisville, Texas, USA): IL12Rβ1 forward primer 5′–CAGTGGCTCTGAATATCAGCGTC–3′, and backward primer 5′–AGGACCGTAGACCACAAGGTGAG–3′; IL12Rβ2 forward primer 5′–ATGTCTGGTACATGAAACGGCAC–3′, and backward primer 5′–TCTCCATTCCAC CACGTACTCC–3′; GAPDH forward primer 5′–CCATCACCATCTTCCAGGAG–3′, and backward primer 5′–CCTGCTTCACCACCTTCTTG–3. Total RNA was extracted from T‐cell clones using the RNAzol B method (Tel‐Test, Inc., Friendswood, Texas, USA). First strand cDNA was synthesised from total RNA using Superscript II (GIBCO Life Technologies), applying conditions recommended by the manufacturer. PCR amplifications were conducted in buffer (final volume 25 μl) containing 2.5 mmol MgCl2, 2.5 mmol dNTP, 1 unit/ml Taq DNA polymerase (Boehringer Mannheim Corp., Indianapolis, Indiana, USA) and 50 ng IL12Rβ1, β2 primers or GAPDH primers. Reactions were performed separately for each set of primers. Each reaction was overlaid with 100 μl mineral oil, denatured at 94°C for three minutes, and carried out for 30 cycles. Resulting PCR products for IL12Rβ1, IL12Rβ2 and GAPDH were combined, electrophoretically separated in the same lane on a 1.2% agarose gel, stained with ethidium bromide, and band intensity quantified with a phosphorimager (BioRad Laboratories, Emeryville, California, USA). The relative amounts of IL12Rβ1 and IL12Rβ2 chain were assessed in relationship (ratio) to the amount of GADPH in each amplification reaction.
Quantitative real‐time RT–PCR
Total RNA was extracted from endoscopic mucosal biopsies with RNAzol B (Tel‐Test, Inc.), and 1 μg was used to make cDNA (1st Strand cDNA Synthesis Kit for RT–PCR (AMV); Roche, Indianapolis, Indiana, USA), which was checked for quality and quantity by conventional PCR for 18S. For each sample this reaction was scaled up so enough cDNA was generated at the same time for all the required real‐time PCR reactions. In addition to the biopsy‐derived RNA samples, control RNA was obtained from stimulated PBMC, and one reaction without enzyme was carried out to serve as a negative control.
For production of the IL12p40 standard DNA, RNA was isolated from PBMC stimulated with IFN‐γ (100 ng/ml) for seven hours and lipopolysaccharide (1 μg/ml) for two hours.22 For production of the IL12Rβ2 chain standard DNA, RNA was isolated from PBMC stimulated with recombinant IL12 (2 ng/ml), anti‐IL4 antibody (125 ng/ml) and 5% phytohemagglutinin for five to six days followed by a second stimulation with IL2 (200 U/ml) for two days.23 18S standard DNA was similarly obtained. At the end of the respective incubation periods, RNA was extracted from PBMC, cDNA generated, and conventional PCR amplification was performed for target molecules and control 18S. Products were run on 1% agarose gel electrophoresis, stained with ethidium bromide and, after visualisation of a single band of the correct size, the remaining amplicon was used to clone in plasmid (TOPO‐4 cloning kit; Invitrogen, Carlsbad, California, USA) and purify all three molecules (Qiagen plasmid purification; Qiagen, Valencia, California, USA). All products were sequenced to ensure the accuracy of inserted DNA.
The following primers and probes were designed using Primer Express on the same portion of genomic RNA used for the cloning of standards, and obtained from Applied Biosystems. For IL12Rβ2 chain: forward primer, CCGTGAGCAGATGTACCCTTTATT; reverse primer, GGTTTCAGATCCAGCAAATCATG; probe: 6FAMCTGGTACTGCTTAATCGACTCA GATATCGGCCTAMRA. For IL12p40: forward primer, ATGCCGTTCACAAGCTCAAGTAT; reverse primer, GACCTCCACCTGCCGAGA; probe, 6FAMACATCATCAACCCTGACCCACC CAAGATAMRA. The primers and probes for 18S were a kind gift from Dr Z. Toossi (Case Western Reserve University): forward primer, CGCCGCTAGAGGTGAAATTC; reverse primer, CATTCTTGGCAAATGCTTTCG; probe, VICACCGGCGCAAGACGGACCAGATAMRA.
For real‐time PCR amplification, all reactions were carried out in triplicate within 24 hours and using equal volumes, and all plates included no template control and serial dilutions (10−108 with 50 μg/ml yeast RNA; Ambion, Austin, Texas, USA) of the standards described above. Optimised concentrations of 300 nmol for the primers and 150 nmol for the probes were used. Forty cycles of amplification were performed using the ABI prism 7700 sequence detector (Applied Biosystems). Standard curves for each amplicon were comparable and, according to company specifications, results were expressed as values (relative values) relative to the mean values of IL12p40 and IL12Rβ2 obtained from mucosal biopsies of four normal control children.24
Statistical analysis
Multiple replicates of cytokine concentrations were obtained from clones of each patient and converted to the log scale. The mean of the replicate on the log scale was used as a point estimate for each patient for the purpose of analysis. Comparison of each disease state with normal, early with late IBD for unmodulated cytokine production, and for the IL12Rβ1 and β2 chain mRNA expression level was performed using a two‐sample t‐test. Cytokine modulation (addition of IL12 or IL4) was defined as a ratio of baseline to post‐addition concentration greater than one (on the raw scale), or similarly, log(addition) − log(baseline) >0. Because the test of significance for the effect of modulation is against a constant (0) a one‐sample t‐test was used. As multiple comparisons were performed for each analysis, the level of significance was set at 0.05 divided by the number of comparisons. Figures show the values on the raw scale as means ± SE. Comparisons among the IL4 concentrations in supernatants of T‐cell clones from control, infectious and eosinophilic colitis, and comparison of the relative values of IL12p40 and IL12Rβ2 chain mRNA measured by real‐time RT–PCR between early and late Crohn's disease were performed by the Mann–Whitney test, with a significance level set at 0.05.
