Abstract
Objective
To determine if an aggressive approach to coronary revascularisation with oversized balloons is counterproductive, we studied the effect of increasing balloon‐to‐artery (B:A) ratio on neointimal hyperplasia following primary stent placement using a non‐atherosclerotic porcine coronary overstretch model.
Methods
60 vessels in 33 Yorkshire swine were randomly assigned to one of five B:A ratios between 1.0:1 and 1.4:1. Intravascular ultrasound (IVUS) imaging was performed before bare‐metal stent placement to accurately determine vessel size, after stent placement, and at 28 days.
Results
The mean prestent vessel diameter was 3.05 (0.31) (SD) mm. In‐stent neointimal volume, in‐stent volume stenosis and cross‐sectional area stenosis at the stent minimum lumen diameter increased significantly with increasing achieved B:A ratio (multilevel regression test for slope, p<0.001, p = 0.002 and p<0.001, respectively) and were independent of vessel size. Even minor vessel overstretch at an achieved B:A ratio of 1.1:1 resulted in significant neointimal hyperplasia. Larger B:A ratios were also associated with more neointima beyond the stent edges (p = 0.008). For vessels from the same animal, neointimal response at a given B:A ratio was dependent upon the animal treated.
Conclusions
In a porcine model of IVUS‐guided coronary primary stent placement, vessel overexpansion is counterproductive. Neointimal hyperplasia at 28 days is strongly associated with increasing B:A ratio. In addition, vessels do not respond independently of each other when multiple stents are placed within the same animal using a range of B:A ratios.
The trauma of coronary stent placement triggers an inflammatory reaction that may culminate in migration and proliferation of cells within the lumen of the vessel, a process termed neointimal hyperplasia. Approximately 10–20% of patients treated with a non‐drug‐eluting, or bare‐metal, stent experience symptomatic restenosis and require revascularisation within 1 year.1,2,3,4,5,6
The non‐atherosclerotic, porcine in vivo coronary artery injury model is the preferred model for studying neointimal hyperplasia because swine coronary artery size and anatomy closely resemble human coronary vasculature.7 This model remains relevant due to the significant delay in neointimal hyperplasia and the requirement for prolonged animal observation associated with the use of drug‐eluting stents. In a previous study using this model, neointimal hyperplasia correlated with the degree of vascular injury using a single balloon‐to‐artery (B:A) ratio.8 The purpose of our study was to use intravascular ultrasound (IVUS) in the porcine overstretch model to determine the extent of neointimal hyperplasia after bare‐metal stent placement using a range of pre‐established and randomised B:A ratios. In addition, we examined the within‐animal correlation between neointimal growth and achieved B:A ratio to determine if data from arteries within the same animal are independent of each other.
Materials and methods
Study design
This study used a prospective cohort of Yorkshire pigs that underwent bare‐metal stent placement in the left anterior descending and left circumflex coronary arteries. Each artery was randomised to one of five stent placement B:A ratios (1.0:1, 1.1:1, 1.2:1, 1.3:1 and 1.4:1). The follow‐up period was 28 days. IVUS imaging was performed before bare‐metal stent placement to accurately determine vessel size, after stent placement, and at 28 days.
Randomisation and blinding
Vessels were block‐randomised in groups of 10 vessels (2 vessels in each of 5 animals assigned to one of 5 B:A ratios) using the pseudo‐random number function of Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Balanced, block randomisation was chosen to assure that there would be roughly equal numbers of vessels randomised to the five B:A ratios in case the experiment had to be terminated early.
The investigators performing stent placement could not be blinded because knowledge of the assigned B:A ratio was required in order to select the correct stent size for the vessel. The investigator performing off‐line analysis of angiographic and IVUS images was blinded to the assigned B:A ratio.
Coronary stent placement and IVUS imaging
The study was approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute and conformed to the “Position of the American Heart Association on Research Animal Use”. Disease‐free Yorkshire pigs, 23–52 kg, received 325 mg of aspirin and 75 mg of clopidogrel per day, starting 2 days prior to the procedure. The animals were fed a non‐atherogenic diet.
After the induction of general anaesthesia with xylazine 1 mg/kg and ketamine 10 mg/kg, the animals were intubated and mechanically ventilated. Arterial access was obtained by surgical exposure of the left carotid artery, and an 8F sheath was placed. Heparin was administered to obtain an activated clotting time of 250–300 s. A sterile 8F hockey stick or AL2 custom 60 cm coronary guide catheter was used to engage the left coronary ostium. A 50 μg aliquot of intracoronary nitroglycerin was administered, and angiographic images of the artery were obtained in two orthogonal projections.
