Abstract
Background
The major potential site of acid nitrosation is the proximal stomach, an anatomical site prone to a rising incidence of metaplasia and adenocarcinoma. Nitrite, a pre‐carcinogen present in saliva, can be converted to nitrosating species and N‐nitroso compounds by acidification at low gastric pH in the presence of thiocyanate.
Aims
To assess the effect of lipid and ascorbic acid on the nitrosative chemistry under conditions simulating the human proximal stomach.
Methods
The nitrosative chemistry was modelled in vitro by measuring the nitrosation of four secondary amines under conditions simulating the proximal stomach. The N‐nitrosamines formed were measured by gas chromatography–ion‐trap tandem mass spectrometry, while nitric oxide and oxygen levels were measured amperometrically.
Results
In absence of lipid, nitrosative stress was inhibited by ascorbic acid through conversion of nitrosating species to nitric oxide. Addition of ascorbic acid reduced the amount of N‐nitrosodimethylamine formed by fivefold, N‐nitrosomorpholine by >1000‐fold, and totally prevented the formation of N‐nitrosodiethylamine and N‐nitrosopiperidine. In contrast, when 10% lipid was present, ascorbic acid increased the amount of N‐nitrosodimethylamine, N‐nitrosodiethylamine and N‐nitrosopiperidine formed by approximately 8‐, 60‐ and 140‐fold, respectively, compared with absence of ascorbic acid.
Conclusion
The presence of lipid converts ascorbic acid from inhibiting to promoting acid nitrosation. This may be explained by nitric oxide, formed by ascorbic acid in the aqueous phase, being able to regenerate nitrosating species by reacting with oxygen in the lipid phase.
Keywords: cancer; diet, gastro‐oesophageal junction, nitrite; nitrosation
Over the last 20 years, a new pattern of gastric carcinogenesis has emerged in the western world, with a decreasing incidence of distal gastric cancer and an alarming increase in the incidence of adenocarcinomas of the proximal stomach, including cardia and adjacent gastro‐oesophageal junction (GOJ).1,2,3,4 The cancers at the GOJ usually occur in healthy acid‐secreting stomachs.5,6 The cause of the increasing incidence of adenocarcinoma of the proximal stomach remains unclear, but the rate of change indicates environmental factors.
For many years, there has been interest in the potential for endogenous generation of carcinogenic N‐nitroso compounds from nitrite within the human upper gastrointestinal tract. This is due to the fact that the acidic pH of gastric juice converts nitrite to nitrous acid and nitrosating species such as N2O3, NO+.7,8 The latter reacts with thiocyanate which is also present in gastric juice to form the particularly potent nitrosating species NOSCN.9,10,11 These nitrosating species can react with secondary amines and amides to form N‐nitroso compounds, many of which are carcinogenic and widely used in animal models of cancer.8,12,13,14,15,16 The nitrosating species N2O3 is itself mutagenic as it can directly deaminate certain DNA bases and inactivate important DNA repair enzymes.17,18,19,20,21 Understanding the factors affecting gastric nitrite chemistry is therefore relevant to our understanding the development of malignancies of the upper stomach.
The main source of nitrite entering the stomach is swallowed saliva. The high level of nitrite in saliva (100 µM under fasting conditions) is derived from the enterosalivary recirculation of dietary nitrate and its reduction to nitrite by buccal bacteria.22,23,24,25,26,27 Consequently, the nitrite level in saliva rises several fold for at least 2 h after ingesting nitrate‐containing foodstuffs. The nitrite concentration in the distal oesophagus is similar to that in saliva.28
A major factor protecting against the generation of N‐nitroso compounds from salivary nitrite on entering the acidic stomach is ascorbic acid present in gastric juice.29,30,31,32,33 Ascorbic acid is actively secreted in gastric juice and effectively competes with secondary amines and amides for reaction with the nitrosating species.32,34,35,36,37,38 In this reaction, the nitrosating species are reduced to nitric oxide and the ascorbic acid oxidised to dehydroascorbic acid.39 We and others have recently demonstrated that high concentrations of nitric oxide are generated in the human upper gastrointestinal tract following nitrate intake by the above mechanism.40
This reduction of acidified nitrite to nitric oxide has been regarded as an effective mechanism for protecting against the generation of N‐nitroso compounds. However, it has recently been recognised that nitric oxide can generate nitrosating species. This arises from the ability of nitric oxide to react with molecular oxygen to form N2O3 – the same species formed by acidification of nitrite.18,41,42 The rate of the reaction between nitric oxide and oxygen to produce N2O3 is proportional to the concentration of oxygen and to the square of nitric oxide concentration.43,44 Consequently, this reaction is most important at high nitric oxide concentrations.
Recent studies have also demonstrated that the reaction between nitric oxide and oxygen is 300 times faster within lipid than within an aqueous phase.41 This is due to the fact that nitric oxide is nine times more soluble in lipid, and oxygen is also more soluble in lipid than aqueous solutions, resulting in both reactants accumulating in the lipid compartment.41 The potential for generation of nitrosating species from nitric oxide will therefore be greatest when high concentrations of nitric oxide are generated close to lipids.
The above chemistry raises the possibility that inhibition of nitrosation reactions by ascorbic acid in the aqueous phase may promote nitrosation within adjacent lipid compartments. This could occur by diffusion of the nitric oxide produced by the reaction between acidified nitrite and ascorbic acid, into adjacent lipid compartments and their reacting with oxygen to form the nitrosating species N2O3. The presence of lipid might therefore have a profound effect on the chemistry occurring between acidified nitrite and ascorbic acid.