Results
Clonality of mucosal T cells to ensure that the T cells used in the modulation experiments were true clonal populations were characterised by phenotypic and molecular criteria. Flow cytometric analysis showed a consistent CD3+, CD4+, CD8− phenotype in six randomly selected T‐cell clones (three normal, three Crohn's disease). Employing oligonucleotides for the amplification of Vβ1‐24, 29 of 29 randomly selected T‐cell clones showed a single dominant but variable Vβ region utilisation, and clonality was confirmed by CDR3 sequencing of four randomly selected Crohn's disease clones expressing Vβ3. For CDR3 display, one clone from a histologically normal control, and three from Crohn's disease mucosa were randomly selected and run individually to display all 24 Vβ family products. Each clone showed only one specific Vβ region.
Representativeness of mucosal T‐cell clones
Because all mucosal biopsies were obtained from areas with a typical endoscopic appearance of Crohn's disease, it was assumed that the derived T‐cell clones were representative of the pathogenic T cells involved in the disease process. To confirm that this was indeed the case, T‐cells clones were also derived from mucosal biopsies of two children with eosinophilic colitis (26 clones), two children with infectious colitis (two C. difficile colitis; 27 clones), and three control children without colonic inflammation (38 clones). When IL4 was measured in the culture supernatants, 73% of the T‐cell clones from normal children produced IL4 (596 ± 155 pg/ml), and 60% of the clones from children with infectious colitis produced IL4 (463 ± 139 pg/ml); in contrast, all (100%) mucosal T‐cell clones from children with eosinophilic/allergic colitis produced IL4 and in substantially (three to fourfold increase) higher concentration (2171 ± 411 pg/ml; p<0.001 compared with both control and infectious colitis). No significant differences were observed with regard to IL2 production among the T‐cell clones of the same three groups (data not shown).
Cytokine production by mucosal T‐cell clones
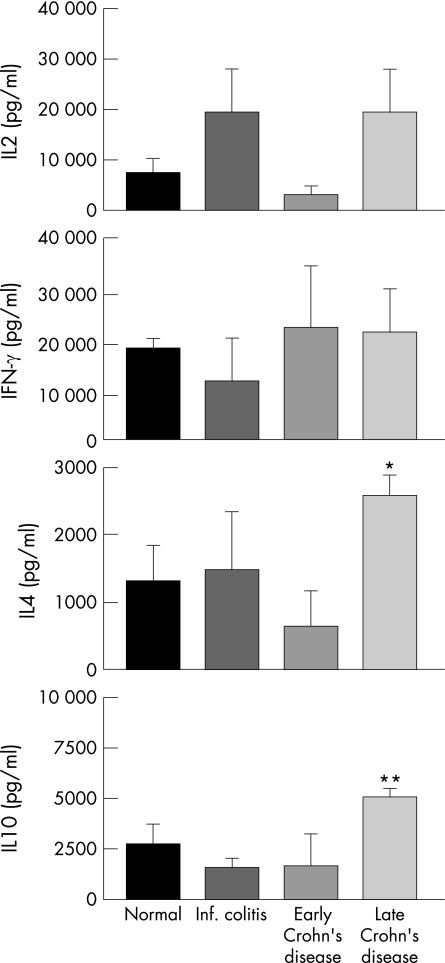
The capacity of IL2‐derived T cells to produce IL2, IFN‐γ, IL4 and IL10 was investigated using T‐cell clones derived from the histologically normal mucosa of control children and the inflamed mucosa of children with inflammatory conditions. IL2 production showed variability among T cells from normal children and children with infectious colitis, as well as early and late Crohn's disease, but statistically significant differences were not observed (fig 1). IFN‐γ production showed less variability, also without statistically significant differences (fig 1). In contrast, the amounts of IL4 and IL10 were significantly different in early and late Crohn's disease (p = 0.001 for IL4, and p = 0.002 for IL10), with no statistically significant differences detected in the other groups (fig 1).
Figure 1 Cytokine production by mucosal T‐cell clones. Production of IL2, IFN‐γ, IL4 and IL10 by mucosal T‐cell clones from children with normal mucosa (26 clones from three separate patients; 8, 6 and 12, respectively), children with acute infectious colitis (16 clones from two patients; 10 and 6, respectively), and early (39 clones from five patients; 8, 12, 7, 6, and 6, respectively) and late Crohn's disease (21 clones from three patients; 10, 6, and 5, respectively). T‐cell clones were derived from mucosal biopsies in the presence of IL2, stimulated by anti‐CD3 antibody plus phorbol ester for 48 hours to obtain supernatants for cytokine measurement. Data are expressed as mean ± SE. *p = 0.001 for IL4 production between early and late Crohn's disease; **p = 0.002 for IL10 production between early and late Crohn's disease.