Single bare‐metal stents were placed in both the left anterior descending and left circumflex arteries using the same procedure for each artery. Stents were deployed without obstruction of a side branch. IVUS images were acquired with a Discovery™ 2.6F 40 MHz IVUS imaging catheter (Boston Scientific/SCIMED, Maple Grove, MN, USA). Imaging began in the distal third of the vessel and ended at the guide catheter using an automatic pullback device at a speed of 0.5 mm/s. Two automatic pullback IVUS runs were performed and stored on S‐VHS tape for off‐line analysis.
The average vessel diameter was determined by IVUS at the site where stent placement was intended. A stent size was selected (3.0, 3.5 or 4.0 mm) that was less than or equal to the intended final stent diameter, based on the randomised B:A ratio assigned to the vessel. The nominal balloon size was used for the purpose of stent size selection. If the assigned B:A ratio could not be achieved at the selected site in the vessel, then a second site was identified by IVUS to best achieve the assigned B:A ratio with the available stents. Vessels with a maximum appropriate diameter of <3.0 mm were treated to meet or exceed the assigned B:A ratio based upon available stent sizes. A single 16 mm long NIR® stent (Boston Scientific/SCIMED) was placed at the selected position and the balloon was inflated to 8 atm for 30 s. Coronary angiography in one projection was performed when the stent placement balloon had achieved maximum diameter. A final IVUS automatic pullback was performed after stent placement.
The vascular sheath was removed, and the carotid artery was ligated. Pigs were monitored in an observation unit until stable and then returned to the housing unit. Discomfort and injury to animals was limited to that which is unavoidable in the conduct of scientifically valuable research. Aspirin 325 mg and clopidogrel 75 mg were administered daily.
Twenty‐eight days after stent implantation the animals were returned to the catheterisation laboratory. Anaesthesia was established as above. Vascular entry was made from the right femoral artery after surgical exposure. An 8F hockey stick or JL 3.5 guide catheter was used to cannulate the left coronary ostium selectively. Two automatic pullback IVUS runs were performed in the left anterior descending and left circumflex arteries. Angiography and IVUS pullbacks were performed as described above.
Angiographic image analysis
To determine the maximum balloon diameter, coronary angiography was performed in a single projection when the stent placement balloon had achieved maximum diameter. Orthogonal angiographic views were not obtained due to the prolonged balloon inflation time required and the increased likelihood of ventricular fibrillation. The images were digitised off‐line from S‐VHS tape. The maximum diameter of the fully inflated stent balloon was measured using digital calipers. The stent balloon was then compared with the diameter of the 8F guide catheter as a measurement reference.
IVUS image analysis
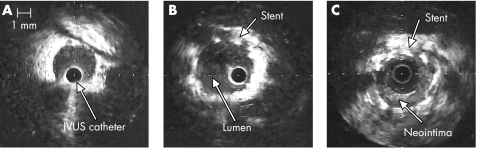
Vessel lumen diameter, stent diameter and neointimal thickness were measured off‐line for each vessel using TapeMeasure™ software (INDEC Medical Systems, Santa Clara, CA, USA). Figure 1 shows representative images.
Figure 1 Neointimal hyperplasia in a porcine model visualised by intravascular ultrasound (IVUS). (A) IVUS image of the left anterior descending coronary artery (diameter 2.9 mm) prior to stent placement. (B) After 4 mm diameter by 16 mm long NIR® stent placement at a balloon‐to‐artery ratio of 1.3:1. The stent is well apposed to the vessel wall. (C) At day 28, the stent diameter is unchanged, but there is eccentric neointimal hyperplasia. The lumen has decreased to 2.75 mm, with a minimum of 0.25 mm and a maximum of 1 mm thickness of neointimal hyperplasia.
Off‐line, computer‐generated, three‐dimensional IVUS image reconstruction was performed using echoPlaque™ (INDEC Medical Systems). Cross‐sectional area measurements were made every 1 mm for images obtained using automatic IVUS pullback, and volume was calculated using Simpson's rule. Table 1 shows the study variable definitions.
Table 1 Definition of study variables.
