Abstract
Objective
To compare bleeding complications and results of percutaneous coronary intervention (PCI) between patients treated by radial and femoral approaches for acute myocardial infarction (AMI,) and using abciximab and 5 French guiding‐catheters.
Patients
114 consecutive patients with AMI were prospectively randomised. Exclusion criteria were a history of coronary artery bypass graft, cardiogenic shock, atrioventricular block, and contraindication to abciximab or a negative Allen test. Local haemostasis was achieved by manual compression.
Results
Baseline characteristics were similar between the two groups. Peripheral arterial complication rates and delays to patient ambulation were significantly lower in the radial group than in the femoral group, whereas in‐hospital stay was similar between the two groups. A cross over was more often necessary in the radial group than in the femoral group. Coronary angiography duration and fluoroscopy time were significantly longer in the radial group than in the femoral group, whereas PCI duration was similar in both groups.
Conclusions
The FARMI trial showed that the radial route lowered peripheral arterial complication rates and allowed earlier ambulation, despite no significant benefit on the duration of hospitalisation.
Keywords: GpIIb/IIIa antagonist, percutaneous coronary intervention, acute myocardial infarction, radial
Percutaneous coronary intervention (PCI) is accepted as the optimal strategy to recanalise culprit coronary arteries in acute myocardial infarction (AMI).1 Acute and long‐term results of PCI are optimised both by stents and by an aggressive medical regimen (ie, antiplatelets such as abciximab, aspirin and thienopyridines).2,3 As PCI is routinely performed through the femoral approach, this medical regimen increases the risk of peripheral arterial complications due to access site trauma and delayed healing, associated morbidity and/or mortality rates, and subsequently prolongs bed rest duration. Recent progress in developing alternative PCI techniques to lower arterial access complication rates, and the resultant duration of bed rest include the miniaturisation of guiding‐catheters in combination with alternative interventional access through the radial artery. Five French guiding‐catheters limit vascular damage with respect to direct stenting strategies, despite reduced coronary artery opacification and back‐up support.4,5 The trans‐radial approach is a safe alternative to femoral access, virtually eliminating local peripheral arterial complications but requiring greater technical skills and, consequently, has lower procedural success rates.6,7,8 Moreover, the radial route precludes the otherwise routine use of the larger guiding‐catheters (ie, 8 French or more) and specific ancillary devices such as a temporary pacemaker or intra‐aortic balloon pump. As far as we know a randomised comparison of the femoral and radial approaches to PCI using abciximab for AMI has never been made.
The FARMI (Five French Arterial access with Reopro in Myocardial Infarction) prospective and randomised trial was therefore designed to compare the radial and femoral approaches to PCI using abciximab and 5 French guiding‐catheters, in AMI.
Patients and methods
Patients
All patients in the FARMI trial were consecutively and prospectively enrolled between January 2004 and September 2005. Inclusion criteria were defined by acute coronary syndrome with ST segment elevation associated with sustained chest pain. Patients were recruited either from the emergency unit of our institution, mobile intensive care units, or were referred from other hospitals to our institution for emergent PCI. Emergent PCIs were defined as primary, rescue or facilitated PCI as follows: primary PCI was defined as mechanical coronary recanalisation without previous thrombolysis or pretreatment by glycoprotein IIb/IIIa (GpIIb/IIIa) inhibitors; rescue PCI was defined as mechanical coronary recanalisation when thrombolytic treatment had failed; facilitated PCI was defined as mechanical coronary recanalisation after successful thrombolytic treatment, assessed by clinical and electrocardiographic criteria.
All the patients underwent coronary angiography and ad hoc PCI. Patients were therefore randomised before the catheterisation procedure. Procedures were performed by five interventional cardiologists routinely using both arterial routes and 5 French guiding‐catheters through these routes (ie, each operator was required to have undertaken more than 100 previous successful transradial coronary procedures before being allowed to participate in the trial). All patients underwent an Allen test and pulse oximetry analysis to assess dual palmar arch supply before randomisation.
