Despite the decrease in overall mortality from coronary artery disease, the number of out‐of‐hospital deaths from myocardial infarction is in the range of 60% of all infarct related case fatalities.1 In patients with known risk of sudden cardiac death (SCD), such as survived resuscitation, left ventricular aneurysm or low left ventricular ejection fraction, the incidence of SCD is in the region of 30% per year. In the general population, it is only 0.5% per year.2 However, the absolute number in this group is 10 times higher than in the patient population with known SCD risk, reaching more than 300 000 case fatalities per year in the USA.2 Even renowned cardiologists such as Ronald W Campbellw1 and Jeffry M Isnerw2, who were experts on the topic of arrhythmias and myocardial infarction, suffered SCD. The MONICA (Monitoring trends and determinants in Cardiovascular disease) study reported that of all coronary heart disease (CHD) patients who die within 28 days after onset of chest pain, two thirds die before reaching the hospital.w3 Accordingly, the main task has been to strengthen primary and secondary prevention.w3 This strategy brings about a major challenge: how can we define who is at risk?
In 1978, Mason Sones, the father of coronary angiography, asked for “a way of recognizing these people before they drop dead”. He noted, “We are still living in a world, where almost one third of the patients die before we are aware that these people were ill or that their lives were in jeopardy”.w4
For identifying people at risk the Framingham risk score,3 the Prospective Cardiovascular Münster (PROCAM) Score,4 the Systemic Coronary Risk Evaluation (SCORE) of the European Society of Cardiology,5 as well as other more specific scores like the Reynold's Risk Score for women,w5 have been proposed. In young adults in particular, risk prediction based on such global risk scores provides for only limited prognostic accuracy, as has been analysed in patients with myocardial infarction under the age of 55 years.w6 Indeed, improved risk prediction may be obtained by detecting signs of subclinical atherosclerosis. The Third National Cholesterol Education Program Adult Treatment Program (NCEP ATP III) as well as the Third Joint Task Force of European and other Societies of Cardiovascular Disease Prevention in Clinical Practice (European Society of Cardiology (ESC) guidelines) have suggested the use of additional imaging and non‐imaging tests in order to detect signs of subclinical atherosclerosis or inflammation for further risk stratification.3,5,6,7,8,9,10,11w7 w8
Coronary artery disease risk assessment algorithms
For individual risk assessment current recommendations suggest the use of algorithms provided by international and national societies as a first step. The most widely used algorithm is based on the Framingham study and is incorporated into the NCEP ATP III.6 Recently, an update was provided which used four categories of 10 year absolute event risk.7
I. High risk: >20% 10 year risk for hard cardiac events (cardiac death or non‐fatal myocardial infarction) resulting from CHD (history of myocardial infarction, unstable angina, stable angina, coronary artery revascularisation) or from cardiac risk equivalents (peripheral artery disease, aortic aneurysms, carotid artery disease (transient ischaemic attacks, stroke or >50% carotid stenosis)) or diabetes or ⩾2 major risk factors (smoking, hypertension, hypercholesterolaemia, low high density lipoprotein (HDL cholesterol <40 mg/dl, 1.0 mmol/l), family history of premature CHD (men <55 years, women <65 years)) with 10 year risk >20%.
II. Moderately high risk (also called “intermediate risk”): ⩾2 major risk factors with 10–20% 10 year risk for hard cardiac events.
III. Moderate risk: ⩾2 major risk factors with <10% 10 year risk of hard cardiac events.
IV. Lower risk: no or 1 risk factor (usually <10% risk of hard cardiac events).
An ESC task force has developed the SCORE risk model,12 which estimates the 10 year risk of a cardiovascular death based on age, sex, blood pressure, cholesterol, and smoking. The cut off value for high risk was set at 5%.6w7 Due to differences and the decline in cardiovascular disease mortality in Europe, risk charts have been adapted to regional variations such as in Norwayw7 and Germany.w9 In Norway, however, the SCORE high risk function overestimates the risk for cardiovascular death in men as well as the elderly and underestimates it in women and young adults.w7
The PROCAM (Program for Coronary Artery disease Münster) study is based on a cohort of working men up to the age of 65 years.4w10 The study is ongoing and now also includes women. High risk is presumed when the 10 year cardiac mortality exceeds 20%.
Recently, further risk score modifications have been suggested, taking into account exercise, body mass index and a more detailed history of smoking (CARRISMA score), but this approach has not yet been prospectively tested.w11
Advanced coronary artery risk assessment
Both the NCEP ATP III and the ESC suggest using additional tests in order to achieve better differentiation, particularly in the moderately high risk group (also called the intermediate risk group in the previous version).5,6 Non‐imaging tests include the measurement of C reactive protein, the ankle–arm index, and stress ECG in men between 45–65 years. For imaging tests, carotid ultrasound based intima–media thickness and coronary artery calcium (CAC) scoring have been proposed.
Compared to other methods under discussion, CAC has the advantage of having been tested intensively. Signs of coronary atherosclerosis are visualised directly and can also be precisely localised and quantified. The technology behind CAC scoring and the clinical understanding, use and consequences of preventive care will be addressed.