| Variable | Definition |
|---|---|
| Absolute stent recoil | (Balloon diameter by angiography) − (stent diameter by IVUS after stent placement) |
| Achieved balloon‐to‐artery ratio | (Maximum stent balloon diameter by angiography in a single view) ÷ (mean prestent vessel diameter by IVUS) |
| Cross‐sectional area stenosis at stent minimum LD | (1 − (Minimum in‐stent lumen area)/(stent area)) × 100 |
| Immediate LD gain | (Minimum LD immediately after stent placement) − (minimum LD before stent placement) |
| In‐stent volume stenosis | (1 − (stent lumen volume)/(in‐stent neointimal volume + stent lumen volume)) × 100 |
| Late‐loss index | (Late lumen loss) ÷ (immediate lumen gain) |
| Late lumen loss | (Minimum LD immediately after stent placement) − (minimum LD at day 28) |
| Neointima beyond the stent edges | Total distance from proximal and distal stent edges that displayed neointimal thickness of ⩾0.25 mm by IVUS at day 28 |
| Neointimal thickness | Average neointimal thickness based on four measurements made every 1 mm along the length of the stent |
| Net lumen diameter change | (Minimum LD at day 28) − (prestent LD) |
| Percentage cross‐sectional area stenosis | (1 −(average cross‐sectional area at stent minimum LD by IVUS at day 28) ÷ (average prestent vessel area by IVUS)) × 100 |
| Percentage LD stenosis | (Average of major and minor axes at the lesion minimum LD by IVUS at day 28) ÷ (average prestent vessel diameter by IVUS) × 100 |
| Prestent vessel diameter by IVUS | Average of major and minor axis measurements, from external elastic membrane to external elastic membrane in the mid‐portion of the region where stent placement was intended |
| Relative stent recoil | (Absolute stent recoil) ÷ (balloon diameter by angiography) |
| Stent symmetry index | (Minor axis stent minimum LD) ÷ (major axis stent minimum LD). Measured at the stent mid‐length |
IVUS, intravascular ultrasound; LD, lumen diameter.
The cross‐sectional view of each vessel was divided into four quadrants, one immediately adjacent to the myocardium and a second adjacent to the pericardium. For the myocardial and pericardial quadrants, the maximum linear thickness of neointima was measured along a line perpendicular to the tangent of the myocardium. The ratio of these measurements yielded the myocardial/pericardial ratio.
Sample size
Thirty animals (60 vessels) were required to detect a 30% absolute change in in‐stent stenosis over the range of B:A ratios to be studied. The sample size was calculated using a B:A ratio SD of 0.14, based on equal allocation of vessels to the five B:A ratios, an estimated stenosis SD of 30%, α equal to 0.05 and power equal to 80%.9 To accommodate for early mortality prior to 28 days, the sample size was increased to 33 animals.
Statistical analysis
Continuous variables are expressed as mean (SD), or, for non‐normal data, as the median and interquartile range. The primary outcome variables were in‐stent neointimal volume, in‐stent volume stenosis, cross‐sectional area stenosis at the stent minimum lumen diameter and neointima beyond the stent edges. The secondary outcome variables were immediate lumen diameter gain, absolute stent recoil, minimum lumen cross‐sectional area, late lumen loss and late‐loss index.
The separate effects of B:A ratio and prestent lumen diameter on each of the primary and secondary outcome variables were evaluated with multilevel, univariable regression analysis using BMDP‐5V.10 This program for unbalanced repeated measures takes into account non‐independence of data obtained from multiple vessels (level 1) within the same animal (level 2).11,12 Multilevel, multivariable regression analysis was performed in which achieved B:A ratio and prestent lumen diameter were considered together as predictor variables for each of the four primary outcome variables.
To determine if achieved B:A ratio and prestent lumen diameter were separately associated with each of the primary and secondary outcome variables within the individual animal, within‐animal correlations (rw) were calculated with SPSS 12.0 (SPSS Inc., Chicago, IL, USA) using the method of Bland and Altman.13 Two‐sided p values <0.05 were regarded as statistically significant.
Results
Baseline and procedural characteristics
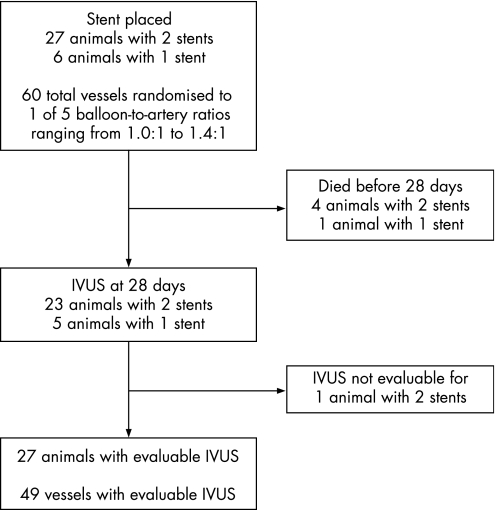
We performed stent placement in 60 coronary arteries of 33 pigs whose mean weight was 40.9 (6.7) kg. Twelve vessels were assigned to each of five B:A ratios from 1.0:1 to 1.4:1 (study flow diagram, fig 2). Three of the animals underwent stent placement in a single vessel because the second vessel was an inappropriate diameter (>4.0 mm) for the randomised B:A ratio and the size of available stents. Five animals died prior to 28 days, four on postprocedure day 1 and one on day 2. Three of the animals that died on day 1 weighed <30 kg on the day of stent placement. IVUS images suitable for quantitative analysis were available for 49 of 51 vessels at the 28‐day end‐point.
