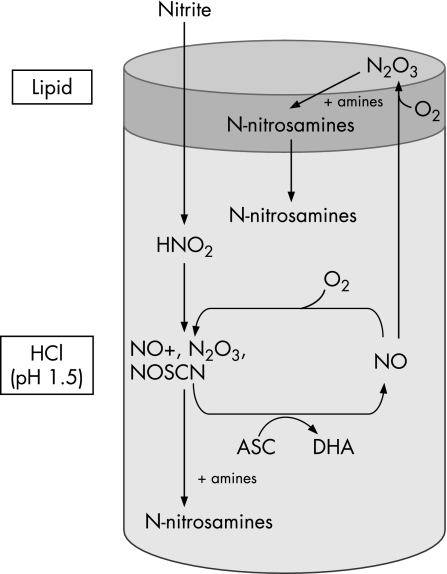
Figure 5 Proposed mechanism of N‐nitrosamine formation in a dual‐phase system with ascorbic acid present in the aqueous phase. Nitrite forms nitrous acid and nitrosating species in the acidic aqueous environment. In the presence of ascorbic acid (ASC), the nitrosating species are reduced to nitric oxide which diffuses into the lipid where it reacts with oxygen reforming nitrosating species such as N2O3. Ascorbic acid is reduced to dehydroascorbic acid. Secondary amines present within the lipid are nitrosated by N2O3 to form N‐nitrosamines. The latter then diffuse back to the aqueous phase.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.