Exclusion criteria were haemodynamic instability (ie, Killip state >2 or cardiogenic shock), the need for an intra‐aortic balloon pump or temporary pacemaker, a history of a coronary artery bypass graft (CABG) or intolerance to abciximab. The study complied with the declaration of Helsinki, and informed written consent was obtained from each patient.
Percutaneous coronary interventions
Radial artery cannulation was performed as previously described.9 To summarise, the right radial puncture was performed under local anaesthesia (subcutaneous 1 ml of 2% lidocaine) using a 20‐gauge needle and a 0.022 inch hydrophilic guide‐wire. Short 5 French sheaths (Terumo, Japan) were used, and a solution of unfractionated heparin (3000 IU bolus) and 2 mg verapamil was injected intra‐arterially. Coronary angiograms were performed using conventional 5 French diagnosis catheters (ie, Judkins or Amplatz curve catheters).
Femoral arteries were cannulated using a 17‐gauge needle and a 0.035 inch guide‐wire. Conventional 5 French sheaths (Terumo, Japan) were used. Coronary angiograms were performed using mainly 5 French Judkins curves diagnosis catheters.
PCIs were performed using 5 French guiding‐catheters (Launcher or Zuma; Medtronic Vascular, Santa Rosa, CA, USA), immediately after the coronary angiographies. If necessary, adjunctive heparinisation before PCI was obtained by complementary intravenous unfractionated heparin bolus to reach 50 IU/kg, except in patients treated by continuous infusion of unfractionated heparin or those with preliminary thrombolytic treatment. According to the practices in our country, activated clotting time was not assessed during PCI. PCIs were achieved using rapid‐exchange devices, balloon catheters and bare metal stents.
Anticoagulation and antiplatelet treatments
Before PCI, patients were pretreated by either an intravenous bolus of heparin as follows: unfractionated heparin 50 IU/kg with an upper limit of 4000 IU in patients more than 75 years old, or low molecular weight heparin (enoxaparin) 30 mg intravenously and 1 mg/kg subcutaneously in patients aged less than 75 years, and a bolus of aspirin (250 mg intravenously). This protocol was used irrespective of arterial access since it was started by emergency units, mobile intensive care units or hospitals referring patients to our institution for emergent PCI. When complementary PCI was required, abciximab was conventionally given (ie, a 0.25 mg/kg bolus followed by a 0.125 μg/kg/min infusion during 12 hours).10 After completion of PCI, subcutaneous enoxaparin (100 IU/kg) was injected twice a day at the most during the first 72 hours, if necessary. Furthermore, all patients received oral clopidogrel (300 mg), followed by 75 mg daily for 1 year, plus 75–300 mg/day oral aspirin.
Sheath management
In the radial group, the arterial sheath was removed after completion of PCI. Local haemostasis was obtained by radial compression using elastic straps for 3 hours. In the femoral group, the arterial sheath was withdrawn within the 4 hours after PCI. Local haemostasis was achieved by manual compression without arterial closure devices. In both groups, systemic anticoagulation was continued as previously mentioned. Patients were allowed to ambulate 6 hours after radial and 12 hours after femoral sheath removal, unless their clinical status dictated otherwise.
Post‐procedural outcome
This study aimed at assessing peripheral arterial complication rates, and PCI efficiency and tolerance of the procedure using radial arterial access as compared with femoral access. Thrombolysis in myocardial infarction (TIMI) major bleeding involved a haemoglobin drop of >50 g/l or intracranial haemorrhage or cardiac tamponade. TIMI minor bleeding involved a haemoglobin drop >30 g/l but <50 g/l, with bleeding from a known site or spontaneous gross haematuria, haemoptysis or haematemesis. Moreover, groin haematoma and ecchymosis were assessed clinically by doctors other than interventional cardiologists. Groin haematoma was defined as local induration of >4 cm diameter. In contrast, ecchymosis was defined as cutaneous bruise or induration of <4 cm diameter, or both. In cases of haematoma, an ultrasonic‐guided examination was needed to differentiate between haematoma and false aneurysm. Durations of supine bed rest and hospitalisation were also assessed. PCI efficiency was characterised by PCI success rates, fluoroscopy duration and procedural duration, from peripheral arterial puncture to the end of PCI, excluding sheath removal. The number of guiding‐catheters or other devices used during PCI was considered. The volume of contrast medium used during PCI and kidney tolerance (defined by variations in plasma creatinine concentration) were also considered. Deaths, as well as clinical and electrocardiographic evidence of sustained or recurrent ischaemia after PCI, were also assessed.