We have demonstrated that luminal nitrosative chemistry in the acid‐secreting stomach is maximal where swallowed saliva first encounters acidic gastric juice and may therefore be contributing to the high incidence of metaplasia and neoplasia in the proximal stomach and GOJ.28,40 The aim of this study was to investigate the influence of lipids on this luminal nitrosative chemistry.
Methods
Assay set‐up
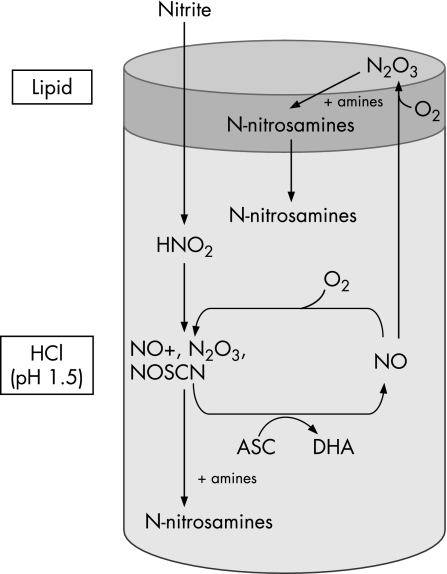
Studies were undertaken in a benchtop model representing the GOJ, with and without lipid added to the acidic aqueous solution. The aqueous phase (0.1 M HCl, pH 1.5) contained 1 mM EDTA, 1 mM sodium thiocyanate, and 5 mM of each secondary amine (dimethylamine, diethylamine, morpholine, piperidine), in the presence or absence of ascorbic acid (1 or 2 mM). The lipid phase contained 5 mM of each secondary amine (dimethylamine, diethylamine, morpholine and piperidine). Lipid (5 ml glycerol trioctanoate, glycerol tributyrate or glycerol triacetate) was added to give a ratio of aqueous to lipid of 10:1, as fat comprises 20–30% of solid food ingested, and aqueous drinks along with aqueous gastric juice will further reduce the fat content to approximately 10%. A low pH of 1.5 was used for the aqueous phase, as this has been shown to be the postprandial pH of the cardia region of the stomach.45 Both phases were combined and placed in a water bath at 37°C and the aqueous phase was stirred with a magnetic stirrer. The reaction was started by adding sodium nitrite (100 µM) to the aqueous phase. The standard assay was carried out for a period of 15 min, after which N‐nitrosamines were extracted from each phase (aqueous and lipid).
Nitric oxide measurement
Nitric oxide dissolved in the aqueous phase was continuously monitored using a nitric oxide electrode and meter (ISO NO Mark II; World Precision Instruments, Sarasota, Florida, USA). The nitric oxide electrode was calibrated by addition of sodium nitrite at varying concentrations (10, 25, 50, 75 and 100 µM) to simulated gastric juice (0.1 M HCl, pH 1.5, ascorbic acid 2 mM). Under these conditions, 1 mol nitrite yields 1 mol nitric oxide. The nitric oxide meter was interfaced to a computer, converting the electrical signal (pA) to nitric oxide concentrations in µM; the response was linear for the range of concentrations tested.
Oxygen measurement
Oxygen dissolved in the aqueous phase was continuously monitored with an oxygen electrode and meter (Mark II; World Precision Instruments). Electrode calibration was performed in simulated gastric juice (0.1 M HCl, pH 1.5, ascorbic acid 2 mM) according to the manufacturer's instructions.
Nitrosamines extraction
A 1 ml aqueous phase aliquot was added to 0.5 ml 0.08 M HCl and 5% sulphamic acid solution in saturated NaCl in a glass tube, and mixed prior to the addition of 10 μl internal standard (0.01% deuterated N‐nitrosodimethylamine (NMDA)‐d6 and deuterated N‐nitrosomorpholine (NMOR)‐d8). The sample was mixed well, and 0.5 ml extraction solvent mix (45:55 v/v dichloromethane:diethyl‐ether, 0.0025% butylhydroxytoluene) was added. The sample was mixed and the upper layer was transferred to a tapered vial. This step was repeated once more, and the sample was concentrated to 100 μl under a gentle stream of nitrogen, capped and stored at −20°C until analysis.
A 0.5 ml lipid phase aliquot was added in a glass tube to 2 ml 88:12 hexane:ethyl acetate supplemented with 0.025% butylhydroxytoluene. The sample was mixed before addition of 10 μl internal standard (0.01% deuterated NMDA and deuterated NMOR). DSC‐Diol solid‐phase extraction cartridges (500 mg, 3 ml) were installed on a vacuum manifold (Phenomenex, Macclesfield, UK) and equilibrated with 3 ml methanol, followed by 2 ml 85:15 v/v ethyl acetate:methanol and 3 ml hexane. All fractions were applied dropwise. The cartridges were washed twice with 3 ml hexane, and dried under vacuum for 30 s. N‐Nitrosamines were eluted into 1.1 ml tapered vials (following the addition of 0.5 ml elution solvents, 85:15 v/v ethyl acetate:methanol), twice. A gentle vacuum was pulled through the cartridges for 30 s while the receiving vials remained in place. The samples were concentrated down to 100 μl under a gentle stream of nitrogen, capped and stored at −20°C until analysis.
Nitrosamines analysis
Nitrosamines analysis was carried out by gas chromatography–ion‐trap tandem mass spectrometry (GC‐ITMS/MS) on a TRACE GC 2000 Series gas chromatograph interfaced to a Polaris Q ion‐trap mass spectrometer equipped with an AS2000 autosampler and a programmable temperature vaporising (PTV) injector (ThermoFinnigan, Hemel Hempsted, UK).
