Abstract
Objectives
The aim of this study was to use late gadolinium hyper‐enhancement cardiac magnetic resonance (LGE‐CMR) imaging to determine if a 72‐h troponin‐I measurement would provide a more accurate estimation of infarct size and microvascular obstruction (MVO) than serial creatine kinase (CK) or early troponin‐I values.
Methods
LGE‐CMR was performed 3.7±1.4 days after medical treatment for acute ST elevation or non‐ST elevation myocardial infarction. Infarct size and MVO were measured and correlated with serum troponin‐I concentrations, which were sampled 12 h and 72 h after admission, in addition to serial CK levels.
Results
Ninety‐three patients, of whom 71 had received thrombolysis for ST elevation myocardial infarction, completed the CMR study. Peak CK, 12‐h troponin‐I, and 72‐h troponin‐I were related to infarct size by LGE‐CMR (r = 0.75, p<0.0001; r = 0.56, p = 0.0003; r = 0.62, p<0.0001 respectively). Serum biomarkers demonstrated higher values in the group with MVO compared with those without MVO (Peak CK 3085±1531 vs 1471±1135, p<0.001; 12‐h troponin‐I 58.3±46.9 vs 33.4±40.0, p = 0.13; 72‐h troponin‐I 11.5±9.9 vs 5.5±4.6, p<0.005). The correlation between the extent of MVO and 12‐h troponin‐I was not significant (r = 0.16), in contrast to the other serum biomarkers (peak CK r = 0.44, p<0.0001; 72‐h troponin‐I r = 0.46, p = 0.0002).
Conclusion
A single measurement of 72‐h troponin‐I is similar to serial CK measurements in the estimation of both myocardial infarct size and extent of MVO, and is superior to 12‐h troponin‐I measurements.
Prognosis after acute myocardial infarction is closely related to the extent of myocardial damage. The degree of damage can be estimated by serological testing and non‐invasive imaging methods (such as echocardiography). Measurement of cytosolic enzymes such as creatine kinase (CK) and the isoenzyme CK‐MB is still common clinical practice.1 However, the utility of these markers in determining infarct size is limited by the requirement for multiple sampling to determine the peak values or area under the curve, and their lack of specificity for myocardial damage.2
Twelve‐hour serum troponin measurements are routinely used in the diagnosis of myocardial ischaemia or infarction. Although such measurements are highly specific for myocardial damage, they may provide unreliable estimates of infarct size.3 Troponin is a structural protein of the contractile apparatus which is released into the circulation in a biphasic fashion after acute myocardial infarction (AMI). An initial peak occurs in the first 24 h, due to release from the cytosolic pool. While this peak provides a reliable marker of infarct size in an animal model of non‐reperfused infarction,4 the complex release kinetics result in a high variability in early troponin concentrations following reperfusion therapy.2
In AMI, the presence of microvascular obstruction (MVO) could cause impaired wash‐out of cardiac biomarkers in the early phase, so that the acute concentrations may not accurately reflect the true extent of myocardial damage. A second phase of troponin release occurs after 72 h, resulting from intramyocardial protein degradation,5 and this “plateau” value seems to be relatively unaffected by early reperfusion therapy.2 The troponin concentration at 72 h may therefore be less affected by release kinetics and coronary reperfusion, and hence provide a more reliable method to quantify myocardial damage and predict the presence of MVO.
Cardiac magnetic resonance (CMR) can be used to image both acute and chronic myocardial infarction using the technique of late gadolinium hyper‐enhancement (LGE).6,7 Several animal studies have validated this technique,8,9 and in the canine model CMR quantification of infarct size has been shown to correlate closely (r = 0.99) with histological measurements using triphenyltetrazolium chloride staining.10 Areas of severe MVO within the infarct core can also be demonstrated by LGE‐CMR in man.11 The high spatial resolution of the LGE‐CMR technique allows accurate measurement of in vivo infarct size.
The aim of this study was to determine if a 72 h troponin (72‐h troponin‐I) measurement would provide a more accurate estimation of infarct size than serial CK or early troponin values.