Differential modulation of IFN‐γ production by mucosal T‐cell clones in early and late Crohn's disease
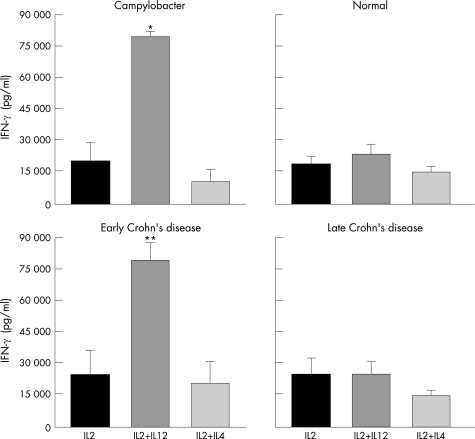
To investigate whether T‐cell cytokine production was susceptible to immunomodulation, the same clones used in the previous experiment (fig 1) were concomitantly grown from the beginning of the culture in the presence of IL12 or IL4, which are responsible for the differentiation of Th1 and Th2 effector cells, respectively. When T cells from normal children were examined, no significant modulatory effect of IL12 or IL4 on IFN‐γ production was observed (p<0.5 and p<0.6, respectively; fig 2). When cells derived from children with acute infectious colitis were evaluated, a striking modulatory effect was observed. In Campylobacter colitis, a fourfold increase in IFN‐γ production was observed when T cells were grown in the presence of IL12 compared with those grown in IL2 (p = 0.0004; fig 2), whereas IL4 had no modulatory effect (p<0.2). An identical pattern of response to IL12 and IL4 was seen with T cells from another child with Aeromonas colitis (not shown).
Figure 2 Modulation of IFN‐γ production by mucosal T‐cell clones. Effect of IL12 and IL4 on IFN‐γ production by mucosal T‐cell clones from children with normal mucosa (19 clones from three patients; 5, 9 and 10, respectively), a child with Campylobacter colitis (10 clones from one patient), and children with early (39 clones from five patients; 8, 12, 7, 6, and 6, respectively) and late (21 clones from three patients; 10, 6, and 5, respectively) Crohn's disease. Clones were separately derived from mucosal biopsies in the presence of IL2, IL2 plus IL12, and IL2 plus IL4 from the beginning of the culture, stimulated by anti‐CD3 antibody plus phorbol ester for 48 hours to obtain supernatants for IFN‐γ measurement. Data are expressed as mean ± SE. For Campylobacter colitis the displayed SE are within patient. *p = 0.0004, **p = 0.0001, and ***p = 0.0001, compared with IL2 alone in Campylobacter colitis, and early Crohn's disease, respectively.
Next, we compared the modulatory effect of IL12 and IL4 on T cells derived from children with early and late Crohn's disease. In early Crohn's disease, IL12 induced a fourfold increase in the level of IFN‐γ production (p = 0.0001), whereas IL4 had no significant modulatory effect (p<0.5; fig 2), a response mimicking that seen with T cells from acute infectious colitis. In sharp contrast, when T cells were derived from children with late Crohn's disease, both IL12 and IL4 failed to modulate their IFN‐γ production (p<0.8 and p<0.3, respectively; fig 2).
Differential expression of mucosal T‐cell clone IL12Rβ2 chain in early and late Crohn's disease
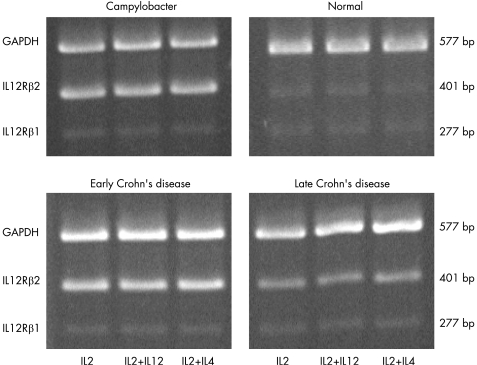
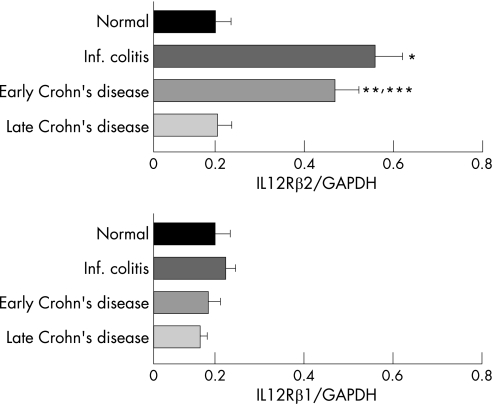
The above results suggest that the mucosal T‐cell response to IL12 depends on the stage of Crohn's disease. If true, the T cells from early but not late Crohn's disease that upregulate IFN‐γ production should display the high affinity IL12 receptor. Therefore, we investigated whether the IL12Rβ2 chain, expressed exclusively by Th1 cells,25,26 was differentially expressed by T cells in early and late Crohn's disease. When the levels of IL12Rβ1 and β2 chain mRNA were assessed by RT–PCR, a pattern emerged showing a clear and consistent correlation between β2 chain expression level and IL12 responsiveness (fig 3). Regardless of the cytokines used to derive the clones, the expression level of the β1 chain mRNA was low in all clones from all groups (fig 3 and fig 4). In contrast, all T cells that upregulated their IFN‐γ production in response to IL12 displayed a two to threefold increase in β2 chain mRNA, regardless of the cytokines used. These included T cells from acute infectious colitis as well as early Crohn's disease (p = 0.03 for infectious colitis versus normal, p<0.05 for early Crohn's disease versus normal, and p<0.05 for early Crohn's disease versus late Crohn's disease, respectively; fig 3 and fig 4). The level of β2 chain mRNA in T cells from late Crohn's disease failed to increase in response to IL12 even though all cells were derived from actively inflamed mucosa (fig 4).