Nitrosamines (NDMA, N‐nitrosodiethylamine (NDEA), NMOR and N‐nitrosopiperidine (NPIP)) were separated by chromatography on a ZB‐5MS fused‐silica capillary column, 30 m×0.25 mm internal diameter, 0.5 µm film thickness (Phenomenex). The oven temperature programme was as follows: 37°C (held for 2 min) to 160°C at 10°C/min, to 250°C at 30°C/min (held for 2 min). Helium was used as a carrier gas at a flow rate of 1 ml/min. The PTV injector was used in splitless mode. The PTV temperature was programmed from 36°C to 115°C (held for 2 min) at 10°C/min and a pressure of 22 psi (151 kPa), prior to a cleaning phase at 325°C (held for 12 min).
Ion‐trap tandem mass spectrometry was carried out by positive‐electron ionisation at an ionisation energy of 70 eV, with the ion source temperature maintained at 220°C, and the transfer‐line at 275°C. Acquisition was performed in three segments, segment 1 for NDMA and NDMA‐d6 (start of acquisition at 4.5 min), segment 2 for NDEA (start of acquisition at 7.3 min), and segment 3 for NMOR, NMOR‐d8 and NPIP (start of acquisition at 10.9 min) with a trap offset voltage of +100 V on segment 3. Precursor ions, excitation voltage and q values, as well as product ions for each N‐nitrosamine, are listed table 1.
Table 1 GC‐ITMS/MS ionisation conditions for analysis of selected N‐nitrosamines.
| Target compound | Precursor ion (m/z) | Product ion (m/z) | Excitation voltage | Excitation q value |
|---|---|---|---|---|
| NDMA | 74 | 44 | 0.85 | 0.45 |
| NDMA‐d6 | 80 | 50 | 0.85 | 0.45 |
| NDEA | 102 | 85 | 0.79 | 0.3 |
| NMOR | 116 | 86 | 0.63 | 0.3 |
| NMOR‐d8 | 124 | 94 | 0.63 | 0.3 |
| NPIP | 114 | 97 | 1 | 0.45 |
Acquisition and treatment of GC‐ITMS/MS data was performed on Xcalibur version 2. All samples were spiked with 10 μl internal standard (0.01% deuterated NMDA and deuterated NMOR), and GC‐MS/MS spectra were calibrated with spiked standard N‐nitrosamines solutions covering concentrations from 0 to 50 µM. Each calibration sample was extracted according to the protocol used for assay samples, and the response was linear for the concentrations studied.
Analysis of ascorbic acid/total vitamin C/dehydroascorbic acid
The aqueous phase was sampled at t = 0, 1, 2, 5, 10 and 15 min post addition of NaNO2 for ascorbic acid and total vitamin C (TVC) measurements. Sampling for TVC allows us to calculate the amount of dehydroascorbic acid present by subtracting ascorbic acid from TVC. The aqueous phase samples (100 µl) were added to deionised water (900 µl). An aliquot of each diluted sample (500 µl) was added to MS solution (500 µl, 2% metaphosphoric acid/0.5% sulphamic acid, 1:1 v/v), while the remainder (500 µl) was added to dithiothreitol/MS solution (500 µl, 2% metaphosphoric acid/0.5% sulphamic acid, 1:1 v/v, supplemented with 6 mg/ml dithiothreitol). The dithiothreitol regenerates ascorbic acid from any dehydroascorbic acid for ascorbic acid and TVC respectively. Ascorbic acid and TVC levels were measured by high‐performance liquid chromatography as previously described46 based upon the method described by Sanderson and Schorah.47
Reagents and chemicals
All solvents and reagents were obtained from Sigma Aldrich (Poole, UK), except for the EDTA (BDH Ltd, Liverpool, UK) and deuterated N‐nitrosamine standards (Qmx laboratories, Thaxted, UK). Solid‐phase extraction cartridges were obtained from Supelco (Poole, UK). Tapered vials were obtained from Chromacol (Welwyn Garden City, UK).
Statistical analysis
Results are presented as mean values ± SE. Statistical analysis of all data was performed by ANOVA or Student's t test. Tukey's HSD test was used for comparison of means within treatments. Mean values were associated to symbols as an indication of significance (p<0.001).
Results
Nitrosation in aqueous solution without lipid present
In the absence of ascorbic acid
When nitrite was added to HCl pH 1.5 containing thiocyanate (1 mM) without ascorbic acid, nitrosation of the secondary amines was observed. After 15 min, 3.43±0.12 µM NMOR was preferentially formed, while only 0.02±0.007 µM NDMA, 0.001±0.000 µM and 0.002±0.001 µM NPIP were detected (table 2).
Table 2 N‐nitrosamine concentrations (µM) in single and dual‐phase experiments 15 min after addition of nitrite (100 µM) in presence or absence of ascorbic acid (2 mM) (n = 12).
| Aqueous phase only | Aqueous phase + 10% lipid | |||
|---|---|---|---|---|
| −Ascorbic acid | +Ascorbic acid | −Ascorbic acid | +Ascorbic acid | |
| Aqueous phase | ||||
| NDMA | 0.02±0.01* | 0.004±0.003* | 0.07±0.02* | 1.19±0.13** |
| NDEA | 0.001±<0.001* | ND | 0.01±0.003* | 0.56±0.07** |
| NMOR | 3.43±0.12* | 0.003±0.003** | 3.37±0.07* | 0.75±0.1*** |
| NPIP | 0.002±0.001* | ND | 0.04±0.002* | 2.78±0.92** |
| Lipid phase | ||||
| NDMA | 1.16±0.27* | 4.77±0.4** | ||
| NDEA | ND | 1.18±0.55** | ||
| NMOR | ND | 4.9±0.41** | ||
| NPIP | 0.06±0.01* | 29.3±7.09** | ||
For the same N‐nitrosamine, concentrations on the same row followed by the same number of asterix are not significantly different according to the Tukey's HSD test (p<0.001).