Angiographic patterns of culprit coronary artery lesions before PCI were classified using the American College of Cardiology/American Heart Association classification.11 TIMI flow grade before and after PCI and TIMI myocardial perfusion grade after PCI were evaluated by visual assessment. Angiographic success after PCI was defined by the combination of a TIMI flow grade 3 and a reduction in the percentage diameter stenosis of <30%.
Statistical analysis
All the data are presented as mean (SD). Differences in categorical variables were analysed by the χ2 or Fisher exact tests, and differences in continuous variables were analysed by the unpaired Student t tests, as required. Pre‐ and post‐procedural variations of biological marker plasma concentrations were assessed using the paired Student t test. The study sample sizes were estimated on the basis of the primary end point (ie, the incidence of peripheral arterial complication rate when considering an expected rate of 1% arterial complication in the radial group and 15% arterial complication in the femoral group). When a one‐sided test with a power of 90% was used, the number of patients required to detect a 5% difference between the two groups was calculated as 60 in each. All data were analysed by intention to treat. Statistical analyses were performed using the SAS 8.0 software (SAS institute, Cary, NC, USA). A p value <0.05 was considered significant.
Results
Population
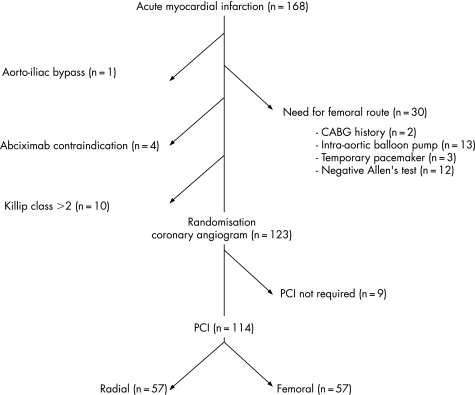
Among 168 patients admitted for emergent PCI because of AMI, 114 fulfilled the inclusion criteria (fig 1). Table 1 summarises the clinical baseline characteristics of the patients.
Figure 1 Study flow chart of the FARMI trial. CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Table 1 Baseline clinical characteristics.
| Characteristics | Radial (n = 57) | Femoral (n = 57) | p Value |
|---|---|---|---|
| Age (years), mean (SD) | 60 (12) | 58 (13) | 0.27 |
| Male, n (%) | 49 (86.0) | 47 (82.5) | 0.80 |
| Cardiovascular risk factors, n (%) | |||
| Diabetes | 12 (21.1) | 9 (15.8) | 0.48 |
| Hypertension | 30 (52.6) | 17 (29.8) | 0.01 |
| Current smoker | 42 (73.7) | 45 (78.9) | 0.66 |
| Hypercholesterolaemia | 28 (49.1) | 22 (38.6) | 0.26 |
| Weight (kg), mean (SD) | 80 (14) | 79 (17) | 0.71 |
| Height (cm), mean (SD) | 170 (7) | 171 (7) | 0.68 |
| Systolic blood pressure (mm Hg), mean (SD) | 123 (21) | 123 (25) | 0.93 |
| Delay: pain onset‐cath‐lab (min), mean (SD) | 409 (305) | 358 (14) | 0.33 |
| LVEF (%), mean (SD) | 46 (9) | 49 (11) | 0.18 |
| Myocardial infarction topography, n (%) | |||
| Anterior | 31 (54.4) | 26 (45.6) | 0.53 |
| Posterior | 23 (40.4) | 27 (47.4) | |
| Lateral | 3 (5.3) | 5 (8.8) |
Cath‐lab, catheterisation laboratory; LVEF, left ventricular ejection fraction.