Figure 3 Expression of IL12Rβ2 mRNA by mucosal T‐cell clones. Representative display of reverse transcriptase–polymerase chain reaction amplification products showing mRNA expression levels for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), IL12Rβ2 and β1 chain in mucosal T‐cell clones from a normal child without any evidence of gastrointestinal inflammation, and one each with infectious colitis (Campylobacter), early Crohn's disease, and late Crohn's disease. T‐cell clones were derived as detailed in the legend of fig 2.
Figure 4 Expression of IL12R β2 mRNA by mucosal T‐cell clones. Relative amounts of IL12Rβ1 and β2 chain mRNA in IL2‐derived mucosal T‐cell clones from two normal children without any evidence of gastrointestinal inflammation (10 clones), and two children with infectious colitis (nine clones), three early Crohn's disease (19 clones), and thee late Crohn's disease (11 clones). Comparable results were observed with clones derived in the presence of IL2 plus IL12 and IL2 plus IL4. Amounts are measured as ratios of IL12Rβ2/glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (A) and IL12Rβ1/GAPDH (B) and expressed as mean ± SE. *p = 0.03 for infectious colitides versus normal; **p<0.05 for early Crohn's disease versus normal; ***p<0.05 for early Crohn's disease versus late Crohn's disease, respectively.
Differential mucosal levels of IL12p40 and IL12Rβ2 chain mRNA in early and late IBD
To confirm that the enhanced levels of IL12Rβ2 expressed by the mucosal T‐cell clones reflected the in‐vivo situation, and learn whether this was in response to a higher production of IL12 in early IBD, we quantified IL12Rβ2 chain and IL12p40 mRNA by real‐time RT–PCR in whole mucosal biopsies from 15 children with early Crohn's disease and six children with late Crohn's disease. The levels of IL12p40 mRNA in biopsies of children with early Crohn's disease were eight times greater than those in biopsies of children with late Crohn's disease (6.6 ± 1.8 versus 0.8 ± 0.9; p = 0.033), and those of IL12Rβ2 mRNA were four times as high (1.9 ± 0.6 versus 0.5 ± 0.4; p = 0.032).
Modulation of mucosal T‐cell clone IL4 and IL10 production in early Crohn's disease
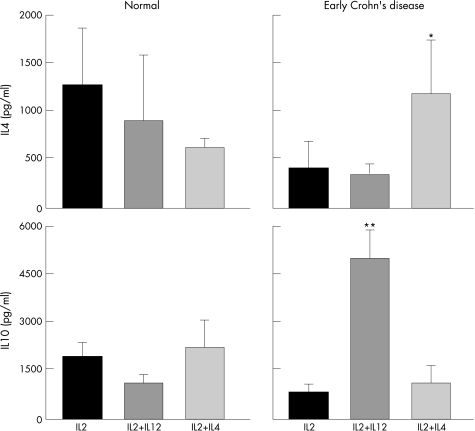
In view of the differential modulation of IFN‐γ production by mucosal T‐cell clones in early and late IBD, we investigated whether a similar response was noted with other cytokines. The levels of IL2, IL4 and IL10 were measured in the same clones modulated by IL12 or IL4 (fig 2). Among all groups (normal children and children with infectious colitis, early and late Crohn's disease) significant modulatory effects were observed exclusively in clones from early Crohn's disease. In these T cells, IL4 induced a fourfold increase in IL4 production compared with that of IL2‐driven clones (p = 0.0025; fig 5), but not in late Crohn's disease clones (not shown). In the same cells, IL12 induced a sevenfold increase in IL10 production (p<0.0001; fig 5), but not in late Crohn's disease T cells (not shown).
Figure 5 Modulation of cytokine production by mucosal T‐cell clones. Effect of IL12 and IL4 on IL4 and IL10 production by mucosal T‐cell clones from children with normal mucosa (27 clones from three patients) and children with early Crohn's disease (29 clones from five patients). T‐cell clones were derived and supernatants generated as detailed in the legend of fig 3. Data are expressed as mean ± SE. *p = 0.0025, **p = 0.0001 compared with IL2 alone in early Crohn's disease.