Figure 2 Study flow diagram. IVUS, intravascular ultrasound.
A randomisation schedule was established prior to stent placement in the first pig. However, the randomised B:A allocation assignments could not be adhered to because of three consecutive unexpected animal deaths (animals 10, 11 and 12). Deaths occurred in animals with both vessels randomised to B:A ratios >1.2:1. As a result, the next three animals underwent single‐vessel stent placement. Then, to reduce the likelihood of animal deaths, starting with animal 13, we initiated a rule that only one vessel out of two in any pig could be assigned a B:A ratio >1.2:1. For instance, if one vessel was assigned a B:A ratio of 1.3:1 or 1.4:1, then the maximum B:A ratio in the second vessel was 1.2:1 or less.
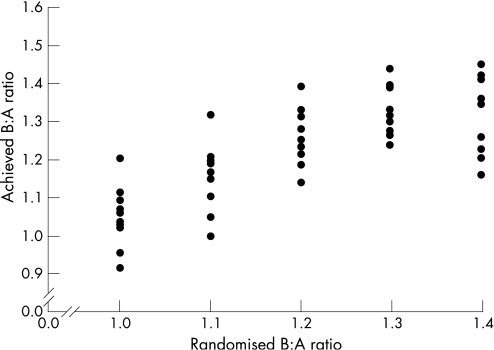
Characteristics of the stented vessels on day 0 and day 28 are given in table 2. It was technically impossible to achieve the exact B:A ratio to which a vessel had been randomised; however, we accomplished our goal of achieving a range of achieved B:A ratios (fig 3).
Table 2 Coronary artery characteristics at day 0 and day 28 after stent placement in 49 vessels from 27 pigs that underwent stent placement and survived to day 28.
| Day 0 | |
| Achieved B:A ratio | 1.23 (0.14) |
| Prestent vessel diameter by IVUS (mm) | 3.05 (0.31) |
| Stent balloon size (mm) | 3.59 (0.42) |
| Absolute stent recoil (mm) | 0.10 (0.29) |
| Relative stent recoil (%) | 2.2 (7.8) |
| Immediate lumen diameter gain (mm) | 0.58 (0.34) |
| Stent symmetry index | Median = 0.96; interquartile range 0.93–1.00 |
| Day 28 | |
| Neointimal thickness (mm) | 0.42 (0.19) |
| In‐stent neointimal volume (mm3) | 54.8 (32.5) |
| Neointima beyond stent edges (mm) | 5.14 (3.28) |
| Late lumen loss (mm) | 1.39 (0.59) |
| Late‐loss index | Median = 2.42; interquartile range 1.72–3.60 |
| Net lumen diameter change | −0.80 (0.45) |
| Percentage lumen diameter stenosis | 26.4 (14.5) |
| Percentage cross‐sectional area stenosis | 43.7 (21.6) |
B:A, balloon‐to‐artery; IVUS, intravascular ultrasound.
Figure 3 Randomised balloon‐to‐artery (B:A) ratio plotted against achieved B:A ratio for 49 vessels in 27 animals that survived to day 28 and had evaluable intravascular ultrasound images.
Primary outcome variables: neointimal response at 28 days
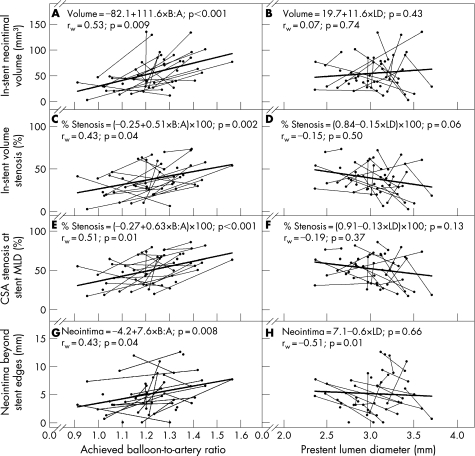
Multilevel, univariable regression analysis and within‐animal correlation analysis both showed that larger B:A ratios yielded greater neointimal responses at day 28 for in‐stent neointimal volume (fig 4A), in‐stent volume stenosis (fig 4C), cross‐sectional area stenosis at the stent minimum lumen diameter (fig 4E) and neointima beyond the stent edges (fig 4G). The data suggest that for vessel sizes ranging from 2.0 to 4.0 mm, increased vessel stretch increased the stimuli for neointimal hyperplasia. Even minor vessel overstretch (B:A ratio of 1.1:1) resulted in significant neointimal hyperplasia (predicted 31% in‐stent volume stenosis and 42% cross‐sectional area stenosis at the stent minimum lumen diameter). IVUS analysis also showed that the zone of neointimal hyperplasia extended beyond both proximal and distal ends of the stent. This was measured in a model where only the stent placement balloon was used and no additional balloon inflations were performed.