Methods
Subjects
Ninety‐seven patients (83 men and 14 women, mean age 58 years, range 30–78 years) with a clinical diagnosis of first AMI were prospectively recruited into this study. The diagnosis of AMI was based on traditional World Health Organization criteria as the presence of two or more of the following: suspected cardiac chest pain, a doubling of the serum creatine kinase levels, and/or ischaemic changes on the electrocardiogram (ST segment elevation/depression or new left bundle branch block). Patients were excluded from the study if they had prior admission with any form of acute coronary syndrome, contraindication to CMR imaging, or previous coronary revascularisation, or had required coronary intervention prior to CMR bring performed. All patients in the study had detailed recording of clinical presentation, risk factors, drugs and ECG variables. Written informed consent was obtained in accordance with a protocol approved by the institutional ethics committee.
Biochemical protocols
All patients had plasma samples taken on admission and 12‐hourly for 36 h for serial creatine kinase levels measured by NAC activated CK at 37°C (Hitachi 747, Roche Diagnostics, Lewes, UK). Seventy‐two‐hour troponin assays were performed in 64 patients (using the Beckman Coulter Accu TnI assay; Beckman Coulter, High Wycombe, UK; inter‐assay CV 4.7 and 7.3% at mean troponin of 1.64 and 33.5 μg/l respectively). These samples were separated and stored at −70°C, then analysed as a single batch at the end of the study. The serial CK and 12‐h troponin‐I assays were analysed individually throughout the study to provide clinical management information. As the clinically available troponin assay changed from troponin‐T to troponin‐I during the study, comparable 12‐h troponin‐I values using the same assay to 72‐h troponin‐I are available only for 37 patients.
CMR protocol and analysis
All patients underwent CMR imaging during their index admission. Patients were studied supine in a 1.5‐T scanner (Philips Medical Systems, Best, The Netherlands) equipped with “Master” gradients (30 mT/m, 150 mT/s slew rate) and a 5‐element cardiac phased array receiver coil. The comprehensive CMR imaging protocol that was used has previously been described in detail.7 In brief, for measurements of ventricular mass and volume, cine imaging covering the whole heart in 10–12 parallel short axis slices was performed using a steady‐state free precession pulse sequence (echo time, 1.4 ms; repetition time, 2.8 ms; flip angle 55°, spatial resolution 2×2×7 mm3, 18 phases per cardiac cycle). During the comprehensive CMR protocol, a cumulative dose of 0.2 mmol/kg Gadolinium‐DTPA (Magnevist, Schering AG, Berlin, Germany) was administered using a power injector (Spectris MR injection system, Medrad, Pittsburgh, PA). Ten to 15 minutes after final contrast injection, late gadolinium hyper‐enhancement imaging was carried out. The images, obtained during breath‐holds and gated to the ECG, were acquired using an inversion‐recovery segmented k‐space gradient‐echo pulse sequence with a non‐selective 180° prepulse (echo time, 3.8 ms; repetition time, 7.5 ms; flip angle 15°), and inversion time was adjusted individually to suppress normal myocardium. Ten to 12 short axis slices with spatial resolution of 1.8×1.8×10 mm3 were obtained.
Analysis was performed off‐line using commercial software (Mass 5.0, Medis, Leiden, The Netherlands), by a single experienced observer blinded to the clinical presentation. For the left ventricular (LV) mass and volumes, epicardial and endocardial contours were traced by planimetry on each short axis slice at end‐diastole and end‐systole. Using a modified Simpson's rule (summation of discs method), LV mass (g) was calculated from the total volume of myocardium at end‐diastole multiplied by the myocardial density of 1.05 g/ml. LGE images were displayed on a grey scale so as to optimally distinguish infarcted tissue (white) from normal myocardium (black) and the blood pool. Regions of interest were drawn by computer‐assisted planimetry around the hyperenhanced tissue (white) in each of the LV short axis slices, summated as above, and expressed in grams of infarcted myocardium. MVO was identified on the late hyper‐enhancement images and defined as subendocardial areas of absent or low signal, surrounded by enhancing myocardium.12 Regions of interest were drawn around areas of hypo‐enhancement, and MVO volume and mass were calculated as described above.