Technology for calcium scoring
It has long been known that fluoroscopy allows the detection of calcification of the coronary arteries, but quantification is not possible and the coronary arteries are not directly visible. Nevertheless, this technique has been used for the diagnosis of coronary artery disease.w12 Clinical cardiology took no advantage of it despite the recommendations of some authors.w13
The development of electron beam computed tomography (EBCT) represented a breakthrough because the coronary arteries could be visualised non‐invasively and calcification detected, localised and quantified.12,13w14–19 EBCT uses an electron sweep of stationary tungsten target rings (210°) in order to generate a cone‐shaped x ray beam. Resulting x ray images can detect even small amounts of calcium. The EBCT can be operated in various scanning modes, using up to four target rings. For coronary artery imaging, the single slice mode, which employs only one target ring with 1728 elements, gives maximum spatial orientation.12 The EBCT examination is performed using C100, C150 or C300 scanners (General Electric GE, Imatron, San Francisco, California, USA). The scanners are operated in the single slice mode with an image acquisition time of 100 ms and a section thickness of 3 mm. Prospective ECG triggering is done at 80% of the R–R interval in diastole. Contiguous slices down to the apex of the heart are obtained. Owing to the favourable temporal resolution, diagnostic scans are possible up to a heart rate of 110 beats/min.12 However, z‐axis spatial resolution is limited to ⩾1.5 mm.
Analyses can be performed with a variety of specific workstations (NetraMD workstation, ScImage, Los Altos, California, USA; Virtuoso, Siemens Medical Solutions, Forchheim, Germany).
Scanning time is between 20–40 s, and examination time 3–4 min. In addition 5–10 min are needed for evaluation, quantification of calcification and generation of a report—ideal for a screening test. Radiation exposure from CAC is about 1 mSv and ranges between 0.8–1.3 mSv.12w18
In many institutions, multislice (detector) computed tomography (MSCT) has either replaced or been installed instead of EBCT, because the systems are cheaper and have an improved spatial resolution. After introduction of the four slice CTs, 16 row, 32 row, 64 row, now dual‐source 64 row, and 256 row CTs can be used for CAC scanning. In the craniocaudal direction, collimation of up to 64×0.6 mm is used, with up to 330 ms for one gantry rotation. With the new scanner generations, temporal resolution has continuously decreased and is now 165 ms for a 64 slice scanner and 83 ms for a dual source scanner.w20 Modern systems have an isotropic spatial resolution of 0.4 mm. For coronary calcium scans, generally a reconstructed slice thickness of 3 mm is chosen. A tube voltage of approximately 120 kV and variable mAs (∼150 mAs) is used. In general, the table is moved continuously. As opposed to EBCT, the tube voltage and current can be modified in order to adjust for body weight or other physiological variables. Total scanning time is only ⩽20 s for the entire chest (table 1). Mostly retrospective ECG gating with single half segment reconstruction is used.w20 With retrospective gating, variable time points can be selected for optimal image reconstruction. Both EBCT and MSCT studies have demonstrated that, in most patients, motion‐free image quality is obtained when selecting images in mid diastole. To reduce the radiation dose it is also possible to perform prospective ECG triggering with MSCT in a fashion similar to EBCT, accepting the lack of opportunity to single out retrospectively certain time points. Because the temporal resolution of all current CT machines is inferior compared with echocardiography or even fluoroscopy, it is useful to reduce the heart rate to ⩽70 beats/min for a coronary calcium scan. Radiation exposure is higher than for EBCT and is in the range of 1–5 mSv for retrospective gating and 1–2 mSv for prospective ECG triggering.12
Table 1 Technical features of multidetector computed tomography (MDCT) systems with different numbers of rows.
| Detector row | 4 | 16 | 32/64 |
| Collimation | 4×2.5 mm | 16×0.75 mm | 32/64×0.625 mm |
| Rotation time | 500 ms | 370–420 ms | 330–400 ms |
| Current | 300 mAs | 300 mAs | 150 mAs |
| Voltage | 80 kV | 80 kV | 120 kV |
| Effective dose | 1–5 mSv | 1–5 mSv | 1–5 mSv |
| Slice thickness | 3 mm | 3 mm | 3 mm |
| Slice overlap | 1.5 mm | 1.5 mm | 1.5 mm |
| ECG pulsing | Yes | Yes | Yes |
Quantification of CAC
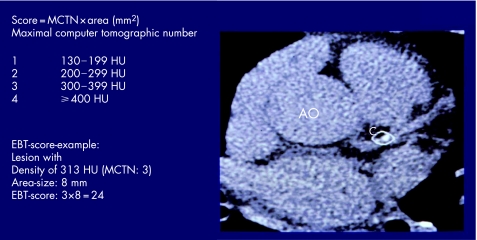
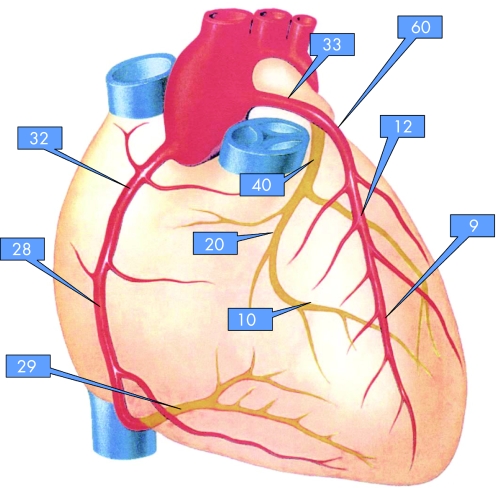
The CAC score can be determined using the methods of Agatston and colleagues13 as well as the CAC volume score.w19 The area of CAC is defined as at least four contiguous pixels (512×512 matrix and a 26 cm field of view) with a CT density ⩾130 Hounsfield Units (HU) (fig 1). This threshold has been shown to provide sufficient differentiation from surrounding tissue and blood, and has been widely accepted as signifying calcification.12w16 All images are analysed separately where areas of CAC are present in ⩾4 pixels. The area size is multiplied by a factor of 1–4 derived from the peak intensity of this focus (1 = 130–199 HU, 2 = 200–299 HU, 3 = 300–399 HU, 4 = ⩾400 HU.13 The total CAC score is computed, comprising all calcified lesions in the epicardial coronary system (fig 1).