ND not detected. Values are means ± SE.
In these experiments, the addition of nitrite produced only a very low concentration of nitric oxide (approximately 3.5 µM) and no discernible fall in oxygen concentration.
In the presence of ascorbic acid
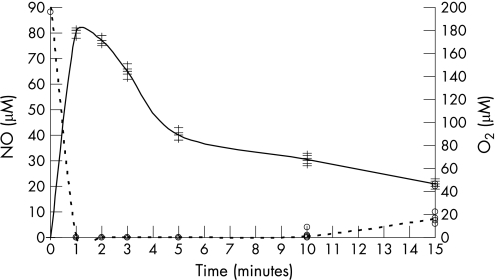
The presence of ascorbic acid markedly inhibited the nitrosation of the secondary amines. The concentration of NDMA formed was reduced by fivefold, while the concentration of NMOR formed was reduced by more than a 1000‐fold. NDEA and NPIP were not detected (table 2). Addition of nitrite produced a rapid increase in nitric oxide concentrations, followed by slow decline. Within 60 s of sodium nitrite addition (100 µM), the dissolved nitric oxide concentration was approximately 80 µM (fig 1). This rapid rise in nitric oxide was accompanied by a rapid fall in the dissolved oxygen concentration (fig 1). Within 60 s of sodium nitrite addition, no dissolved oxygen was detected until 10 min later when the concentration slowly increased to reach 5–10% of its original concentration.
Figure 1 Nitric oxide (continuous line) and oxygen (dashed line) concentrations in the aqueous phase after the addition of nitrite (100 µM) to a dual‐phase system containing ascorbic acid (1 mM) (n = 6).
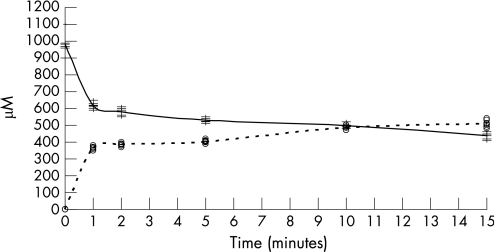
The addition of nitrite (100 µM) triggered a fall in the ascorbic acid concentration, from its original concentration of 970±75 µM to 580±40 µM at 2 min, and then it slowly fell to 480±40 µM at 15 min (fig 2).
Figure 2 Ascorbic acid (continuous line) and dehydroascorbic acid (dashed line) concentrations in the aqueous phase after the addition of nitrite (100 µM) to a dual‐phase system containing ascorbic acid (1 mM) (n = 6).
Nitrosation in a dual‐phase system containing aqueous solution and 10% lipid
In absence of ascorbic acid
When nitrite was added to HCl pH 1.5 containing thiocyanate (1 mM), no ascorbic acid and 10% lipid, nitrosation of the secondary amines was observed. The concentrations of N‐nitrosamines formed in the aqueous phase were similar to those observed in the absence of lipid, with NMOR preferentially formed (3.37±0.07 µM) (table 2). Only NDMA (1.16±0.27 μM) and NPIP (0.06±0.01 μM) were detected in the lipid phase (table 2).
In presence of ascorbic acid
In marked contrast to the observations in the absence of lipid, the addition of ascorbic acid did not inhibit the generation of N‐nitrosamines in the presence of 10% lipid. Indeed, in the presence of lipid and ascorbic acid, substantial concentrations of each of the N‐nitrosamines were detected in both the aqueous and lipid phases (table 2). After 15 min, the concentrations of each N‐nitrosamine in the lipid phase, and the concentration of NDMA, NDEA and NPIP in the aqueous phase, were significantly higher than in the absence of ascorbic acid (p<0.001). In the aqueous phase, 1.19±0.13 µM NDMA, 0.56±0.07 µM NDEA, 0.74±0.1 µM NMOR and 2.78±0.92 µM NPIP were detected. In the lipid phase, 4.77±0.4 µM NDMA, 1.18±0.55 µM NDEA, 4.90±0.41 µM NMOR and 29.3±7.01 µM NPIP were detected (table 2). In the presence of lipid, the addition of nitrite produced similar changes in the concentrations of nitric oxide, oxygen and ascorbic acid to those seen in the absence of lipid.
Effect of the lipid on the influence of ascorbic acid on acid nitrosation
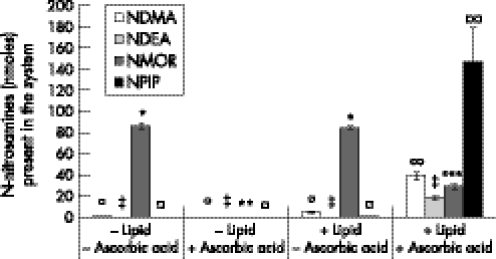
The presence of lipid transformed the overall effect of ascorbic acid from inhibiting to promoting N‐nitrosamine formation. In the absence of lipid, ascorbic acid totally inhibited the formation of NDEA and NPIP, and reduced the formation of NDMA by fivefold and NMOR by more than a 1000‐fold. The addition of lipid to the system, in a 1:10 ratio, overrode the protective effect of ascorbic acid. In the presence of lipid, the addition of ascorbic acid increased the amount of three of the N‐nitrosamines in the dual‐phase system: NDMA by approximately eightfold, from 4.7±0.77 nmol to 39.19±3.49 nmol; NDEA by approximately 60‐fold, from 0.3±0.08 nmol to 18.13±1.98 nmol, and NPIP by approximately 140‐fold, from 1.05±0.07 nmol to 147.35±31.59 nmol (fig 3). In the presence of lipid, the addition of ascorbic acid reduced the total amount of NMOR in the system by approximately threefold, from 84.21±1.78 nmol to 28.78±2.59 nmol (fig 3), levels that were nonetheless significantly higher than in the presence of ascorbic acid and absence of lipid (0.08±0.08 nmol) (p<0.001). In addition, ascorbic acid increased the concentration of NMOR formed in the lipid from undetectable to 4.9±0.4 µM (p<0.001).