Statistics
Data are presented as mean±standard deviation (SD). Differences between means of continuous variables were analysed using an independent samples t test, and Levene's test for equality of variances was calculated to determine the appropriate significance level. The relationship between changes in measured variables was examined by Spearman correlation coefficient (r). The relationships of clinical, biochemical and LGE‐CMR measurements were examined using linear regression analysis. Subgroup analyses were carried out for the thrombolysed and non‐thrombolysed groups of patients. All statistical tests were two‐sided, and values of p<0.05 were considered statistically significant.
Results
Ninety‐seven patients were recruited and 93 patients completed the CMR protocol. Four CMR studies were not completed due to claustrophobia. Images were interpretable in all patients who completed the protocol. The mean time from admission with AMI to the CMR study was 3.7±1.4 days. The majority of patients were scanned between 2 and 5 days after their infarction, with only 7 patients having their CMR outside this 3 day window. Table‐1 summarises the patient demographics and their presenting characteristics. Of the 73 patients who had ST elevation on their presentation ECG and were eligible for thrombolysis, 2 had only transient ECG changes and therefore were not thrombolysed.
Table 1 Demographics including cardiac risk factors and presenting characteristics of the 93 patients included in the analysis.
| Sex | |
| Male | 79 (85%) |
| Female | 14 (15%) |
| Age (years) | 58.2±10.8 |
| Risk factors | |
| BMI (kg/m2) | 26.3±3.5 |
| Current smoker | 54 (58%) |
| Diabetes mellitus | 12 (13%) |
| Hypertension | 17 (18%) |
| Family history of premature CAD | 39 (42%) |
| Prior diagnosis of angina | 7 (8%) |
| Presenting characteristics | |
| STEMI | 73 (78%) |
| NSTEMI | 19 (20%) |
| Bundle branch block | 1 (1%) |
| Thrombolysis | 71 (76%) |
| Time from hospital arrival to thrombolysis (min) | 29±19.1 |
Data presented as n (%) or mean±SD.
BMI, body mass index; NSTEMI, non‐ST elevation myocardial infarction; premature CAD, coronary artery disease in first degree relative before the age of 65 years; STEMI, ST elevation myocardial infarction.
Relationship between infarct size and biochemical markers
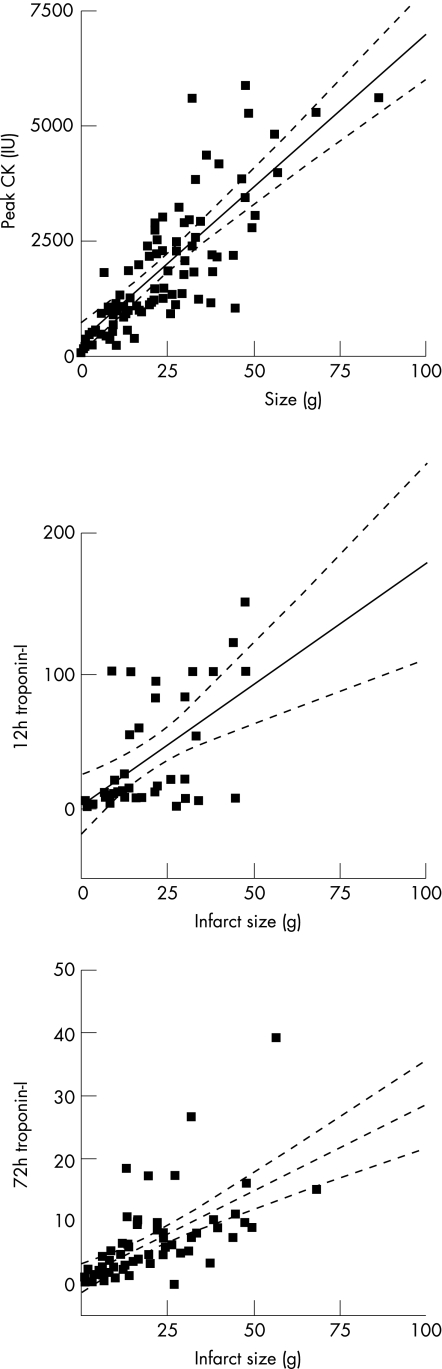
Table 2 details the differences in LV mass, infarct size, percentage LV infarcted and biomarkers for all patients with subdivision into thrombolysed and non‐thrombolysed groups. These CMR and biomarker data are concordant in that the non‐thrombolysed group (predominately NSTEMI) tended to have smaller infarcts in this study. All of the biomarker measurements showed a significant positive correlation to the mass of infarcted myocardium (peak CK r = 0.75, p<0.0001; 12‐h troponin‐I r = 0.56, p = 0.0003; 72‐h troponin‐I r = 0.62, p<0.0001). For those patients who received thrombolysis, the relationship appeared stronger for peak CK (r = 0.73, p<0.0001) and 72‐h troponin‐I (r = 0.60, p<0.001) compared with 12‐h troponin‐I (r = 0.53, p = 0.017). In the 22 patients who did not receive thrombolysis, all three biomarker measurements still remained positively correlated to infarct mass (peak CK r = 0.78, p<0.0001; 12‐h troponin‐I r = 0.52, p = 0.027; 72‐h troponin‐I r = 0.67, p = 0.017). Figure 1 shows example images from different patients, which illustrates LGE and MVO by CMR. Figure 2 demonstrates the relationship by linear regression between infarct size measured by CMR imaging and peak CK, 12‐h troponin‐I and 72‐h troponin‐I.