Figure 1 Principle of calculating the Agatston score. Electron beam computed tomography (EBCT) of the base of the heart with an image of a single coronary calcification with a density of 313, which is scored as 3 according to Agatston, and a pixel area of 8 mm2. Multiplication gives an Agatston score of 24.
In order to improve the accuracy of CAC determination, the calcium mass score has been proposed.12w19 The mass score integrates the signals of pixels for a given threshold and represents the total mineral content.12 This value is independent of slice thickness and spatial resolution of the systems. This parameter has, however, not yet been validated regarding clinical end points.12
The coronary artery tree is subdivided according to the American Heart Association classification into 16 segmentsw21 for which the CAC score is calculated and summed for each coronary artery and for the whole system (“total Agatston score”).13
Regarding the reproducibility of the Agatston score, inter‐reader variability measures about 3%, intra‐reader variability <1%, and inter‐scan variability approximately 15%.12w22 In a group of >500 subjects, the κ value regarding inter‐reader variability was 0.94, suggesting minimal variability.w23 Recently, a mean difference of 20.1% for the Agatston score and 18.3% for the volume score was found.w24 The absolute difference between two scans was 15.8. For an Agatston score of 100, confidence limits measure 77 and 123,w24 while others found 59 and 149.w25 Non‐linear limits help to define the confidence limits dependent on the Agatston score.w26
The resulting CAC score can be classified into five groups:
zero, no coronary calcification
<100, mild coronary calcification
⩾100–399, moderate calcification
⩾400–999, severe calcification
⩾1000, extensive calcification.
In addition to these categories of the CAC score, it needs to be considered that coronary artery calcification is age dependent, as studies comparing pathological–anatomic findings have demonstrated.w15 Intimal thickening of coronary arteries starts with fatty streaks, which can develop into intermediate lesions. At this stage of pre‐atheroma, calcification can be detected, first intracellularly and then extracellularly.w27 In one study, advanced plaque formation with atheroma and fibroatheroma was observed in 20% of young adults, rising to 60% at the age of 30–34 years.w27 In terms of plaque composition, calcification comprises 10–20% of total plaque volume.w28 w29 This percentage, however, is quite variable. In summary, CAC is not part of an end stage process, but is found early in life at the beginning of the development of coronary arteriosclerosis.
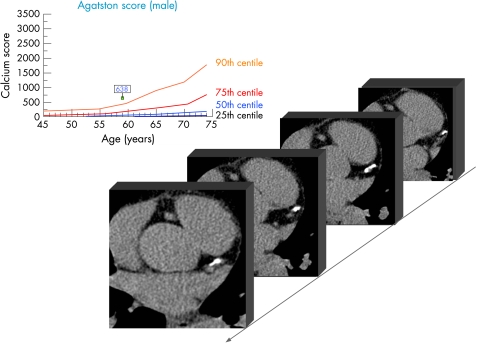
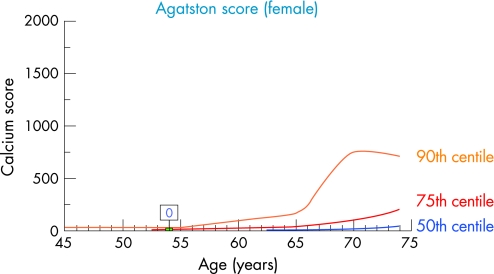
Based on these considerations, CAC quantification (fig 2) also needs to take into account the patient's age and gender. There has to be a comparison of the individual data to a cohort, demonstrating the “normal” age dependent CAC distribution. This helps to explain if an individual CAC result is below, equal, or above the “normal” range. This can be achieved by using centile distribution of CAC (fig 3).14 The 25th, 50th, 75th, and 90th centile are illustrated, showing a continuous increase with age which is strongly gender dependent. In women, CAC develops 10–15 years later in life than in men, and the amount is 5–7 times lower at any given age (fig 4).14 The typical additional information which is provided can be demonstrated in a couple who came for a check‐up. The clinical characteristics are illustrated in table 2 which shows the risk assessment based on different algorithms.4,6w9 The difference between both in relation to family history and cholesterol values is obvious. The 10 year risk is regarded as low and only for the husband as intermediate. After CAC scanning the risk of the husband has to be reclassified as high, with an Agatston score of 638 being above the 90th centile (fig 3), whereas the woman has no CAC despite a cholesterol concentration of 362 mg/dl (9.4 mmol/l).
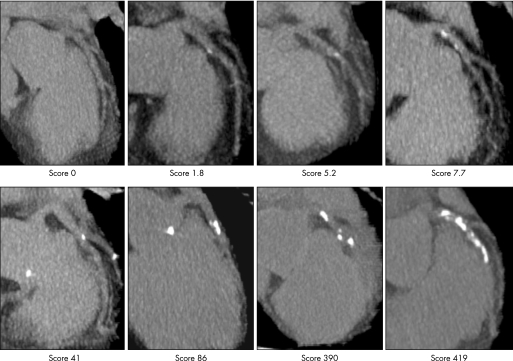
Figure 2 Different degrees of coronary artery calcification imaged by electron beam computed tomography of the left anterior descending coronary artery. The Agatston score is given which was calculated according to the information shown in fig 1, summing up all lesions.