The mean delay between the onset of symptoms and admission to the catheterisation laboratory was longer than 6 hours. Indication for PCI was similar between groups. Moreover, 14 patients were treated with enoxaparin after thrombolytic treatment, according to the ASSENT‐3 plus trial—that is, eight in the radial group and six in the femoral group.12
Percutaneous coronary interventions
Table 2 summarises the angiographic and procedural characteristics.
Table 2 Angiographic and procedural characteristics.
| Characteristics | Radial (n = 57) | Femoral (n = 57) | p Value | ||
|---|---|---|---|---|---|
| Diffusion of the coronary artery disease | |||||
| One‐vessel disease, n (%) | 27 (47.4) | 30 (52.6) | |||
| Two‐vessel disease, n (%) | 16 (28.1) | 20 (35.1) | 0.22 | ||
| Three‐vessel disease, n (%) | 14 (24.6) | 7 (12.3) | |||
| Target lesion, n (%) | |||||
| LAD | 29 (50.9) | 26 (45.6) | |||
| Left circumflex | 8 (14.0) | 8 (14.0) | 0.83 | ||
| Right coronary | 20 (35.1) | 23 (40.4) | |||
| ACC/AHA type of lesion, n (%) | |||||
| A | 5 (8.8) | 4 (7.0) | |||
| B1 | 23 (40.4) | 27 (47.4) | 0.60 | ||
| B2 | 18 (31.6) | 16 (28.1) | |||
| C | 11 (19.3) | 10 (17.5) | |||
| Target vessel diameter (mm), mean (SD) | 3.06 (0.08) | 3.02 (0.06) | 0.75 | ||
| Pre‐PCI TIMI flow grade, n (%) | |||||
| 0 | 27 (47.4) | 31 (54.4) | |||
| 1 | 5 (8.8) | 5 (8.8) | 0.87 | ||
| 2 | 11 (19.3) | 9 (15.8) | |||
| 3 | 14 (24.6) | 12 (21.1) | |||
| Coronary angiography duration (min), mean (SD) | 17 (8) | 13 (6) | <0.01 | ||
| Contrast medium (ml), mean (SD) | 78 (5.2) | 73 (4.6) | 0.47 | ||
| Cross over, n (%) | 7 (12.3) | 1 (1.8) | 0.03 | ||
| Indication of PCI, n (%) | |||||
| Primary PCI | 26 (45.6) | 32 (56.1) | |||
| Rescue PCI | 28 (49.1) | 20 (35.1) | 0.65 | ||
| Facilitated PCI | 3 (5.3) | 5 (8.8) | |||
| Size of guiding‐catheters, n (%) | |||||
| 5 French | 51 (89.5) | 53 (93.0) | 0.66 | ||
| 6 French | 6 (10.5) | 4 (7.0) | |||
| Guiding‐catheters for PCI, mean (SD) | 1.24 (0.68) | 1.11 (0.42) | 0.25 | ||
| Stents (n), mean (SD) | 1.15 (0.36) | 1.28 (0.61) | 0.22 | ||
| Direct stent implantation, n (%) | 29 (50.9) | 27 (47.4) | 0.87 | ||
| Stent with predilatation, n (%) | 25 (43.9) | 27 (47.4) | |||
| Overall stent length (mm), mean (SD) | 17.5 (0.9) | 20.4 (1.8) | 0.22 | ||
| Stent diameter (mm), mean (SD) | 3.17 (0.05) | 3.18 (0.06) | 0.86 | ||
| Angiographic success of PCI, n (%) | 52 (91.2) | 55 (96.5) | 0.43 | ||
| Duration of PCI (min), mean (SD) | 28 (14) | 26 (18) | 0.72 | ||
| Delay: pain onset to TIMI 3 flow (min), mean (SD) | 450 (46) | 381 (31) | 0.22 | ||
| Contrast medium for PCI (ml), mean (SD) | 97 (57) | 91 (47) | 0.45 | ||
| Overall fluoroscopy duration (min), mean (SD) | 13 (9) | 8 (6) | <0.01 | ||
ACC/AHA, American College of Cardiology/American Heart Association; LAD, left anterior descending coronary artery; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Of the 57 patients assigned to the radial group, 7 (12%) required conversion to femoral access despite primary arterial cannulation success. Most radial approach failures resulted from technical difficulties (ie, inability to selectively catheterise the coronary ostia, anatomical variations of aortic roots, painful brachial artery loops).