Table 2 Biomarker and CMR estimation of infarct size in all subjects and their subdivision into thrombolysed and non‐thrombolysed groups.
| All patients | All thrombolysed | Non‐thrombolysed | p Value | |
|---|---|---|---|---|
| Peak CK (IU) | 1905±1438 (93) | 2141±1527 (71) | 1142±692 (22) | p<0.001 |
| 12‐h troponin‐I (mg/ml) | 38.6±42.1 (38) | 55.1±49.5 (20) | 20.3±21.2 (18) | p<0.008 |
| 72‐h troponin‐I (mg/ml) | 6.8±6.5 (64) | 7.4±6.9 (52) | 4.1±3.6 (12) | p<0.108 |
| LV mass (g) | 118.8±27.3 (93) | 119.4±27.6 (71) | 116.7±26.8 (22) | p<0.679 |
| Infarct size (g) | 23.2±16.2 (93) | 25.3±17.1 (71) | 16.4±10.1 (22) | p<0.004 |
| Percentage of LV infarcted (%) | 19.2±11.8 (93) | 20.7±12.2 (71) | 14.2±8.5 (22) | p<0.007 |
| MVO (g) | 5.4±3.7 (25) | 5.7±1.7 (22) | 2.6±2.5 (3) | p<0.011 |
Data presented as mean±SD (number of patients in group).
CK, creatine kinase; LV, left ventricle; MVO, microvascular obstruction; p value, difference between thrombolysed and non‐thrombolysed groups.
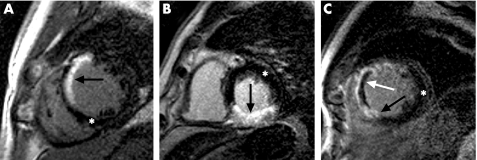
Figure 1 Examples of short‐axis LGE‐CMR images in three separate patients. The white asterix shows normal myocardium; solid black arrows indicate infarcted (hyper‐enhanced) myocardium; the solid white arrow indicates microvascular obstruction as a black core within an area of hyperenhancement. (A) Anteroseptal infarction (white) of the endocardium with some sparing of the epicardial layer. (B) Transmural inferior myocardial infarction. (C) Transmural infarction of the anterior and septal myocardial walls with evidence of microvascular obstruction.
Figure 2 Mass (g) of infarcted myocardium assessed by cardiac magnetic resonance imaging, correlated to biochemical markers of infarct size in all patients studied. CK, creatine kinase.
Relationship between MVO mass, infarct size and biochemical markers
Twenty‐five patients (27%) had evidence of microvascular obstruction, and 68 did not. Three patients with MVO had not received thrombolysis. Data on the mean infarct and MVO mass according to whether thrombolysis was administered are presented in table 2. Infarct mass was greater, and serum biomarkers demonstrated higher values in the group with MVO compared with those without MVO, as detailed in table 3. The extent of MVO was related to infarct size by LGE‐CMR (r = 0.63, p<0.001).