Figure 3 Electron beam computed tomographic images at different scan planes illustrating extended coronary calcification (Agatston score 638) in a man who, since his youth, was an active sportsman with the risk factors shown in table 2. Centile distribution (25th, 50th, 75th, 90th centile) of men between 45–75 years based on the results of the Heinz Nixdorf Recall study are illustrated.14 The resulting coronary artery calcium score was beyond the 90th centile of age adjusted male subjects.
Figure 4 Electron beam computed tomography with 25th, 50th, 75th, 90th centile distribution of coronary artery calcification in women between 45–75 years based on the results of the Heinz Nixdorf Recall study.14 The wife of the husband (table 2) had no coronary artery calcification despite a cholesterol concentration of 362 mg/dl (9.4 mmol/l) and was regarded as below the 25th centile distribution of an age adjusted female cohort.14
Table 2 Clinical characteristics and risk factors of a sporting couple, who were active in sport since their youth, presenting for a check up examination after a son died suddenly at the age of 22 years preparing for an athletic competition.
| Husband | Wife | |
|---|---|---|
| Age (years) | 60 | 54 |
| Height (cm) | 171 | 163 |
| Weight (kg) | 69 | 56 |
| Total cholesterol (mg/dl) | 206 (5.3 mmol/l) | 362 (9.4 mmol/l) |
| LDL cholesterol (mg/dl) | 111 (2.9 mmol/l) | 206 (5.3 mmol/l) |
| HDL cholesterol (mg/dl) | 60 (1.6 mmol/l) | 103 (2.7 mmol/l) |
| Fasting triglyceride (mg/dl) | 77 (0.87 mmol/l) | 79 (0.89 mmol/l) |
| Family history | Yes | No |
| Diabetes mellitus | No | No |
| Systolic blood pressure (mm Hg) | 162 | 117 |
| Smoking | No | No |
| NCEP ATP III (%) | 13 | 1 |
| PROCAM score (%) | 7 | 3 |
| ESC SCORE for Germany (%) | 5 | 1 |
| Agatston score | 638 | 0 |
| Centile distribution | >90th | <25th |
ESC, European Society of Cardiology; NCEP ATP III, Third National Cholesterol Education Program Adult Treatment Program; PROCAM, Prospective Cardiovascular Münster; SCORE, Systemic Coronary Risk Evaluation.
For the Framingham, PROCAM and ESC SCORE the 10 year risk for hard events is listed.
CAC centile values had previously been based on selected groups of individuals and patients, who were recruited by the physician, press media and other forms of advertising, but could include thousands of patients.w30–34 Only recently, the centile distribution of two independent population based cohorts were published: the Multi‐Ethnic Study on Atherosclerosis (MESA) funded by the National Institute of Health in the USA,15w35 and the Heinz Nixdorf Recall (HNR) study funded by the Heinz Nixdorf Foundation, Germany.14w36 Both studies started in 2000 and published their design in 2002. The centile distribution have also been published recently.14,15w35 w36 The HNR study showed that using previous non‐population based analysis would result in an underestimation of the centile rank of CAC in individual patients.14w31–34
The results of the MESA and HNR studies could be compared due to very similar definitions and measurements.8,14 The centile distributions were very similar. However, the curves are shifted to the left and upwards in men and also for women, starting at the age of 65 years.8 This may be related to the higher prevalence of risk factors in Germany compared to the USA: smoking (24% vs 12%), blood pressure (mean (SD) 133 (20)/81 (10) mm Hg vs 124 (20)/70 (10) mm Hg), total cholesterol (mean (SD) 231 (41) mg/dl vs 196 (35) mg/dl (6.0 (1.06) mmol/l vs 5.1 (0.90) mmol/l)), and use of lipid lowering drugs (9.6% vs 18%), despite a lower age of recruited individuals (45–74 years in HNR and 45–85 years in MESA).w37
MESA demonstrates that for the assessment of the degree of CAC, national and ethnic influences have to be taken into account. The highest prevalence of CAC was found in whites, followed by Hispanic, black and the Chinese groups.8 In order to allow the use of these new findings, both MESA and HNR provide a calculator for comparing an individual's CAC score with the centile CAC distribution in the respective population via the internet: <www.mesa‐nhlbi.org> <www.recall‐studie.uni‐essen.de>.
To date, almost all prognostic studies have used EBCT. Therefore, the question arose as to how to handle data which were received from the new MDCT systems. The first comparison was performed with a four slice system.w38 In a cohort of 2030 patients, the 50th, 75th and 90th centile distribution was nearly identical when similar algorithms were used.w48 The variability was reported to be between 17–32%.12w39–41 In MESA, three EBCTs and three MDCTs from different companies were used. The investigators calibrated the Agatston score to an external standard.12w42 The influence of motion artefacts is higher (18.2% vs 11.8%), but noise is lower for MDCTs compared to EBCTs (2.1% vs 11.5%). The absolute difference between two MDCT scans was 16.9.w24 Although the Agatston score cannot be transferred from EBCT to MDCT in an identical fashion, the MDCT “Agatston algorithm” seems to yield very similar results; however, the term “Agatston score” cannot be used.
What does the degree of CAC mean?