Discussion
The results of this study are the first to show clear differences in the production of and response to cytokines by T cells in early and late human Crohn's disease. During the initial manifestation of the disease mucosal T cells are susceptible to the modulatory influence of Th1‐driving cytokines, but not once the disease has been present for a prolonged period of time. This is demonstrated by a striking elevation of IFN‐γ production in response to IL12 by T cells in early, but not late, Crohn's disease. IL12 is essential to differentiate naive helper T cells into Th1 cells and induce Th1 responses in infectious diseases,27 but also to condition or exacerbate autoimmune diseases.28,29 The response of T cells to IL12 is mediated by a receptor complex composed of two subunits named β1 and β2 chains.30 In both human and murine T cells the expression of the IL12Rβ2 chain is restricted exclusively to Th1 cells.25,26 This is important because it proves that in early Crohn's disease the gut still contains T cells that are naive enough to be influenced to differentiate into typical Th1 cells by both phenotypic and functional criteria. This response is identical to that observed with T cells in acute infectious colitis, corroborating the theory that T‐cell clones translate real pathogenic events.26 The elevation of IL12Rβ2 chain expression and IFN‐γ production in infectious colitis and early Crohn's disease suggests that the initial inflammatory events in Crohn's disease are dominated by a mucosal “infectious‐type” Th1 response, a concept supported by studies in experimental colitis31 and recent epidemiological evidence linking acute gastrointestinal infections to an increased risk of IBD.32
In both humans and animals the effect of IL12 may vary depending on the phase of parasitic, allergic, and autoimmune diseases.33,34,35,36 In acute toxoplasmosis, macrophage‐derived IL12 is necessary to induce a Th1 response, but is no longer required to maintain anti‐parasite immunity once this is established.37 In collagen type II arthritis, IL12 accelerates joint destruction in the early phase, whereas in the chronic phase it exerts a beneficial suppressive role.36 In mice with acute enteric infections, IL12 may induce protection or exacerbation depending on the genetic background of the host.38 Particularly significant are observations in IL10‐deficient mice. In this model, colitis is associated with increased IL12 levels only early in the disease process, as shown by the fact that the neutralisation of IL12 prevents and reverses early disease, whereas it is ineffective in late colitis.39 This variability in the type, degree and timing of responsiveness to IL12 is closely correlated with the IL12Rβ2 chain expression level.40,41 This correlation was evident in high IFN‐γ‐producing T cells from children with infectious colitis as well as early Crohn's disease. The reverse was true for normal mucosa and late Crohn's disease T cells, in which low IL12Rβ2 chain expression was associated with little or no IL12‐induced IFN‐γ production.
As high IL12Rβ2 chain expression and IFN‐γ production faded from early to late Crohn's disease, parallel changes in mucosal T‐cell reactivity are expected. In our study, these changes cannot simply result from differences in disease severity because all children with early and late disease had comparable activity by clinical, endoscopic and histological criteria. The possibility that changes are caused by treatment in the late Crohn's disease group cannot be entirely excluded, but this is also improbable given that the time period needed for the generation of T‐cell clones is certainly long enough to eliminate any residual drug‐related effects. In addition, if lack of modulation in late Crohn's disease was caused by immunomodulatory therapy, the normal control group, which was on no such therapy, should have upregulated IFN‐γ production in response to IL12, but it did not, supporting the notion that the lack of modulation is unrelated to therapy. In fact, the opposite may be true. Finding consistent differences in cytokine patterns between early and late disease T cells despite the experimental conditions speak strongly in favor of a built‐in behavior. Changes in the composition of tissue T cells thus appear more likely to be dependent on the basic biology of the disease process. In this regard, the reported accumulation in early Crohn's disease aphthoid ulcers of discrete clonal populations of T cells with a receptor repertoire distinct from that of clones present in advanced lesions is of great interest,42 indicating that T cells activated at the onset of the disease are radically different from those activated in late disease. Those and our observations indicate that changes in Crohn's disease T‐cell reactivity are intimately associated with disease evolution, and correlate with switches between T helper cytokine profiles, as seen in a number of animal and human studies. Switches from a Th1 to a Th2 pattern occur in non‐obese diabetic mice, BB rats, and atherosclerotic apolipoprotein‐E knockout mice.43,44,45 With regard to experimental IBD, in the colitis of IL10‐deficient mice and the ileitis of SAMP1/YitFc mice, both of which are Th1‐mediated initially, there is a marked increase in disease‐mediating Th2 cytokines in late disease, e.g. IL4 and IL13 in the colitis model and IL5 and IL13 in the ileitis model.39,46 In humans with acute allergic dermatitis, a classic Th2‐type condition, the skin is initially infiltrated by IL4‐producing cells, but in the chronic phase IFN‐γ producing Th1 cells predominate.47 In patients with rheumatoid arthritis, a condition in which the notion of distinct early and late pathogenic phases is well established, the disease is characterised by a unique but transient synovial T‐cell cytokine profile (elevated IL2, IL4, IL13, IL15, and IL17), and this profile disappears in established arthritis.48
Considering the complexity of the inflammatory response, one can only speculate about the factors that might induce changes in cytokine profiles during the course of immune‐mediated disorders, but they could include variations in the amount and duration of antigenic stimuli,49 the state of differentiation of inflammatory T cells,50 or even dietary factors.44 In the case of IBD, the recent advances in our understanding of how bacterial–immune interactions are mediated and regulated allows more focused speculations. Changes in the composition of the commensal flora or its recognition by Toll‐like receptors (TLR) could certainly alter the subsequent adaptive immune response.51,52 The phenotype and state of maturation of mucosal dendritic cells is important in the activation of pathogenic and regulatory T cells,53 and TLR expressed on T cells control both the expansion and function of regulatory T cells, which in turn may control the type and activity of cytokine‐producing T helper effector cells.54,55 Variations in the number, type, state of maturation and TLR repertoire expression of dendritic cells and T cells could thus explain disparate or even opposing cytokine patterns during an immune response, more so considering that this response unfolds over a lifetime as in the case of IBD.