Table 3 Infarct size and biomarker concentration according to the presence of microvascular obstruction.
| MVO present | MVO absent | p Value | |
|---|---|---|---|
| Patient number | 25 | 68 | – |
| Peak CK | 3085±1531 (25) | 1471±1135 (68) | p<0.001 |
| 12‐h troponin‐I | 58.3±46.9 (8) | 33.4±40.0 (30) | p = 0.199 |
| 72‐h troponin‐I | 11.5±9.9 (14) | 5.5±4.6 (50) | p<0.002 |
| Infarct size (g) | 37.6±15.8 (25) | 17.8±12.7 (68) | p<0.0001 |
| % LV infarcted | 29.6±9.9 (25) | 15.3±11.9 (68) | p<0.0001 |
The correlation between the extent of MVO and 12‐h troponin‐I was not significant (r = 0.16), in contrast to the other serum biomarkers (peak CK r = 0.44, p<0.0001; 72‐h troponin‐I r = 0.46, p = 0.0002).
Discussion
This is the first study to use LGE‐CMR to investigate the relationship between 72‐h troponin elevation, infarct size and MVO. In addition, our study contributes to the growing body of evidence that CMR imaging is feasible in patients shortly after either ST elevation or non‐ST elevation acute myocardial infarction.7,12,13
For many years, it has been routine clinical practice to estimate the size of a myocardial infarction by the use of biochemical markers, particularly from the peak serum levels of CK or CK‐MB.1 Although these peak values may relate to overall cardiovascular risk, it is well recognised that there are major limitations in using these to quantify infarct size. This was demonstrated by Smith et al,14 who showed that infarct size could be underestimated in up to 47% of cases. The reasons for this underestimation are due largely to complex kinetics relating to the proportion of enzyme depleted from the myocardium, the amount released into the plasma, as well as the effects of clearance and compartmentalisation. Thus, multiple samples have to be taken to ensure that either the true peak CK level is not missed or the area under the curve can be calculated. To complicate matters further, after coronary reperfusion therapy, enzyme kinetics are substantially altered and become even more variable depending on the time delay to reperfusion and its abruptness.15
Troponin's release ratios and kinetics differ from CK, having an early peak after release of the cytosolic pool, and then a plateau phase resulting from intramyocardial protein degradation.5,16 Earlier studies have compared the relationship between histopathological infarct size and early troponin values in animal models,4 and between early troponin values and thallium or sestamibi defects in patients.17 Late troponin measurements, in previous non‐quantitative nuclear imaging studies, correlated well with scintigraphically determined infarct size,18,19 However, until recently, late troponin values had not been assessed by LGE‐CMR.
Ingkanisorn et al13 published the first work investigating the association between early troponin values and CMR infarct size after acute coronary syndromes in humans. The authors found that peak troponin‐I correlated with acute infarct mass in those patients who underwent acute percutaneous coronary intervention (PCI) (r = 0.83, p<0.001, n = 23), which was defined as “PCI within 6 h of presentation”. However, the linear correlation between infarct size and troponin‐I was not statistically significant for non‐reperfused infarcts (p = 0.28, n = 10), and there were no data relating to CK or late troponin values. Ingkanisorn's data are in keeping with the results in our study patients who received thrombolysis, and which showed a significant positive correlation between infarct size and 12‐h troponin‐I. However, with our larger patient sample, we have also been able to identify for the first time a significant relationship between infarct size and 12‐h troponin‐I in a non‐thrombolysed patient group.
Recently, Steen et al tested troponin‐T assessment at 96 h after myocardial infarction and compared it to infarct size as assessed by LGE‐CMR in 23 patients with STEMI and 21 with NSTEMI.20 While no data relating to early troponin or CK values were presented, they showed a strong positive relationship between this late troponin value and infarct size in 23 PCI‐treated STEMI patients (r = 0.91). The correlation for the 21 NSTEMI patients (r = 0.58), is similar to our results in the 22 non‐thrombolysed patients (r = 0.67). The correlation between 96‐h troponin T and infarct mass appears stronger than that obtained for 72‐h troponin‐I for ST elevation infarcts in our study (r = 0.60) Although this discrepancy could be caused by the different assays or sampling times, it could also be explained by the difference in sample size or reperfusion therapy (PCI vs thrombolysis) between the two studies.