There is no correlation between CAC and the development of symptoms, because CAC is a disease of the vessel wall.w28 w29 Particularly in the early stages of coronary atherosclerosis, vascular remodelling compensates for intimal thickening.w43 w44 However, acute coronary syndromes are related to plaque rupture and erosion and not to continuous vessel lumen loss by the atherosclerotic process.w45 w46 Interestingly, newer studies suggest that CAC is a consequence of intramural bleeding during development of vulnerable plaques.w47 w48
The CAC score can range from 0 to >10 000. There is no “normal” Gaussian distribution. Depending on the age range, most subjects have scores <100, and only a few scores are >1000. However, even in asymptomatic subjects, CAC scores >1000 may be found. In a typical cohort of coronary artery disease patients, the median of the Agatston score is 975 for men and 370 for women, and the highest values are found in patients with chronic renal disease.w49
The degree of CAC is related to the risk of cardiovascular events, as studies in large cohorts—whose subjects were, however, largely physician or self referred—have demonstrated.12,16w32 w50–56 Relative risk ratios for high versus low CAC scores ranged between 1.1–26.5 and were generally higher for men than for women.11 In six recent reports,10w52–56 the summary relative risk ratio was 4.3 with a confidence limit between 3.1 and 5.2, comparing events in the high risk (any CAC) with the low risk (most often no CAC) group.11 It was concluded that the risk associated with any degree of CAC compared to no CAC is increased by a factor of 4 over the next 3–5 years.
The summary relative risk ratio was related to the degree of CAC (comparison of the listed groups to the low risk group)11:
CAC 1–100: 1.9 (95% confidence limits (CL) 1.3 to 2.8)*
CAC 100–400: 4.3 (95% CL 3.1 to 6.1)
CAC 400–999: 7.2 (95% CL 5.2 to 9.9)
CAC >1000: 10.8 (95% CL 4.2 to 27.7).
*modified 1–100 instead of 1–112.11
This means that the rate of CVD death and myocardial infarction reaches 4.6% and 7.1% in the two high risk groups, respectively, for the next 3–5 years.11 Interestingly, in the group with extended CAC (>1000) the prognosis was better in symptomatic than asymptomatic cohorts, possibly because in the latter group treatment had not yet been started.17 w57
Ongoing studies such as MESA and HNR will address the risk prediction in a prospective, population based approach with a more accurate determination of cardiovascular risk factors.
Risk stratification based on CAC scoring
In the intermediate (moderate high) risk group, the result of CAC scoring can be used for confirmation or reclassification of the individual risk which has been derived from the first step of risk assessment.5,6,9w58 If the result of the CAC score is zero, a low risk is confirmed, and healthy lifestyle habits recommended. A reassessment after 5 years is suggested.w59 In those reclassified to a high risk group based on CAC scoring, lifestyle changes and drug treatment according to standard guidelines for primary prevention are advised.
Because signs of subclinical atherosclerosis have been detected, this may be regarded as secondary prevention. However, because secondary prevention usually depends on clinical manifestations of heart disease, this treatment concept will need to be further evaluated. In this respect, it is of interest that in a prospective study, Arad et al observed an event rate of >2% per year in patients who appeared to have an intermediate risk on the basis of the Framingham risk score, but who had a CAC score >400.10
Finally, in the group with CAC scores in the intermediate range, an intermediate risk is indeed confirmed.w58
In order to select patients for intensified treatment (exceeding the lifestyle changes), the NCEP ATP III recommends using the 75th CAC centile value based on established age and sex dependent CAC distribution rather than a specific CAC cut off value.6 In the HNR study, the combination of both parameters was used to determine high risk: CAC >100 and/or a level >75th centile.9 This might help to identify young people and women at high risk who otherwise would be disregarded.9
What does a zero CAC score mean?
Depending on age and sex, the percentage of individuals with zero CAC scoring varies. Zero CAC means that no calcification is detectable, but non‐calcified plaques may already be formed. The accuracy of CT is so high that even single spots of CAC can be detected, as comparisons between EBCTw60 w61 and MDCTw61 with intracoronary ultrasound have demonstrated. Only microcalcification may be missed. In the population screened in the HNR study, 327 (6.8%) of 4814 individuals had known coronary artery disease, defined as non‐fatal myocardial infarction or coronary revascularisation procedures. All male subjects with coronary artery disease and 92.8% of the female cohort had signs of CAC.9 These data confirm recent studies in patients with acute coronary syndromes, because all presented with CAC except in rare pathological situations such as myocardial infarction in patients with myocardial bridging.w61 In addition modern technology with contrast MDCT even allows the detection of non‐calcified lesions.w62 The negative predictive value reaches 95–99%. A significant luminal narrowing is unlikely when no CAC is found. The risk for CHD death or non‐fatal myocardial infarction is only 0.1% within the next 2–5 years.11
A zero CAC implies a low risk, but this should not deter patients from making an effort to modify risk factors, such as increasing physical activities or consuming healthier food.3,5,6 It has to be taken into account that during life, CAC may develop as a function of prolonged risk factor exposure. Vice versa, data are currently insufficient to withhold treatment if a high cardiovascular risk is identified on the basis of prevalent risk factors such as diabetes, even if the CAC score is zero.10,11
What does CAC localisation mean?