In addition to the differential expression of IL12Rβ2 chain and the production of IFN‐γ by T cells, unique differences were noted with regard to Crohn's disease. In the absence of immunomodulation, mucosal T cells produced significantly less IL4 and IL10 in children with early compared with late disease. Upon exposure to IL4 and IL12, however, the same cells significantly upregulated the production of IL4 and IL10, respectively. Both IL4 and IL10 are immunosuppressive cytokines with the potential to inhibit IL12 production, suggesting that early in the course of Crohn's disease T cells can still respond to anti‐inflammatory signals and potentially prevent the development of a full‐blown Th1 response. This response is far from universal in adult patients with long‐standing IBD, indicating that the generation of Th1 cells during the initial phase of Crohn's disease is not an irreversible phenomenon intrinsic to either form of IBD. The diverse T helper profiles seen in adults with Crohn's disease must thus involve additional modulatory factors. For example, both the amount and source of specific cytokines are important.56 IL12 is consistently elevated in Crohn's disease mucosa even in the absence of inflammation, but not in the mucosa involved by chronic infections.57,58 IL18 plays a protective role in an early mucosal immune response, when it is produced primarily by epithelial cells, but plays a detrimental role later, when it is produced by lamina propria macrophages and dendritic cells.59 Finally, diverse T helper profiles in adult Crohn's disease could depend on differences in mucosal dendritic cell function.60
Our observations in children with Crohn's disease complement animal studies on the pathogenic role of IL12 in gut inflammation, and rationalise blocking this cytokine for therapeutic purposes. The beneficial effect of IL12 neutralising antibodies, and the little or no effect of IFN‐γ antibodies, in various models of experimental IBD associated with high IL12 production,39,61,62 underscore the crucial importance of downregulating the early inductive phase rather than the late effector phase of gut inflammation. Anti‐IL12 antibodies have been used in adults with long‐standing Crohn's disease and, although they induced a beneficial effect, they failed to show a robust and sustained response.63 One could speculate that antibody blockade of IL12 during the initial manifestations of pediatric IBD could result in better and long‐lasting effects, as reported in children with early Crohn's disease receiving tumor necrosis factor‐α antibodies,64 further illustrating the difference between early and late IBD. Therefore, as recently corroborated by the differential effect of antibody therapy in early versus late murine colitis,65 intervening at the time of the earliest possible immunopathogenic events of IBD might be ideal. The identification of the novel Th‐17 cell subset, which distinctly produces IL17 independently of Th1 and Th2 cells,8 may help identify these very early events. IL17 is a proinflammatory cytokine whose production is mediated by IL23, an IL12 family member.66 There is evidence that inflammation in an infected or injured tissue is first induced by the IL23/IL17 axis, and is later replaced by immune effector functions regulated by the IL12/IFN‐γ axis.67 IL23 is apparently essential to induce colitis in IL10‐deficient mice, suggesting that the IL23/IL17 pathway may actually be responsible for mucosal inflammation.68 Because the p40 subunit is shared by IL12 and IL23,66 it is conceivable that the elevated IL12p40 mRNA levels we detected in children with early Crohn's disease are also caused by increased IL23 production in the mucosa. For the same reason, it is also conceivable that IL12 antibodies, which are directed at the p40 subunit, also block IL23 and this is responsible, at least partly, for its therapeutic effect. Whatever the case, IL23‐mediated events may precede IL12‐dependent inflammation in early IBD, and this possibility should be actively pursued in children with newly diagnosed Crohn's disease.
Acknowledgements
The authors would like to thank Dr B Dahms, Rainbow Babies and Children's Hospital, for histopathological analyses, and Ms M O'Riordan and Dr H Souza for statistical analyses.
Abbreviations
CD - Crohn's disease
CDR3 - complementary determining region 3
GAPDH - glyceraldehyde‐3‐phosphate dehydrogenase
IBD - inflammatory bowel disease
PBMC - peripheral blood mononuclear cell
PBS - phosphate‐buffered saline
PCR - polymerase chain reaction
RT - reverse transcriptase
Th - T helper
TLR - Toll‐like receptor
Footnotes
Funding: This work was supported by grants from the National Institutes of Health (DK30399, DK50984 and DK57756 to C F; DK54213 to A D L; DK44319 and DK51362 to R B). L J S is a Howard Hughes Medical Institute Physician Postdoctoral Fellow.
Competing interests: None.
References
- 1.Barton J R, Gillon S, Ferguson A. Incidence of inflammatory bowel disease in Scottish children between 1968 and 1983; marginal fall in ulcerative colitis, threefold increase in Crohn's disease. Gut 198930618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawczenko A, Sandhu B K, Logan R F A.et al Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet 20013571093–1094. [DOI] [PubMed] [Google Scholar]
- 3.Kugathasan S, Judd R H, Hoffmann R G.et al Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population‐based study. J Pediatr 2003143525–531. [DOI] [PubMed] [Google Scholar]
- 4.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998115182–205. [DOI] [PubMed] [Google Scholar]
- 5.Desreumaux P, Brandt E, Gambiez L.et al Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology 1997113118–126. [DOI] [PubMed] [Google Scholar]
- 6.Pappa H M, Semrin G, Walker T R.et al Pediatric inflammatory bowel disease. Curr Opin Gastroenterol 200420333–340. [DOI] [PubMed] [Google Scholar]
- 7.Kugathasan S, Willis J, Dahms B B.et al Intrinsic hyperreactivity of mucosal T‐cells to interleukin‐2 in pediatric Crohn's disease. J Pediatrics 1998133675–681. [DOI] [PubMed] [Google Scholar]
- 8.Wynn T A. TH‐17: a giant step from TH1 and TH2. Nat Immunol 200561069–1070. [DOI] [PubMed] [Google Scholar]
- 9.Iwakura Y, Ishigame H. The IL‐23/IL‐17 axis in inflammation. J Clin Invest 20061161218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuss I J, Neurath M, Boirivant M.et al Disparate CD4+ lamina propria lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN‐γ, whereas ulcerative colitis LP cells manifest increased secretion of IL‐5. J Immunol 19961571261–1270. [PubMed] [Google Scholar]
- 11.Annunziato F, Cosmi L, Santarlasci V.et al Phenotypic and functional features of human Th17 cells. J Exp Med 20072041849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuss I J, Heller F, Boirivant M.et al Nonclassical CD1d‐restricted NK T cells that produce IL‐13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 20041131490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strober W, Fuss I J, Blumberg R S. The immunology of mucosal models of inflammation. Annu Rev Immunol 200220495–549. [DOI] [PubMed] [Google Scholar]
- 14.Murphy K M, Reiner S L. The lineage decisions of helper T cells. Nat Rev Immunol 20022933–944. [DOI] [PubMed] [Google Scholar]
- 15.Antonioli D A. Colitis in infants and children. In: Dahms BB, Qualman SJ, eds. Gastrointestinal diseases. Basel: Karger 199777–110.