Figure 3 N‐nitrosamines present in the single and dual‐phase systems in the presence and absence of ascorbic acid (2 mM) 15 min after addition of nitrite (100 µM) (n = 12).
Variations in the nature of the lipid phase
The conversion of the inhibiting effect of ascorbic acid on nitrosation to a promoting effect was observed with three different lipid phases (glycerol trioctanoate, a low‐density lipid, and glycerol tributyrate and glycerol triacetate, two high‐density lipids). While glycerol trioctanoate provided a physiologically accurate representation of a floating lipid layer, glycerol tributyrate and glycerol triacetate were included in the study in order to test the robustness of our benchtop system when lipids are not in direct contact with air. Each of the three lipids generated similar results (data not shown).
Time course study of N‐nitrosamines diffusion in a dual‐phase system
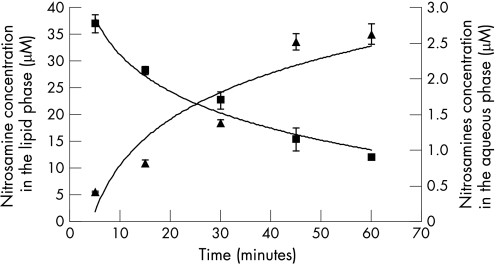
Diffusion of the four N‐nitrosamines from the lipid phase to the acidic aqueous phase under conditions similar to the previous experiment (0.1 M HCl, pH 1.5, at 37°C, in the presence of EDTA, thiocyanate and ascorbic acid) was monitored by adding 10 µM each N‐nitrosamine to the lipid phase at t = 0, and measuring their concentration in each phase during a 60 min time course. The concentrations of all four N‐nitrosamines decreased in the lipid phase and increased in the aqueous phase over time (60 min) (table 3). The total N‐nitrosamine concentration of the lipid phase decreased from 40 µM down to approximately 12 µM, while the total N‐nitrosamine concentration in the aqueous phase rose to approximately 2.6 µM (fig 4). The diffusion pattern of all four N‐nitrosamines was comparable, although NPIP tended to remain at higher levels in the lipid phase (table 3).
Table 3 Time course study of standard N‐nitrosamine concentration in a dual‐phase system (n = 12).
| Standard N‐nitrosamine | Concentration (μM) | ||||
|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 45 min | 60 min | |
| Aqueous phase | |||||
| NDMA | 0.15±0.01 | 0.29±0.06 | 0.40±0.04 | 0.48±0.07 | 0.54±0.01 |
| NDEA | 0.08±0.01 | 0.18±0.02 | 0.3±0.01 | 0.49±0.15 | 0.64±0.02 |
| NMOR | 0.09±0.02 | 0.25±0.07 | 0.33±0.03 | 0.89±0.28 | 0.70±0.01 |
| NPIP | 0.09±0.01 | 0.09±0.03 | 0.34±0.03 | 0.68±0.08 | 0.75±0.1 |
| Lipid phase | |||||
| NDMA | 6.64±0.40 | 4.03±0.25 | 1.97±0.24 | 0.86±0.24 | 0.39±0.01 |
| NDEA | 6.29±0.48 | 5.11±0.67 | 5.86±1.74 | 4.61±1.51 | 2.98±0.67 |
| NMOR | 10.62±0.61 | 8.75±0.51 | 5.86±0.24 | 3.71±0.12 | 2.96±0.20 |
| NPIP | 13.34±0.73 | 9.75±0.9 | 8.95±0.57 | 6.49±0.4 | 5.69±0.20 |
10 µM of each N‐nitrosamine was added to the lipid phase at time 0 min, and concentrations in each phase were monitored over the subsequent 60 min.
Values are means ± SE.
Figure 4 Diffusion of total N‐nitrosamines in a dual‐phase system. N‐nitrosamine concentration in the lipid phase (square), N‐nitrosamine concentration in the aqueous phase (triangle) (n = 12).
Discussion
These studies indicate that the presence of lipid profoundly alters acid‐catalysed nitrosative chemistry. The lipid phase is able to convert the influence of ascorbic acid from one that protects against nitrosation to one that promotes it. This effect is likely to be due to the ability of the nitric oxide formed by ascorbic acid within the aqueous phase to regenerate nitrosative species by reacting with oxygen within the lipid phase.
In the studies performed without lipid, and without ascorbic acid, the addition of nitrite to the HCl pH 1.5 containing thiocyanate resulted in nitrosation of the secondary amines. The main nitrosating species formed under these conditions is NOSCN.9,10,11 NOSCN reacts with the secondary amine in its unprotonated uncharged state.48 The amount of the secondary amine in its nitrosatable form depends upon its −log dissociation constant (pKa). The pKa values of the amines studied are 8.33 for morpholine, 11.22 for piperidine, 10.73 for dimethyamine and 11.09 for diethylamine. The amount of the secondary amine in a form available for nitrosation by NOSCN will therefore be greatest for morpholine and least for piperidine. The differences in pKa can partially explain why different concentrations of the four N‐nitrosamines were generated.