During follow up the first signs of CAC appear in the proximal part of the left anterior descending coronary artery (fig 4), which is typical and reflects the natural history of coronary artery disease, which has been described previously.w62–64 Coronary angiographyw65 and now EBCT16 confirmed these observations. In the majority of patients, CAC was first found to appear in the proximal part of the left coronary artery, followed by the right and circumflex coronary artery, then involving more distal parts and the main stem.16
The natural history determines the presence and location of CAC (fig 5). However, all attempts to relate CAC scoring to the presence of coronary artery disease or coronary luminal narrowing assessed during coronary angiography have failed to show clinically relevant associations. Importantly, CAC scanning is not recommended to establish the presence of obstructive coronary disease.11 Even in the presence of extensive calcification, there may be no luminal narrowing due to the compensatory remodelling process of the arterial wall.
Figure 5 Distribution of calcium in 269 patients with calcific deposits in the major coronary arteries. Numbers indicate the percentage of calcification in different coronary segments detected by electron beam computed tomography.16
Should CAC scoring be repeated?
The physician will be confronted with the question of whether the study should be repeated and when, independently from the results initially obtained.
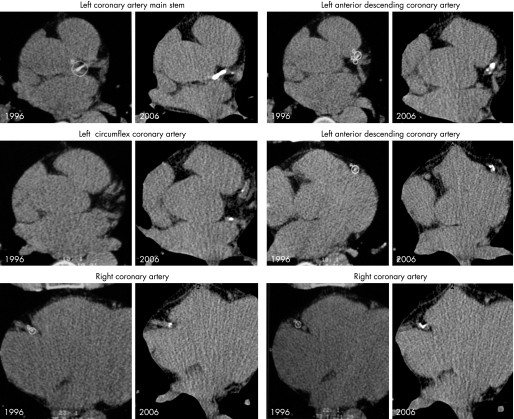
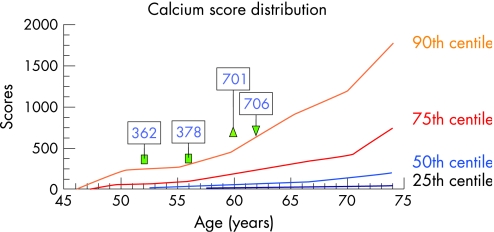
A typical example is shown in fig 6 of a man who was followed over 10 years. Table 3 lists his clinical characteristics and shows that lifestyle changes and statin treatment effectively lowered the low density lipoprotein (LDL) cholesterol values and even lipoprotein Lp(a). His CAC score increased according to the centile distribution (fig 7). The natural history (increase of the Agatston score) followed the population based centile distribution of CAC corrected for age and sex. This result confirms the value of the established CAC centile distribution.
Figure 6 Continuous progression of coronary calcification in a man who came for a check up examination in 1996 (table 3 lists the subject's clinical characteristics) in whom a follow up over 10 years by electron beam tomography was possible, illustrating an increase in the density and calcification of the coronary arteries.
Table 3 Clinical characteristic of a 53‐year‐old man who underwent a first check up examination in 1996.
| 1996 | 1999 | 2004 | 2006 | |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 251 (6.5 mmol/l) | 143 (3.7 mmol/l) | 149 (3.9 mmol/l) | 121 (3.1 mmol/l) |
| HDL cholesterol (mg/dl) | 64 (1.7 mmol/l) | 43 (1.1 mmol/l) | 62 (1.6 mmol/l) | 50 (1.3 mmol/l) |
| LDL cholesterol (mg/dl) | 176 (4.6 mmol/l) | 72 (1.9 mmol/l) | 104 (2.7 mmol/l) | 45 (1.2 mmol/l) |
| Triglycerides (mg/dl) | 61 (0.69 mmol/l) | 73 (0.82 mmol/l) | 74 (0.84 mmol/l) | 72 (0.81 mmol/l) |
| Lipoprotein Lp(a) (mg/dl) | 21.5 (0.77 mmol/l) | 18 (0.64 mmol/l) | 18 (0.64 mmol/l) | 16 (0.57 mmol/l) |
| PROCAM score | 4.8% | 4.2% | 5.1% | 4.8% |
| NCEP ATP III score | 8% | 8% | 8% | 8% |
| ESC SCORE for Germany | 2% | 2% | 3% | 4% |
| Agatston score | 362 | 378 | 701 | 706 |
ESC, European Society of Cardiology; HDL, high density lipoprotein; LDL, low density lipoprotein; NCEP ATP III, Third National Cholesterol Education Program Adult Treatment Program; PROCAM, Prospective Cardiovascular Münster; SCORE, Systemic Coronary Risk Evaluation.
The subject was seen many times over the next 10 years. He was 189 cm in height and his body weight was 87 kg in 1996 and 85 kg in 2006. A progression of coronary artery calcification was seen despite starting statin treatment in 1998 and also receiving ezetimibe since 2004. The CAC score followed the line of the centile distribution which was based on the results of the Heinz Nixdorf Recall study.14
Figure 7 Continuous progression of coronary artery calcification along the 90th centile distribution (shown in fig 6), based on the analysis of the population based Heinz Nixdorf Recall study,14 without any attenuation despite lowering of total cholesterol and low density lipoprotein cholesterol using statin treatment and more recently additionally ezetimibe. No event occurred during the 10 year follow up time.