Delay between onset of symptoms to TIMI 3 flow was similar between the two groups (450 (46) minutes vs 381 (31) minutes, p = NS, in the radial and femoral groups, respectively) and according to indication of PCI (ie, primary PCI, facilitated PCI and rescue PCI).
The duration of coronary angiography was significantly longer in the radial group than in the femoral group, with a subsequent increase in medium contrast volume consumption. Interestingly, radial approach failures occurred only during the diagnosis phase. Indeed, during PCI via the radial approach, the PCI duration, the success rate, the post‐PCI TIMI flow grade 3 rates, the medium contrast volume consumption, and the number of guiding‐catheters and stents used were similar in the two groups. Moreover, larger guiding‐catheters (ie, 6 French) were required in six patients of the radial group and four patients of the femoral group in order to use specific ancillary devices (ie, clot aspiration devices).
In contrast, a conversion from the femoral to the radial group was required in one case, due to severe aorto‐iliac loops.
Finally, the use of thrombolytic agents, unfractionated heparin and low molecular weight heparin was similar in the two groups.
Post‐procedural outcomes
Table 3 summarises the post‐procedural outcomes.
Table 3 Post‐PCI characteristics.
| Characteristics | Radial (n = 57) | Femoral (n = 57) | p Value |
|---|---|---|---|
| Post‐PCI systolic blood pressure (mm Hg), mean (SD) | 116 (22) | 113 (24) | 0.42 |
| Ischaemic complication due to in‐stent thrombosis, n (%) | 4 (7.0) | 4 (7.0) | NS |
| In‐hospital death, n (%) | 3 (5.3) | 3 (5.3) | NS |
| Delay to ambulation (hours), mean (SD) | 22 (9) | 42 (27) | <0.001 |
| Duration of hospitalisation (days), mean (SD) | 7.2 (0.5) | 7.5 (0.4) | 0.59 |
| TIMI major bleeding, n (%) | 3 (5.3) | 3 (5.3) | NS |
| TIMI minor bleeding, n (%) | 0 (0) | 1 (1.8) | NS |
| Peripheral arterial complications, n (%) | |||
| Haematoma | 2 (3.5) | 11 (19.3) | 0.05 |
| Ecchymosis | 6 (10.5) | 9 (15.8) | NS |
| Transfusion, n (%) | 1 (1.8) | 0 (0) | NS |
| Biological parameters marker | |||
| Creatinine plasma concentration (mmol/l), mean (SD) | |||
| Pre‐procedural | 91 (23) | 81 (17) | 0.12 |
| Post‐procedural | 91.5 (28) | 82 (22.5) | 0.07 |
| Delta | 1.1 (25) | 1.8 (12) | 0.85 |
| Haemoglobin plasma concentration (g/l), mean (SD) | |||
| Pre‐procedural | 1.42 (0.14) | 1.42 (0.11) | 0.76 |
| Post‐procedural | 1.35 (0.13) | 1.34 (0.14) | 0.76 |
| Delta | −0.07 (0.07) | −0.08 (0.1) | 0.57 |
TIMI, thrombolysis in myocardial infarction.