In the experiments performed in the absence of lipid, the addition of ascorbic acid effectively prevented the nitrosation of the amines. This can be explained by the ascorbic acid competing with the secondary amines for the NOSCN.36,38,49 In the reaction between ascorbic acid and NOSCN, the latter is reduced to nitric oxide and the former oxidised to dehydroascorbic acid. Consistent with this, we observed a burst of nitric oxide accompanied by a fall in the ascorbic acid and oxygen concentrations. This can be explained by the nitric oxide reacting with dissolved oxygen to form N2O3.43,44 The N2O3 formed in this way is a nitrosating species and again preferentially reacts with ascorbic acid and is reduced back to nitric oxide.39 This recycling continues until either the oxygen or the ascorbic acid is consumed. Under our experimental conditions, the oxygen was the first to be depleted. Stoichemically, 1 mol ascorbic acid can reduce 2 mol nitrite to nitric oxide. The greater consumption of ascorbic acid observed is due to this recycling of nitric oxide.
In the presence of the lipid, N‐nitrosamines were formed despite the presence of ascorbic acid. Indeed, the presence of lipid transformed the effect of the ascorbic acid from effectively inhibiting nitrosation of each amine to powerfully enhancing nitrosation of three of the four amines. (The overall amounts of NDMA, NDEA and NPIP in the system were increased by 8‐, 60‐ and 140‐fold, respectively, in the dual‐phase system when ascorbic acid was present versus absent; fig 3.) The overall amount of NMOR formation was inhibited by ascorbic acid in both the absence and presence of lipid, although the inhibitory effect of ascorbic acid was markedly reduced by the presence of lipid, from over 1000‐fold in absence of lipid, to only threefold in the presence of lipids. In addition, the presence of ascorbic acid increased the concentration of NMOR in the lipid from undetectable to 4.9±0.4 µM.
What is the explanation for the ability of the lipid to convert the effect of the ascorbic acid from being an inhibitor to a promoter of nitrosation? The effect observed is likely to be mediated by the nitric oxide produced by the reaction between the ascorbic acid and the nitrosating species within the aqueous phase. The nitric oxide will diffuse into to the lipid phase and within it react with oxygen to form N2O3.50 Liu et al. demonstrated that the reaction between nitric oxide and oxygen is 300 times faster in lipid than in aqueous solutions, due to the increased solubility of both gases in lipids.41 As ascorbic acid is not lipophilic, it is unable to enter the lipid and thus the N2O3 generated within the lipid will be able to nitrosate the secondary amines within the lipid. The N‐nitrosamines generated within the lipid in this way will then diffuse out of the lipid and produce the rise in their concentration observed in the aqueous compartment (fig 5).
Figure 5 Proposed mechanism of N‐nitrosamine formation in a dual‐phase system with ascorbic acid present in the aqueous phase. Nitrite forms nitrous acid and nitrosating species in the acidic aqueous environment. In the presence of ascorbic acid (ASC), the nitrosating species are reduced to nitric oxide which diffuses into the lipid where it reacts with oxygen reforming nitrosating species such as N2O3. Ascorbic acid is reduced to dehydroascorbic acid. Secondary amines present within the lipid are nitrosated by N2O3 to form N‐nitrosamines. The latter then diffuse back to the aqueous phase.
In the dual‐phase studies without ascorbic acid, low concentrations of NDMA and NPIP were detected in the lipid phase, whereas NDEA and NMOR were both undetectable in the lipid phase. NDMA and NPIP are more lipid soluble than NMOR (ClogP 0.01, 0.73 and −0.33, respectively) and are likely to have diffused into the lipid following their generation in the aqueous phase. Low levels of nitric oxide are present in the aqueous phase even in the absence of ascorbic acid and this may also have nitrosated dimethylamine and piperidine within the lipid phase. The dual‐phase experiments with ascorbic acid indicated that dimethylamine and piperidine were the amines most nitrosated by nitric oxide in the lipid phase. Piperidine showed the greatest degree of nitrosation in the dual phase; this may be due to its lipophilicity (ClogP 0.52) and thus it is the one most available for nitrosation within the lipid compartment.51
In the dual‐phase system, the concentrations of three of the N‐nitrosamines detected in the aqueous phase were significantly greater in the presence versus absence of ascorbic acid. The one exception was NMOR and a number of factors may explain this. First, the amount of NMOR formed in the aqueous phase in the absence of ascorbic acid is considerably greater than the amount of other N‐nitrosamines, due to its lower pKa and thus higher proportion in the nitrosatable unprotonated form.52 Second, the aqueous concentrations of each N‐nitrosamine were similar in presence of both ascorbic acid and lipid compared to aqueous concentrations in the absence of ascorbic acid (table 2). This is because the N‐nitrosamines detected in the aqueous phase in presence of both ascorbic acid and lipid will have been formed mainly in the lipid phase, where the pKa of the amine is not relevant. The decrease of NMOR aqueous concentration in the dual‐phase system in presence of ascorbic acid compared to when ascorbic acid is absent is therefore mainly explained by the inhibition of the aqueous nitrosation of morpholine when ascorbic acid is present. In addition, differences in partitioning of the different amines and N‐nitrosamines between the lipid and aqueous phase may contribute to the different results.
The above studies indicate that the presence of lipid transforms the regulation of nitrosative chemistry in conditions simulating the proximal stomach. The presence of lipid overcomes the protective effect of ascorbic acid and indeed transforms ascorbic acid from an inhibitor to a promoter of nitrosation. The transforming role of lipids is likely to be relevant to the in vivo situation as lipid is present in the proximal stomach for a considerable time after eating and is also an important component of the epithelial membranes.