Figure 4 Primary outcome variables. Balloon‐to‐artery (B:A) ratio and prestent lumen diameter (LD) plotted against in‐stent neointimal volume (A, B), in‐stent volume stenosis (C, D), cross‐sectional area (CSA) stenosis at the stent minimum lumen diameter (MLD) (E, F) and neointima beyond the stent edges (G, H). Data are from 49 vessels in 27 animals that survived to day 28 and had evaluable intravascular ultrasound images. Thin lines connect data points (solid circles) from the same animal. Data points with no connecting line are from five animals that received a single stent. Linear regression lines (thick lines), linear regression equations and p values for test of slope (first p value in each panel) are based on multilevel regression analysis, which takes into account non‐independence of data obtained from multiple vessels within the same animal. A test‐of‐slope p value <0.05 indicates that the slope of the regression line is significantly different from zero. Within‐animal correlations (rw) and their associated p values are based on data from 22 animals that underwent stent placement in two vessels. A within‐animal correlation p value <0.05 indicates that there is a significant association between the x‐axis and y‐axis variables within the animal. A larger B:A ratio was associated with greater in‐stent neointimal volume (A), in‐stent volume stenosis (C), CSA stenosis at the stent MLD (E) and neointima beyond the stent edges (G). The regression equation in C predicts a 31% in‐stent volume stenosis at a B:A ratio of 1.1:1. The regression equation in E predicts a 42% CSA stenosis at the stent minimum lumen diameter at a B:A ratio of 1.1:1. Note that in H, prestent LD was not significantly associated with neointima beyond the stent edges using multilevel regression analysis, but there was a significant negative within‐animal correlation; within an individual animal, larger prestent LD was associated with less neointima beyond the stent edges. This finding shows that data points obtained from multiple vessels within the same animal cannot be assumed to be independent of each other.
For a given B:A ratio, animals varied with respect to the amount of neointima their vessels produced (fig 4A, C, E and G). Animals that produced a relatively larger amount of neointima or had greater stenosis at a lower B:A ratio tended to have a relatively greater neointimal response at a higher B:A ratio compared with other animals.
Prestent lumen diameter was not predictive of neointimal response by both multilevel, univariable regression analysis and within‐animal correlation analysis for in‐stent neointimal volume (fig 4B), in‐stent volume stenosis (fig 4D) and cross‐sectional area stenosis at the stent minimum lumen diameter (fig 4F). Prestent lumen diameter was not predictive of neointima beyond the stent edges based on multilevel regression analysis (p = 0.66, fig 4H); however, by within‐animal correlation analysis, larger prestent lumen diameter was associated with less neointima beyond the stent edges (p = 0.01). In other words, prestent lumen diameter was not predictive of neointima beyond the stent edges across animals, but it was predictive within the individual animal.
In the multilevel, multivariable regression analysis that considered achieved B:A ratio and prestent lumen diameter together as predictor variables for each of the primary outcome variables (results not shown), both B:A ratio and prestent lumen diameter were predictive of in‐stent neointimal volume (p<0.001 and p = 0.01, respectively). However, only the B:A ratio was a significant predictor for in‐stent volume stenosis (B:A ratio p = 0.01; prestent lumen diameter p = 0.31), cross‐sectional stenosis at the stent minimum lumen diameter (B:A ratio p<0.001; prestent lumen diameter p = 0.67) and neointima beyond the stent edges (B:A ratio p = 0.004; prestent lumen diameter p = 0.21).
Secondary outcome variables
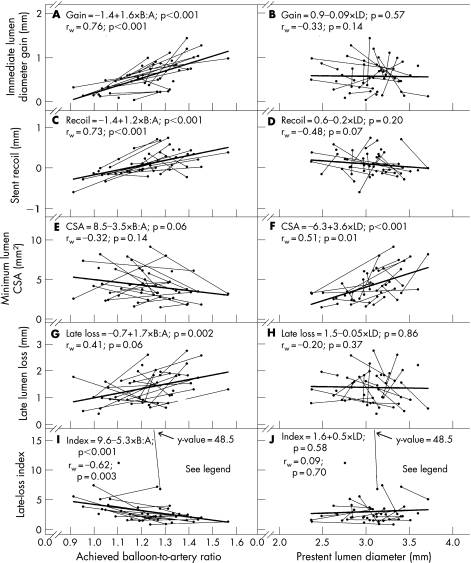
On day 0, immediate lumen diameter gain and absolute stent recoil increased with increasing achieved B:A ratio (fig 5A and C) but were independent of prestent lumen diameter (fig 5B and D). At day 28, the association between achieved B:A ratio and minimum lumen cross‐sectional area was not statistically significant (fig 5E; p = 0.06), but minimum lumen cross‐sectional area was significantly larger for vessels with a larger prestent lumen diameter (fig 5F; p<0.001). A larger B:A ratio was associated with greater late lumen loss and lower late‐loss index values (fig 5G and I). Late lumen loss and late‐loss index were independent of prestent lumen diameter (fig 5H and J).