In our study, we have confirmed the relationship of CMR infarct size to peak CK. As the relationship is stronger for peak CK than those of early or late troponin measurement, this supports the continued use of serial CK measurements to assess infarct size in routine clinical practice. However, when serial CK samples cannot be obtained (such as late patient presentation) or when they may be inaccurate due to concurrent skeletal muscle damage (such as in the postoperative period) an alternative biomarker such as 72‐h troponin‐I could be useful.
In previous studies, MVO appears to have been a better predictor of cardiovascular complications than absolute infarct size.21,22,23 Although the reasons for this are unclear, it may be that the persistent blockage of the microvasculature reduces the benefit of epicardial revascularisation. Hence, in addition to the prognostic information, it is possible that the presence of MVO in combination with the transmurality of the infarct and assessment of myocardial perfusion by CMR may in the future help identify patients in whom coronary intervention would be most beneficial.
This persistent obstruction of the microvasculature could also prevent rapid “washout” of the cardiac enzymes after reperfusion, thus blunting the early serum measurement of CK and 12‐h troponin‐I, thereby giving a falsely small impression of infarct size. Indeed, we have shown no relationship between MVO extent and 12‐h troponin‐I in this study, but a significant positive relationship between MVO and 72‐h troponin‐I and peak CK from serial measurements.
We hypothesised that a late “plateau” troponin value would be less dependent on the timing and abruptness of reperfusion therapy and hence would provide a more accurate marker of final infarct size than early troponin values. Our study supports the hypothesis that in both ST and non‐ST segment elevation acute myocardial infarction, a 72‐h troponin‐I can provide a reliable estimate of infarct size and indicator of MVO.
Limitations
Twelve‐ and 72‐h troponin‐I measurements were available on only 37 and 64 patients respectively as detailed above. The data concerning early troponin is therefore not as complete as for the other measurements, but this group is still larger than recently published studies relating CMR measurements to troponin values.13,20
The results from this study have been analysed as thrombolysed vs non‐thrombolysed, rather than STEMI vs NSTEMI. Thrombolysed patients were analysed separately because the utility of timed biomarker values was one of the key variables being assessed, and as reperfusion therapy alters the kinetics of enzyme release, it was thought appropriate to consider all of the non‐reperfused patients separately. For the purpose of analysis, we have assumed that administration of thrombolysis leads to resolution of ischaemia, although this may not always be the case. The efficacy and rate of action of thrombolysis vary between patients and is difficult to make allowance for in small studies. However, patients who failed to reperfuse after thrombolysis and required rescue PCI were excluded from this study.
Finally, by definition, the non‐thrombolysed group is heterogeneous, containing patients with ST depression myocardial infarction and late‐presentation infarcts. However, this is a valid group to consider, as it closely represents real‐life clinical practice where biomarkers are used with some difficulty to determine infarct size.
Conclusion
A single measurement of 72‐h troponin‐I is similar to serial CK measurements in the estimation of both myocardial infarct size and extent of MVO, and is superior to 12‐h troponin‐I measurements.
Acknowledgements
The research programme was supported by the British Heart Foundation.
Abbreviations
AMI - acute myocardial infarction
CK - creatine kinase
LGE‐CMR - late gadolinium hyper‐enhancement cardiac magnetic resonance
LV - left ventricular
MVO - microvascular obstruction
NSTEMI - non‐ST elevation myocardial infarction
PCI - percutaneous coronary intervention
STEMI - ST elevation myocardial infarction
Footnotes
Funding: Dr Younger and Professor Ball are supported by the British Heart Foundation. Dr Plein is supported by the Wellcome Trust.
Competing interests: None.