Acknowledgements
The authors wish to thank Mrs Valerie Fyfe and Mr David Hughes for the technical support provided.
Abbreviations
GC‐ITMS/MS - gas chromatography–ion‐trap tandem mass spectrometry
GOJ - gastro‐oesophageal junction
NDEA - N‐nitrosodiethylamine
NDMA - N‐nitrosodimethylamine
NMOR - N‐nitrosomorpholine
NPIP - N‐nitrosopiperidine
PTV - programmable temperature vaporising
TVC - total vitamin C
Footnotes
This study was supported financially by the World Cancer Research Fund, and the Medical Research Council (a Personal Research Fellowship awarded to SP)
Competing interests: None
References
- 1.Powell J, McConkey C C, Gillison E W.et al Continuing rising trend in oesophageal adenocarcinoma. Int J Cancer 2002102422–427. [DOI] [PubMed] [Google Scholar]
- 2.Blot W J, Devesa S S, Kneller R W.et al Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 19912651287–1289. [PubMed] [Google Scholar]
- 3.Botterweck A A M, Schouten L J, Volovics A.et al Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol 200029645–654. [DOI] [PubMed] [Google Scholar]
- 4.McKinney P A, Sharp L, Macfarlane G J.et al Oesophageal and gastric cancer in Scotland 1960–90. Br J Cancer 199571411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McColl K E L. Cancer of the gastric cardia. Best Pract Res Clin Gastroenterol 200620687–696. [DOI] [PubMed] [Google Scholar]
- 6.Hansen S, Vollset S E, Derakhshan M H.et al Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut 200756918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach S. Mechanisms of endogenous N‐nitrosation. In: Hill J, ed. Nitrosamines; toxicology and microbiology. Chichester: Ellis Horwood, 198869–72.
- 8.Fischermann K, Bech I, Andersen B. Diagnostic value of augmented histamine test in cancer of upper part of stomach. Scand J Gastroenterol 19694517–519. [DOI] [PubMed] [Google Scholar]
- 9.Fan T Y, Tannenbaum S R. Factors influencing rate of formation of nitrosomorpholine from morpholine and nitrite – acceleration by thiocyanate and other anions. J Agric Food Chem 197321237–240. [DOI] [PubMed] [Google Scholar]
- 10.Boulos P B, Whitfield P F, Dave M.et al Thiocyanate as a marker of saliva in gastric‐juice. Gut 19802118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyland E, Walker S A. Effect of thiocyanate on nitrosation of amines. Nature 1974248601–602. [DOI] [PubMed] [Google Scholar]
- 12.Kokkinakis D M, Subbarao V. The significance of DNA‐damage, its repair and cell‐proliferation during carcinogen treatment in the initiation of pancreatic‐cancer in the hamster model. Cancer Res 1993532790–2795. [PubMed] [Google Scholar]
- 13.Peto R, Gray R, Brantom P.et al Nitrosamine carcinogenesis in 5120 rodents – chronic administration of 16 different concentrations of ndea, ndma, npyr and npip in the water of 4440 inbred rats, with parallel studies on ndea alone of the effect of age of starting (3, 6 or 20 weeks) and of species (rats, mice or hamsters). IARC Sci Publ 1984627–665. [PubMed]
- 14.Verna L, Whysner J, Williams G M.N‐nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA‐adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 19967157–81. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal M, LaRusso N F, Burgart L J.et al Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide‐dependent mechanism. Cancer Res 200060184–190. [PubMed] [Google Scholar]
- 16.Ruiz F, Corrales F J, Miqueo C.et al Nitric oxide inactivates rat hepatic methionine adenosyltransferase in vivo by S‐nitrosylation. Hepatology 1998281051–1057. [DOI] [PubMed] [Google Scholar]
- 17.Laval F, Wink D A, Laval J. A discussion of mechanisms of NO genotoxicity: Implication of inhibition of DNA repair proteins. Rev Physiol Biochem Pharmacol 1997131175–191. [DOI] [PubMed] [Google Scholar]
- 18.Wink D A, Feelisch M, Vodovotz Y.et al In: Gilbert DL, Colton CA, eds. Reactive oxygen species in biological systems. New York: Kluwer Academic/Plenum, 1999245–291.