Follow up studies have been performed, and the rate of progression of CAC determined. In a group with a mean age of 59 years, the mean annual relative progression of calcification was 51% for the total CAC score, and the median progression was 32%.w66 Other authors found a mean annual increase in the range of 24–33%.w67–71 In men and women the smallest statistically significant interval change is ± (4.930× square root of baseline Agatston score) or ± (3.445× square root of baseline volumetric CAC score).w25 Based on the analysis of accuracy and reproducibility, others defined a limit of 15% or ⩾2.5 mm2 of the square root from the starting level in order to differentiate a random from a non‐random change. For example, at a zero level this limit is calculated as ⩾6.25 ( = 2.52) or at 100 CAC score 156.25 (12.52).w26
During follow up, the changes of CAC did not differ within the coronary tree, but were related to the typical predilection site of coronary atherosclerosis in the proximal left coronary segments.16w59–62 Progression was evenly distributed in the right coronary artery, whereas in the left coronary artery it was mainly related to the proximal part of the left anterior descending and circumflex coronary artery.w66
The following percentage CAC progression was found per year at the level of the 75th centile:
62% proximal left anterior descending coronary artery (LAD)
31% proximal right coronary artery (RCA)
31% left main stem
25% left circumflex coronary artery
21% mid RCA
19% distal RCA
14% mid LAD.
Absolute and relative changes of CAC are very different and strongly dependent on the baseline values. Median increase and annual percentage change of CACw66:
+ 3.1 for Agatston score 1–30, +57%
+26.1 for Agatston score >30–100, +49%
+58.9 for Agatston score >100–400, +32%
+109.7 for Agatston score >400, +15%.
Is there any effect of treatment on CAC scores?
Rhesus monkeys received a cholesterol‐rich diet that induced progression of overall coronary plaque burden and CAC. After a change of diet that resulted in an overall regression of coronary plaques, the area of CAC stabilised. Thus, CAC progression was stopped following a reduction of cholesterol ingestion. At the same time, the percentage plaque area that comprised CAC increased in relation to the other plaque components because there was an overall regression of coronary plaque burden.w72
An early report seemed to be able to relate the value of LDL cholesterol to CAC progression.w73 The progression was 52% in an untreated group with an LDL of 147 mg/dl (3.8 mmol/l), 25% in a treated group with LDL cholesterol values of 139 mg/dl (3.6 mmol/l), and regression of 7% was observed in those with LDL cholesterol below 120 mg/dl (3.1 mmol/l).w73 Also an open, non‐controlled study with the cholesterol lowering drug cerivastatin appeared to demonstrate that statins are able to attenuate CAC progression or even induce a regression.w74 However, four randomised, double blind, controlled studies were unable to show any effect of this form of treatment on CAC progression using atorvastatin in comparison to placebo,18w75 simvastatin in comparison to pravastatinw76 and atorvastatin in low and high dosages,19 despite a study duration of up to 4.3 years.w75
Comparing high (80 mg) and low (10 mg) dosages of atorvastatin, progression was 27% and 25% within 12 months.19 In the St Francis Heart study, the progression over 4.3 years was 81% in the atorvastatin (20 mg) group and 73% in the control group receiving only aspirin.w75 With pravastatin (40 mg) and atorvastatin (80 mg), a progression of 15.1% and 14.3%, respectively, within 1 year was observed.w76 Finally, in another study, a CAC score progression of 26% in the (80 mg) atorvastatin group and 18% in the placebo group was observed within 1 year.18
Accordingly, we have not found a way to stop the progression of CAC. Statins alone are not able to attenuate CAC progression. Research needs to investigate different ways to influence this process. Of note, however, vascular risk during statin treatment is decreased by 30–35%.w77
Is there any prognostic implication of CAC progression?
Progression of CAC seems to be an independent prognostic marker. In subjects with an increase in CAC progression above 15% per year, a higher rate of myocardial infarction was found.20 In the St Francis Heart Study, CAC score was determined again after 2 years.10 Those individuals with cardiovascular events had a higher CAC progression than those without. CAC progression was significantly related to outcome, as well as age, gender, LDL and HDL cholesterol values. However, the number of reports is small, and only a few patients were included in these studies. Also, only highly selected subjects have been scanned sequentially. The MESA and HNR studies will be able to address this topic in a more definitive manner.w32 w78
Repetitive CAC scanning can be performed with high accuracy and reproducibility, but currently no intervention has been identified which may be able to attenuate or even stop CAC progression.11 It seems to be similar to the ageing process. The vascular ageing represented by the CAC is a continuum, and those individuals with the greatest progression have the greatest risk.
Additional references appear on the Heart website— http://heart.bmj.com/supplemental.
INTERACTIVE MULTIPLE CHOICE QUESTIONS
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs).
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
Copyright © 2007 BMJ Publishing Group and British Cardiovascular Society.