Figure 5 Secondary outcome variables. Balloon‐to‐artery (B:A) ratio and prestent lumen diameter (LD) plotted against immediate lumen diameter gain (A, B), absolute stent recoil (C, D), minimum lumen cross‐sectional area (CSA) (E, F), late lumen loss (G, H) and late‐loss index (I, J). Other details are as in fig 4. Because of a single very large late‐loss index value of 48.5 for one vessel, the multilevel regression analysis and the within‐animal correlation analysis reported in I and J were performed with the outlier removed. A larger B:A ratio was associated with greater immediate lumen diameter gain (A), greater absolute stent recoil (C), greater late lumen loss (G) and lower late‐loss index values (I). A larger LD was associated with larger minimum lumen cross‐sectional area (F).
In a post hoc analysis, we compared neointimal thickness on the myocardial and pericardial sides of the stented vessel. The ratio of neointimal thickness in the myocardial quadrant of the stented vessel to neointimal thickness in the pericardial quadrant had a mean of 1.39 (0.59). This documents a difference in the proliferative response of the vessel, which may be dependent on extravascular factors and different stresses on the vessel wall from the myocardial and pericardial surfaces.
Discussion
In this study we used IVUS in the non‐atherosclerotic porcine coronary artery overstretch model to determine the extent of neointimal hyperplasia after bare‐metal stent placement using a range of B:A ratios. Our results indicate that there is a strong association between coronary artery overexpansion and neointimal hyperplasia. In‐stent neointimal volume, in‐stent volume stenosis and cross‐sectional area stenosis at the stent minimum lumen diameter are strongly associated with increasing B:A ratio. Surprisingly, even minor vessel overstretch (eg, an achieved B:A ratio equal to 1.1:1) results in significant neointimal hyperplasia (predicted 31% in‐stent volume stenosis).
Previous studies on balloon overstretch
In several previous studies, coronary artery overstretch was used to create vascular injury and the resulting neointimal growth was evaluated using the porcine model. The B:A ratio used in these studies ranged from 1.1:1 to 2.0:1, with a mean follow‐up of 28 days.8,14,15,16,17,18,19,20 Neointimal response is difficult to compare from study to study but appears to be in the range of 0.2–1.4 mm in thickness for a 2.75–3.00 mm coronary artery.
In a previous study, IVUS imaging was used to measure neointimal hyperplasia after bare‐metal stent placement in a non‐atherosclerotic porcine model.21 A single B:A ratio of 1.3:1 was used. At 28 days of follow‐up, there was a strong correlation between IVUS and histological measurements of neointimal hyperplasia. There is only one previous study that used a non‐atherosclerotic porcine model to investigate the effect of arterial stretch and deep vessel injury on neointimal hyperplasia.20 Stent placement methods were based on angiographic rather than IVUS measurements of prestent vessel diameter. Results showed that in‐stent neointimal hyperplasia after stent placement can result from coronary artery stretch in the absence of deep vessel injury. Deep injury, however, stimulates a more prolific neointimal response compared with arterial stretch only.20
IVUS is superior to coronary angiography for evaluating coronary stenosis
IVUS has been shown to be superior to coronary angiography for accurate measurement of vessel diameter.22,23 Despite this knowledge, visual estimatation of reference vessel diameter by angiography has been the traditional method for vessel sizing at the time of stent placement. In the present study, we used IVUS in a porcine model of neointimal hyperplasia before and after stent placement for precise measurement of preprocedure vessel diameter and selection of an appropriate stent size. To our knowledge, our study is the first to use IVUS to examine the extent of neointimal hyperplasia after stent placement using a range of pre‐established and randomised B:A ratios.
Use of the porcine coronary overstretch model
The porcine coronary model remains the standard for the preclinical assessment of new therapies for the treatment of obstructive coronary disease and for the evaluation of restenosis after stent placement. Drug‐eluting stent therapies are being evaluated for their effect on restenosis and the results compared with previous studies using bare‐metal stents in a porcine model. With an increase in preclinical studies to examine the efficacy of new agents and combinations of agents carried by drug‐eluting devices, the porcine model will remain the preferred model for preclinical testing.