- 16.Rothenberg M E. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 200411311–28. [DOI] [PubMed] [Google Scholar]
- 17.Hanauer S, Schwartz J, Robinson M.et al Mesalamine capsules for treatment of acute ulcerative colitis: results of a controlled trial. Am J Gastroenterol 1993881188–1197. [PubMed] [Google Scholar]
- 18.Hermida‐Rodriguez C, Perona J C, Garcia‐Valriberas R.et al High‐dose intravenous cyclosporine in steroid refractory attacks of inflammatory bowel disease. Hepatogastroenterology 1999462265–2268. [PubMed] [Google Scholar]
- 19.Parronchi P, Macchia D, Piccinini M ‐ P.et al Allergen‐ and bacterial antigen‐specific T‐cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A 1991884538–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang J C, Smith L R, Proning K J.et al CD8+ T‐cells in psoriatic lesions preferentially use T‐cell receptor VB3 and/or VB13.1 genes. Proc Natl Acad Sci U S A 1994919282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saubermann L J, Probert C S J, Christ A D.et al Evidence of T cell receptor β‐chain patterns in inflammatory and noninflammatory bowel disease states. Am J Physiol 1999276G613–G621. [DOI] [PubMed] [Google Scholar]
- 22.Hayes M P, Wang J, Norcross M A. Regulation of interleukin‐12 expression in human monocytes: selective priming by interferon‐gamma of lipopolysaccharide‐inducible p35 and p40 genes. Blood 199586646–650. [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Mackay C R.et al Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 1998187875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tichopad A, Dilger M, Schwarz G.et al Standardized determination of real‐time PCR efficiency from a single reaction set‐up. Nucleic Acids Res 200331e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogge L, Barberis‐Maino L, Biffi M.et al Selective expression of an interleukin‐12 receptor component by human T helper 1 cells. J Exp Med 1997185825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabo S J, Dighe A S, Gubler U.et al Regulation of the interleukin (IL)‐12 β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med 1997185817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trinchieri G, Scott P. The role of interleukin‐12 in the immune response, disease and therapy. Immunol Today 199415460–463. [DOI] [PubMed] [Google Scholar]
- 28.Trembleau S, Germann T, Gately M K.et al The role of IL‐12 in the induction of organ‐specific autoimmune diseases. Immunol Today 199516383–386. [DOI] [PubMed] [Google Scholar]
- 29.Segal B M, Shevach E M. IL‐12 unmasks latent autoimmune disease in resistant mice. J Exp Med 1996184771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Presky D H, Yang H, Minetti L J.et al A functional interleukin‐12 receptor complex is composed of two β‐type cytokine receptor subunits. Proc Natl Acad Sci U S A 19969314002–14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins L M, Frankel G, Douce G.et al Cytrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun 1999673031–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia‐Rodriguez L A, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 20061301588–1594. [DOI] [PubMed] [Google Scholar]
- 33.Seder R A, Kelsall B L, Jankovic D. Differential roles for IL‐12 in the maintenance of immune responses in infectious versus autoimmune disease. J Immunol 19961572745–2748. [PubMed] [Google Scholar]
- 34.Gudmundsson G, Monick M M, Hunninghake G W. IL‐12 modulates expression of hypersensitivity pneumonitis. J Immunol 1998161991–999. [PubMed] [Google Scholar]
- 35.Marshall J D, Secrist H, DeKruyff R H.et al IL‐12 inhibits the production of IL‐4 and IL‐10 in allergen‐specific human CD4+ T lymphocytes. J Immunol 1995155111–117. [PubMed] [Google Scholar]
- 36.Joosten L A B, Lubberts E, Helsen M M A.et al Dual role of IL‐12 in early and late stages of murine collagen type II arthritis. J Immunol 19971594094–4102. [PubMed] [Google Scholar]
- 37.Gazzinelli R T, Wysocka M, Hayashi S.et al Parasite‐induced IL‐12 stimulates early IFN‐γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 19941532533–2543. [PubMed] [Google Scholar]
- 38.Bohn E, Schmitt E, Bielfeldt C.et al Ambigous role of interleukin‐12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect Immun 1998662213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer D M, Veldman G M, Banerjee S.et al Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology 200212294–105. [DOI] [PubMed] [Google Scholar]
- 40.Himmerlich H, Parra‐Lopez C, Tacchini‐Cottier F.et al The IL‐4 rapidly produced in BALB/c mice after infection with Leishmania major down‐regulates IL‐12 receptor β2‐chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL‐12. J Immunol 19981616156–6163. [PubMed] [Google Scholar]
- 41.Gorham J D, Guler M L, Fenoglio D.et al Low dose TGF‐β attenuates IL‐12 responsiveness in murine Th cells. J Immunol 19981611664–1670. [PubMed] [Google Scholar]
- 42.Nakajima A, Kodama T, Yazaki Y.et al Specific clonal T cell accumulation in intestinal lesions of Crohn's disease. J Immunol 19961575683–5688. [PubMed] [Google Scholar]