- 19.Wink D A, Hanbauer I, Grisham M B.et al Chemical biology of nitric oxide: regulation and protective and toxic mechanisms. Curr Top Cell Regul 199634159–187. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T, Brunson D, Crespi C L.et al DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A 1992893030–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wink D A, Kasprzak K S, Maragos C M.et al DNA deaminating ability and genotoxicity of nitric‐oxide and its progenitors. Science 19912541001–1003. [DOI] [PubMed] [Google Scholar]
- 22.Bos P M J, Vandenbrandt P A, Wedel M.et al The reproducibility of the conversion of nitrate to nitrite in human‐saliva after a nitrate load. Food Chem Toxicol 19882693–97. [DOI] [PubMed] [Google Scholar]
- 23.Wagner D A, Schultz D S, Deen W M.et al Metabolic fate of an oral dose of N‐15‐labeled nitrate in humans – effect of diet supplementation with ascorbic acid. Cancer Res 1983431921–1925. [PubMed] [Google Scholar]
- 24.Granli T, Dahl R, Brodin P.et al Nitrate and nitrite concentrations in human‐saliva – variations with salivary flow‐rate. Food Chem Toxicol 198927675–680. [DOI] [PubMed] [Google Scholar]
- 25.Tannenbaum S R, Weisman M, Fett D. Effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol 197614549–552. [DOI] [PubMed] [Google Scholar]
- 26.Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine – what does this measure tell us about the activity of the endogenous nitric oxide system? Curr Opin Nephrol Hypertens 1998759–62. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes P M, Leone A M, Francis P L.et al The l‐arginine:nitric‐oxide pathway is the major source of plasma nitrite in fasted humans. Biochem Biophys Res Commun 1995209590–596. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Iijima K, Moriya A.et al Conditions for acid catalysed luminal nitrosation are maximal at the gastric cardia. Gut 2003521095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartsch H, Ohshima H, Pignatelli B. Inhibitors of endogenous nitrosation – mechanisms and implications in human cancer prevention. Mutat Res 1988202307–324. [DOI] [PubMed] [Google Scholar]
- 30.Leaf C D, Vecchio A J, Roe D A.et al Influence of ascorbic acid dose on N‐nitrosoproline formation in humans. Carcinogenesis 19878791–795. [DOI] [PubMed] [Google Scholar]
- 31.Mirvish S S, Salmasi S, Cohen S M.et al Liver and forestomach tumors and other forestomach lesions in rats treated with morpholine and sodium‐nitrite, with and without sodium ascorbate. J Natl Cancer Inst 19837181–85. [PubMed] [Google Scholar]
- 32.Mirvish S S. Inhibition by vitamins C and E of in vivo nitrosation and vitamin C occurrence in the stomach. Eur J Cancer Prev 19965131–136. [PubMed] [Google Scholar]
- 33.Ohsawa K, Nakagawa S, Kimura M.et al Detection of in vivo genotoxicity of endogenously formed N‐nitroso compounds and suppression by ascorbic acid, teas and fruit juices. Mutat Res 200353965–76. [DOI] [PubMed] [Google Scholar]
- 34.Schorah C J, Sobala G M, Sanderson M.et al Gastric juice ascorbic acid – effects of disease and implications for gastric carcinogenesis. Am J Clin Nutr 199153287–93S. [DOI] [PubMed] [Google Scholar]
- 35.Sobala G M, Schorah C J, Sanderson M.et al Ascorbic acid in the human stomach. Gastroenterology 198997357–363. [DOI] [PubMed] [Google Scholar]
- 36.Bunton C A, Dahn H, Loewe L. Oxidation of ascorbic acid and similar reductones by nitrous acid. Nature 1959183163–165. [Google Scholar]
- 37.Mirvish S S, Grandjean A C, Reimers K J.et al Dosing time with ascorbic acid and nitrate, gum and tobacco chewing, fasting, and other factors affecting N‐nitrosoproline formation in healthy‐subjects taking proline with a standard meal. Cancer Epidemiol Biomarkers Prev 19954775–782. [PubMed] [Google Scholar]
- 38.Mirvish S S. Blocking formation of N‐nitroso compounds with ascorbic acid in vitro and in vivo. Ann N Y Acad Sci 1975258175–180. [DOI] [PubMed] [Google Scholar]
- 39.Tannenbaum S R, Wishnok J S, Leaf C D. Inhibition of nitrosamine formation by ascorbic acid. Am J Clin Nutr 199153247–50S. [DOI] [PubMed] [Google Scholar]
- 40.Iijima K, Henry E, Moriya A.et al Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology 20021221248–1257. [DOI] [PubMed] [Google Scholar]
- 41.Liu X P, Miller M J S, Joshi M S.et al Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A 1998952175–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford P C, Wink D A, Stanbury D M. Autoxidation kinetics of aqueous nitric‐oxide. FEBS Lett 19933261–3. [DOI] [PubMed] [Google Scholar]
- 43.Wink D A, Darbyshire J F, Nims R W.et al Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media – determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol 1993623–27. [DOI] [PubMed] [Google Scholar]
- 44.Awad H H, Stanbury D M. Autoxidation of NO in aqueous solution. Int J Chem Kinet 199325375–381. [Google Scholar]
- 45.Fletcher J, Wirz A, Young J.et al Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology 2001121775–783. [DOI] [PubMed] [Google Scholar]
- 46.Mowat C, Carswell A, Wirz A.et al Omeprazole and dietary nitrate independently affect levels of vitamin C and nitrite in gastric juice. Gastroenterology 1999116813–822. [DOI] [PubMed] [Google Scholar]
- 47.Sanderson M J, Schorah C J. Measurement of ascorbic acid and dehydroascorbic acid in gastric juice by HPLC. Biomed Chromatogr 19872197–202. [DOI] [PubMed] [Google Scholar]
- 48.Challis B C, Kyrtopoulos S A. Chemistry of nitroso‐compounds. 11. Nitrosation of amines by the 2‐phase interaction of amines in solution with gaseous oxides of nitrogen. J Chem Soc Perkin Trans I 1979299–304.
- 49.Mirvish S S, Shubik P, Wallcave L.et al Ascorbate‐nitrite reaction – possible means of blocking formation of carcinogenic N‐nitroso compounds. Science 197217765–68. [DOI] [PubMed] [Google Scholar]
- 50.Wink D A, Mitchell J B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 199825434–456. [DOI] [PubMed] [Google Scholar]
- 51.Garcia‐Rio L, Herves P, Mejuto J C.et al Comparative study of nitroso group transfer in colloidal aggregates: micelles, vesicles and microemulsions. New J Chem 200327372–380. [Google Scholar]
- 52.Challis B C, Edwards A, Hunma R R.et al Rapid formation of N‐nitrosamines from nitrogen oxides under neutral and alkaline conditions. IARC Sci Publ 1978127–142. [PubMed]