In animal studies of neointimal formation in response to coronary stent placement, the animals often receive more than one stent in an effort to minimise the number of animals and the resulting research costs. The assumption is usually made that two data points from the same animal are independent of each other for data analysis purposes. In our study, a significant positive within‐animal correlation was noted between neointimal growth and achieved B:A ratio when two stents were placed in the same animal. Data points from the same animal, therefore, cannot be assumed to be independent of each other when the B:A ratio varies. Neointima beyond the stent edges was also dependent on prestent lumen diameter within the animal. These findings, along with the observation that animals that produced relatively larger amounts of neointima and stenosis at a lower B:A ratio tended to produce relatively more neointima at a higher B:A ratio, need to be considered when analysing data from similar studies involving the porcine model.
Prestent lumen diameter was not predictive of neointima beyond the stent edges based on multilevel regression analysis, yet, within the individual animal, larger prestent lumen diameter was clearly associated with less neointima. These conflicting findings illustrate the importance of conducting both multilevel regression and within‐animal correlation analyses in studies where multiple stents are placed within the same animal.
Study limitations
Although our study was performed using bare‐metal stents, we believe that it provides an essential baseline for future comparison with similar studies using drug‐eluting stents in the porcine model. Disadvantages of the non‐atherosclerotic porcine coronary overstretch model may include differences in vessel compliance and the amount, geometric pattern and time course of neointimal hyperplasia after coronary stent placement when compared with a diseased arterial segment. However, the non‐atherosclerotic porcine model of coronary restenosis is a well‐accepted research tool for the evaluation of in‐stent restenosis much referenced in the literature over the last decade.7,24
Compared with the experimental treatment of a non‐atherosclerotic porcine artery, stent placement within a human coronary artery may require a different approach with respect to balloon sizing. In some cases, a balloon‐to‐lumen ratio of 1.4:1 or greater may be required at the lesion minimum lumen diameter within a diseased human artery to achieve the desired angiographic/clinical result. However, rarely is a balloon‐to‐artery ratio (media to media) of >1.4:1 utilised for human coronary stent placement. This approach would result in significant balloon overexpansion and probable vascular complication (eg, a 4.0 mm balloon inflated within a 2.8 mm artery).
Clinical implications
In clinical practice, no guidelines have been established for matching stent size to vessel size during coronary revascularisation. Many have suggested that it is preferable to overstretch vessels to achieve the largest possible lumen and to accommodate the anticipated late lumen loss after stent placement. This approach is based upon the belief that there will always be a constant amount of neointimal hyperplasia after stent placement that is independent of vessel size or vascular trauma. However, our study showed that this approach is counterproductive and results in an increase of neointimal hyperplasia with only minor vessel overstretch.
The elastic properties of the non‐atherosclerotic porcine coronary artery produced significant vascular recoil after stent placement, with a mean immediate lumen diameter gain of 0.58 (0.34) mm. Larger B:A ratios produced increasing vessel trauma within a segment of normal artery. At 28‐day follow‐up, late lumen loss increased and late‐loss index decreased with increasing B:A ratios within the range of 1.0:1–1.4:1 when the balloon size is compared with the vessel size. However, one should remember in this non‐atherosclerotic porcine model that vessel size (media‐to‐media) is essentially the same as lumen size due to the absence of coronary plaque.
The clinical implication of this study is that careful matching of vessel diameter (media‐to‐media), not lumen diameter, to the desired final stent size, in some cases by preprocedure IVUS measurement, should minimise neointimal hyperplasia and the resulting symptoms of in‐stent restenosis. While angiography can be used to measure lumen size, IVUS may be required in many cases to measure vessel media‐to‐media diameter accurately. IVUS measurements may allow the operator to avoid vessel overstretch of 1.2:1 (eg, a 3.0 mm balloon in a 2.5 mm artery), a treatment ratio that resulted in significant neointimal hyperplasia in the present study.
Recently, a significant clinical trend has been observed with a greater use of drug‐eluting stents with a gradual decline in the use of bare‐metal stents. However, recent data indicate that approximately one‐third to one‐half of percutaneous coronary interventions worldwide involve the use of bare‐metal stents. Thus, our results continue to be relevant to current clinical practice and provide an important foundation for future animal studies using the porcine model that examine implantation of drug‐eluting stents and the resulting neointimal hyperplasia.
Abbreviations
B:A ratio - balloon‐to‐artery ratio
IVUS - intravascular ultrasound
Footnotes
Funding: This study was funded by grants from the Skaggs Clinical Scholars Program in Translational Research at The Scripps Research Institute, La Jolla, CA, USA; the Hewitt Foundation for Medical Research, Irvine, CA, USA; and The Vascular Surgery Foundation of San Diego, La Jolla, CA, USA. During the course of this work, M.Y. was an Established Investigator of the American Heart Association and a recipient of a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund. The authors thank Boston Scientific/SCIMED for supplying coronary stents and intravascular ultrasound catheters, and Cordis Corporation for providing custom coronary guide catheters. The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Competing interests: None.