- 43.Polanski M, Melican N S, Zhang J.et al Oral administration of the immunodominant B‐chain of insulin reduces diabetes in a co‐transfer model of diabetes in the NOD mouse and is associated with a switch from Th1 to Th2 cytokines. J Autoimmunity 199710339–346. [DOI] [PubMed] [Google Scholar]
- 44.Scott F W, Cloutier H E, Kleemann R.et al Potential mechanisms by which certain foods promote or inhibit the development of spontaneous diabetes in BB rats: dose, timing, early effect on islet area, and switch in infiltrate from Th1 to Th2 cells. Diabetes 199746589–598. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Paulsson G, Stemme S.et al Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo‐E‐knockout mice. J Clin Invest 19981011717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bamias G, Martin C, Mishina M.et al Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology 2005128654–666. [DOI] [PubMed] [Google Scholar]
- 47.Thepen T, Langeveld‐Wildschut E G, Bihari I C.et al Biphasic response against areoallergen in atopic dermatitis showing a switch from an initial Th2 response to a Th1 response in situ: an immunohistochemical study. J Allergy Clin Immunol 199697828–837. [DOI] [PubMed] [Google Scholar]
- 48.Raza K, Falciani F, Curnow S J.et al Early rheumatoid arthritis is characterized by a distinct and transient synovial cytokine profile of T cell and stromal cell origin. Arthritis Res Ther 20057R784–R795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C ‐ Y, Kirman J R, Rotte M J.et al Distinct lineage of TH1 cells have differential capacities for memory cell generation in vivo. Nat Immunol 20023852–858. [DOI] [PubMed] [Google Scholar]
- 50.Manetti R, Gerosa F, Giudizi M G.et al Interleukin 12 induces stable priming for interferon γ (IFN‐γ) production during differentiation of human T helper (Th) cells and transient IFN‐γ production in established Th2 cell clones. J Exp Med 19941791273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rakoff‐Nahoum S, Paglino J, Eslami‐Varzaneh F.et al Recognition of commensal microflora by toll‐like receptors is required for intestinal homeostasis. Cell 2004118229–241. [DOI] [PubMed] [Google Scholar]
- 52.Abreu M T, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol 20051744453–4460. [DOI] [PubMed] [Google Scholar]
- 53.Kelsall B L, Rescigno M. Mucosal dendritic cells in immunity and inflammation. Nat Immunol 200451091–1095. [DOI] [PubMed] [Google Scholar]
- 54.Crellin N K, Garcia R V, Hadisfar O.et al Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 and CD4+CD25+ T regulatory cells. J Immunol 20051758051–8059. [DOI] [PubMed] [Google Scholar]
- 55.Sutmuller R P M, denBork M H M G M, Kramer M.et al Toll‐like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest 2006116485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caspi R R. IL‐12 in autoimmunity. Clin Immunol Immunopathol 1998884–13. [DOI] [PubMed] [Google Scholar]
- 57.Monteleone G, Biancone L, Marasco R.et al Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology 19971121169–1178. [DOI] [PubMed] [Google Scholar]
- 58.Berrebi D, Besnard M, Fromont‐Hankard G.et al Interleukin‐12 expression is focally enhanced in the gastric mucosa of pediatric patients with Crohn's disease. Am J Pathol 1998152667–672. [PMC free article] [PubMed] [Google Scholar]
- 59.Reuter B K, Pizarro T T. The role of the IL‐18 system and other members of the IL‐1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol 2004342347–2355. [DOI] [PubMed] [Google Scholar]
- 60.Hart A L, Al‐Hassi H O, Rigby R J.et al Characteristics of intestinal dendritic cells in inflammatory bowel disease. Gastroenterology 200512950–65. [DOI] [PubMed] [Google Scholar]
- 61.Neurath M F, Fuss I, Kelsall B L.et al Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 19951821281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simpson S J, Shah S, Comiskey M.et al T cell‐mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/signal transducer and activator of transcription (stat)‐4 pathway, but is not conditional on interferon γ expression by T cells. J Exp Med 19981871225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mannon P J, Fuss I J, Mayer L.et al Anti‐interleukin‐12 antibody for active Crohn's disease. N Engl J Med 20043512069–2079. [DOI] [PubMed] [Google Scholar]
- 64.Kugathasan S, Werlin S L, Martinez A.et al Prolonged duration of response to infliximab in early but not late pediatric Crohn's disease. Am J Gastroenterol 2000953189–3194. [DOI] [PubMed] [Google Scholar]
- 65.Rivera‐Nieves J, Ho J, Bamias G.et al Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine colitis. Gastroenterology 20061311518–1529. [DOI] [PubMed] [Google Scholar]
- 66.Brombacher F, Kastelein R A, Alber G. Novel IL‐12 family members shed light on the orchestration of Th1 responses. Trends Immunol 200324207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKenzie B S, Kastelein R A, Cua D J. Understanding the IL‐23/IL‐17 immune pathway. Trends Immunol 20062717–23. [DOI] [PubMed] [Google Scholar]
- 68.Yen D, Cheung J, Scheerens H.et al IL‐23 is essential for T cell‐mediated colitis and promotes inflammation via IL‐17 and IL‐6. J Clin Invest 20061161310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]