References
- 1.Apple F S, Murakami M, Panteghini M.et al International survey on the use of cardiac markers. Clin Chem 200147587–588. [PubMed] [Google Scholar]
- 2.Katus H A, Remppis A, Scheffold T.et al Intracellular compartmentation of cardiac troponin‐T and its release kinetics in patients with reperfused and nonreperfused myocardial‐infarction. Am J Cardiol 1991671360–1367. [DOI] [PubMed] [Google Scholar]
- 3.Katus H A, Looser S, Hallermayer K.et al Development and in vitro characterization of a new immunoassay of cardiac troponin‐T. Clin Chem 199238386–393. [PubMed] [Google Scholar]
- 4.Metzler B, Hammerer‐Lercher A, Jehle J.et al Plasma cardiac troponin T closely correlates with infarct size in a mouse model of acute myocardial infarction. Clin Chim Acta 200232587–90. [DOI] [PubMed] [Google Scholar]
- 5.Remppis A, Scheffold T, Greten J.et al Intracellular compartmentation of troponin‐T—release kinetics after global‐ischemia and calcium paradox in the isolated‐perfused rat‐heart. J Mol Cell Cardiol 199527793–803. [DOI] [PubMed] [Google Scholar]
- 6.Wagner A, Mahrholdt H, Holly T A.et al Contrast‐enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003361374–379. [DOI] [PubMed] [Google Scholar]
- 7.Plein S, Greenwood J P, Ridgway J P.et al Assessment of non‐ST‐segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004442173–2181. [DOI] [PubMed] [Google Scholar]
- 8.Kim R J, Chen E L, Lima J A C.et al Myocardial Gd‐DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 1996943318–3326. [DOI] [PubMed] [Google Scholar]
- 9.Judd R M, Lugoolivieri C H, Arai M.et al Physiological‐basis of myocardial contrast enhancement in fast magnetic‐resonance images of 2‐day‐old reperfused canine infarcts. Circulation 1995921902–1910. [DOI] [PubMed] [Google Scholar]
- 10.Kim R J, Fieno D S, Parrish T B.et al Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 19991001992–2002. [DOI] [PubMed] [Google Scholar]
- 11.Lima J A, Judd R M, Bazille A.et al Regional heterogeneity of human myocardial infarcts demonstrated by contrast‐enhanced MRIs. Potential mechanisms. Circulation 1995921117–1125. [DOI] [PubMed] [Google Scholar]
- 12.Tarantini G, Cacciavillani L, Corbetti F.et al Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty—A study performed with contrast‐enhanced magnetic resonance. J Am Coll Cardiol 2005461229–1235. [DOI] [PubMed] [Google Scholar]
- 13.Ingkanisorn W P, Rhoads K L, Aletras A H.et al Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J Am Coll Cardiol 2004432253–2259. [DOI] [PubMed] [Google Scholar]
- 14.Smith J L, Ambos H D, Gold H K.et al Enzymatic estimation of myocardial infarct size when early creatine‐kinase values are not available. Am J Cardiol 1983511294–1300. [DOI] [PubMed] [Google Scholar]
- 15.Vatner S F, Baig H, Manders W T.et al Effects of coronary‐artery reperfusion on myocardial infarct size calculated from creatine‐kinase. J Clin Invest 1978611048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavin F, Kane M, Forde A.et al Comparison of five cardiac markers in the detection of reperfusion after thrombolysis in acute myocardial‐infarction. Br Heart J 199573422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmarian J J, Pratt C M, Borgesneto S.et al Quantification of infarct size by Tl‐201 single‐photon emission computed‐tomography during acute myocardial‐infarction in humans—comparison with enzymatic estimates. Circulation 198878831–839. [DOI] [PubMed] [Google Scholar]
- 18.Panteghini M, Cuccia C, Bonetti G.et al Single‐point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem 2002481432–1436. [PubMed] [Google Scholar]
- 19.Licka M, Zimmermann R, Zehelein J.et al Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 200287520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steen H, Giannitsis E, Futterer S.et al Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006482192–2194. [DOI] [PubMed] [Google Scholar]
- 21.Wu K C, Zerhouni E A, Judd R M.et al Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 199897765–772. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Maruyama A, Iwakura K.et al Clinical implications of the “no reflow” phenomenon—A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 199693223–228. [DOI] [PubMed] [Google Scholar]
- 23.Kramer C M. The prognostic significance of microvascular obstruction after myocardial infarction as defined by cardiovascular magnetic resonance. Eur Heart J 200526532–533. [DOI] [PubMed] [Google Scholar]