References
- 1.Stone G W, Ellis S G, Cox D A.et al A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med 2004350221–231. [DOI] [PubMed] [Google Scholar]
- 2.Holmes D R, Leon M B, Moses J W.et al Analysis of 1‐year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus‐eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 2004109634–640. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald P J, Oshima A, Hayase M.et al Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation 2000102523–530. [DOI] [PubMed] [Google Scholar]
- 4.Macaya C, Serruys P W, Ruygrok P.et al Continued benefit of coronary stenting versus balloon angioplasty: one‐year clinical follow‐up of Benestent trial. J Am Coll Cardiol 199627255–261. [DOI] [PubMed] [Google Scholar]
- 5.Morice M C, Serruys P W, Sousa J E.et al A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med 20023461773–1780. [DOI] [PubMed] [Google Scholar]
- 6.George C J, Baim D S, Brinker J A.et al One‐year follow‐up of the Stent Restenosis (STRESS I) Study. Am J Cardiol 199881860–865. [DOI] [PubMed] [Google Scholar]
- 7.Lowe H C, Schwartz R S, Mac Neill B D.et al The porcine coronary model of in‐stent restenosis: current status in the era of drug‐eluting stents. Catheter Cardiovasc Interv 200360515–523. [DOI] [PubMed] [Google Scholar]
- 8.Kornowski R, Hong M K, Tio F O.et al In‐stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 199831224–230. [DOI] [PubMed] [Google Scholar]
- 9.Dupont W D, Plummer W D. Power and sample size calculations for studies involving linear regression. Control Clin Trials 199819589–601. [DOI] [PubMed] [Google Scholar]
- 10. BMDP‐5V for DOS. Los Angeles, CA: BMDP Statistical Software Inc., 1991
- 11.Schluchter M D. Unbalanced repeated measures models with structured covariance matrices. In: Dixon WJ, ed. BMDP Statistical software manual: Volume 2. Berkeley, CA: University of California Press, 19881081–1114.
- 12.de Leeuw J, Kreft I G. Software for multilevel analysis. In: Leyland AH, Goldstein H, eds. Multilevel modelling of health statistics. Chichester: John Wiley & Sons, Ltd, 2001187–204.
- 13.Bland J M, Altman D G. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ 1995310446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna C J, Burke S E, Opgenorth T J.et al Selective ETA receptor antagonism reduces neointimal hyperplasia in a porcine coronary stent model. Circulation 1998972551–2556. [DOI] [PubMed] [Google Scholar]
- 15.Huber K C, Schwartz R S, Edwards W D.et al Effects of angiotensin converting enzyme inhibition on neointimal proliferation in a porcine coronary injury model. Am Heart J 1993125695–701. [DOI] [PubMed] [Google Scholar]
- 16.Morton A C, Arnold N D, Crossman D C.et al Response of very small (2 mm) porcine coronary arteries to balloon angioplasty and stent implantation. Heart 200490324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yorozuya M, Suzuki H, Iso Y.et al Comparison of the morphological changes of restenosis after the implantation of various types of stents in a swine model. Coron Artery Dis 200213305–312. [DOI] [PubMed] [Google Scholar]
- 18.Strehblow C, Gyongyosi M, Sperker W.et al Usefulness of intravascular ultrasound‐guided histological measurements after stenting in porcine coronary artery. Coron Artery Dis 200213291–294. [DOI] [PubMed] [Google Scholar]
- 19.Carrozza J P, Hosley S E, Cohen D J.et al In vivo assessment of stent expansion and recoil in normal porcine coronary arteries: differential outcome by stent design. Circulation 1999100756–760. [DOI] [PubMed] [Google Scholar]
- 20.Gunn J, Arnold N, Chan K H.et al Coronary artery stretch versus deep injury in the development of in‐stent neointima. Heart 200288401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehran R, Mintz G S, Hong M K.et al Validation of the in vivo intravascular ultrasound measurement of in‐stent neointimal hyperplasia volumes. J Am Coll Cardiol 199832794–799. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann R, Mintz G S, Popma J J.et al Overestimation of acute lumen gain and late lumen loss by quantitative coronary angiography (compared with intravascular ultrasound) in stented lesions. Am J Cardiol 1997801277–1281. [DOI] [PubMed] [Google Scholar]
- 23.von Birgelen C, Kutryk M J, Gil R.et al Quantification of the minimal luminal cross‐sectional area after coronary stenting by two‐ and three‐dimensional intravascular ultrasound versus edge detection and videodensitometry. Am J Cardiol 199678520–525. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz R S, Chronos N A, Virmani R. Preclinical restenosis models and drug‐eluting stents: still important, still much to learn. J Am Coll Cardiol 2004441373–1385. [DOI] [PubMed] [Google Scholar]