Supplementary Material
Acknowledgements
I thank Dr Björn Plicht and Dr Nico Reinsch for the assistance in preparing the figures and tables.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
References
- 1.Löwel H, Meisinger C, Heier M.et al Herzinfarkt und koronare Sterblichkeit in Süddeutschland – Ergebnisse des bevölkerungsbasierten MONICA/KORA Herzinfarktregisters 1991 bis 1993 und 2001 bis 2003. Dtsch Ärzteblatt 2006103A616–A622.Follow up data in Germany demonstrate a constant decrease of cardiac mortality, but still 60% of all acute myocardial infarction related deaths occur out of the hospital. [Google Scholar]
- 2.Huikuri H V, Castellanos A, Myerburg R J. Sudden death due to cardiac arrhythmias. N Engl J Med 20013451473–1482.The absolute number of sudden deaths in the general population is many times higher than the number of deaths in those who are identified as high risk patients due to recent acute myocardial infarction, malignant arrhythmias, survived resuscitation and other risk factors. [DOI] [PubMed] [Google Scholar]
- 3.Wilson P W, Castelli W P, Kannel W B. Coronary risk prediction in adults (the Framingham Heart Study). Am J Cardiol 19875991G–4G. [DOI] [PubMed] [Google Scholar]
- 4.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10‐year follow‐up of the prospective cardiovascular Munster (PROCAM) study [erratum in Circulation 2002;105:900]. Circulation 2002105310–315. [DOI] [PubMed] [Google Scholar]
- 5.De Backer G, Ambrosioni E, Borch‐Johnsen K.et al Executive Summary. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003241601–1610. [DOI] [PubMed] [Google Scholar]
- 6.National Cholesterol Education Program Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 20012852486–2497. [DOI] [PubMed] [Google Scholar]
- 7.Grundy S M, Cleeman J I, Merz C N.et al Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004110227–239. [DOI] [PubMed] [Google Scholar]
- 8.McClelland R L, Chung H, Detrano R.et al Distribution of coronary artery calcium by race, gender, and age: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation 200611330–37.Demonstration of the influence of ethnicity on the degree of coronary artery calcification in whites, Chinese, Hispanic, and blacks. [DOI] [PubMed] [Google Scholar]
- 9.Erbel R, Möhlenkamp S, Lehmann N.et al on behalf of the Heinz Nixdorf Recall Study Investigative Group. Sex related cardiovascular risk stratification based on quantification of atherosclerosis and inflammation. Atherosclerosis 2007; [Epub ahead of print]Reclassification of individual risk using signs of subclinical atherosclerosis detected by electron beam computed tomography and signs of inflammation measured as C reactive protein. This article shows the value of a combined index of inflammation and atherosclerosis which demonstrated a similar influence in male and female participants of the Heinz Nixdorf Recall study. [DOI] [PubMed]
- 10.Arad Y, Goodman K J, Roth M.et al Coronary calcification, coronary disease risk factors; C‐reactive protein, and atherosclerosis cardiovascular disease events. The St. Francis Heart study. J Am Coll Cardiol 200546158–165.Reclassification of cardiovascular risk based on coronary artery calcification improves risk prediction in those with intermediate risk, but not with low and high risk. [DOI] [PubMed] [Google Scholar]
- 11.Greenland P, Bonow R O, Brundage B H.et al ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 200749378–402. [DOI] [PubMed] [Google Scholar]
- 12.Budoff M J, Achenbach S, Blumenthal R S.et al Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 20061141761–1791. [DOI] [PubMed] [Google Scholar]
- 13.Agatston A S, Janowitz W R, Hildner F J.et al Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 199015827–832.Key reference for the algorithm used for calculation of coronary artery quantification. Most widely used and validated parameter, which has to be age and sex adjusted using population based data of centile distribution of coronary artery calcification. [DOI] [PubMed] [Google Scholar]
- 14.Schmermund A, Möhlenkamp S, Berenbein S.et al Population‐based assessment of subclinical coronary atherosclerosis using electron beam computed tomography. Atherosclerosis 2006185177–182.Publication of the centile distribution of coronary artery calcification adjusted to age and sex in the Heinz Nixdorf Recall study, which can be used for risk assessment in men and women. [DOI] [PubMed] [Google Scholar]
- 15.Bild D E, Detrano R, Peterson D.et al Ethnic differences in coronary calcification: the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation 20051111313–1320.Publication of the centile distribution of coronary artery calcification adjusted to age and sex in the Multi‐Ethnic Study on Atherosclerosis, which can be used for risk assessment in men and women. [DOI] [PubMed] [Google Scholar]
- 16.Schmermund A, Mohlenkamp S, Baumgart D.et al Usefulness of topography of coronary calcium by electron‐beam computed tomography in predicting the natural history of coronary atherosclerosis. Am J Cardiol 200086127–132.Natural history of coronary artery atherosclerosis demonstrating that calcification is first seen in most individuals in the proximal segment of the left anterior descending coronary artery. [DOI] [PubMed] [Google Scholar]
- 17.Möhlenkamp S, Lehmann N, Schmermund A.et al Prognostic value of extensive coronary calcium quantities in symptomatic males – a 5‐year follow‐up study. Eur Heart J 200324845–854. [DOI] [PubMed] [Google Scholar]
- 18.Houslay E S, Cowell S J, Prescott R J.et al on behalf of the Scottish Aortic Stenosis and Lipid Lowering Therapy, Impact on Regression (SALTIRE) Trial Investigators. Progressive coronary calcification despite intensive lipid‐lowering treatment: a randomised controlled trial. Heart 2006921207–1212.Randomised controlled double blind study demonstrating that treatment with atorvastatin did not attenuate progression of coronary artery calcification over a period of 24 months. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmermund A, Achenbach S, Budde T.et al Effect of intensive versus standard lipid‐lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double‐blind trial. Circulation 2006113427–437.Randomised controlled double blind study demonstrating that high dose (80 mg) atorvastatin treatment did not attenuate progression of coronary artery calcification compared to low dose treatment (10 mg) over a 12 month period. [DOI] [PubMed] [Google Scholar]
- 20.Raggi P, Callister T Q, Shaw L I. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol‐lowering therapy. Arterioscler Thromb Vasc Biol 2004241272–1277.In postmenopausal women, intensive statin treatment for 1 year caused a greater LDL reduction than moderate treatment, but did not result in less progression of coronary calcification. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.