Peripheral arterial complication rates
TIMI major and minor bleeding rates were similar in the two groups. However, most bleeding complications occurred in the femoral group. When the major and minor vascular complications (ie, false aneurism, haematoma and ecchymosis, respectively) are added together, the peripheral arterial complication rate was significantly lower in the radial group than in the femoral group (n = 8 (14%) vs n = 20 (35%), p = 0.014, respectively). Two large haematomas occurred in the radial group. Interestingly, these patients received preliminary thrombolytic treatment and presented with tight humeral artery loops requiring use of a hydrophilic guide‐wire. Although these haematomas were confined to the arm without functional symptoms, one patient required blood transfusion. No vascular surgical repair was necessary.
As expected, the delay between PCI and ambulation was significantly lower in the radial group (22 (9) vs 42 (27) hours, p<0.001, respectively). In contrast, the duration of hospital stay was similar between both groups.
PCI efficiency and tolerance
The major complication rates (ie, out‐of‐lab in‐stent thrombosis and deaths) were similar between the groups (table 3). Two out of six deaths were related to in‐stent thrombosis, two patients died owing to major bleeding (one haemorrhagic stroke and one cardiac tamponade) and two patients died owing to cardiogenic shock (one related to postinfarction ventricular septal rupture despite emergency surgery).
There was no significantly increased acute renal failure in the radial group or increased anaemia in the femoral group.
Discussion
Feasibility and efficiency of the radial route to treat coronary artery lesions have been largely documented in various clinical settings.13,14,15 Most benefits of such an approach were shown in patients requiring non‐emergent PCI (ie, patients with stable angina or acute coronary syndrome without persistent ST segment elevation) allowing earlier hospital discharge.16 This benefit is enhanced patients aged over 80 who are receiving aggressive antithrombin and antiplatelet treatment.17 Moreover, since the radial artery is superficial, its compression is easy, allowing easy and powerful haemostasis. To our knowledge, the present study is the first prospective and randomised trial comparing the radial and the femoral routes for PCI in the setting of AMI using abciximab. Previous non‐randomised trials and registries have suggested the benefit of the radial route for preventing peripheral arterial complications and lowering the duration of hospital stay, as compared with the femoral route.18,19,20,21 Moreover, Saito et al demonstrated the feasibility and safety of the radial route for performing PCI in the setting of AMI, leading to reperfusion rates, in‐hospital major adverse cardiac event rates and long‐term follow‐up, similar to those obtained with the femoral route. Nevertheless, PCIs were performed through 6 French guiding‐catheters without GpIIb/IIIa antagonists, as compared with the present study.22
PCI in AMI: the technical challenge of the radial approach
In this study, durations of PCI and cross‐over incidence rates were significantly higher in the radial group than in the femoral group. Several reasons might account for procedural failure during the radial approach. First, the operator might puncture the radial artery, the skill of the operator is important here, or there might be vessel tortuosity or sustained arterial spasm. Second, failure might be related to an inability to cannulate the coronary ostia because of difficulties in rotating and manipulating the catheter. Finally, PCI might fail because of inadequate guiding‐catheter back‐up support or an inability to track the devices to the correct place. Most of the conversions from the radial to the femoral approach in this study were related to difficulties in rotating and manipulating catheters because of inadequate variations of aortic roots. Nevertheless, these differences concerned only the diagnosis phase and were related to increased consumption of the contrast agent. The radial route is associated with increased technical difficulties in performing coronary angiographies, as compared with the femoral route. In contrast, ad hoc PCIs through the radial route are less difficult since most of the technical challenges are resolved during the diagnosis phase.17 Moreover, since diagnosis through the radial route provides experience with the technical difficulties of PCI, a cross over to the femoral route can be promptly achieved, if required.
The strategy of PCI using 5 French guiding‐catheters was supported by the challenge of lowering peripheral arterial complication rates through the femoral route; moreover, the smaller the sheaths, the lower the radial artery spasm rates. The feasibility of using 5 French guiding‐catheters, as compared with 6 French devices, to perform PCI through the radial route, and the safety of the procedure despite less back‐up support, have already been documented, since the procedure can be optimised by using extra‐support guide wires.23 Indeed, cross over to 6 French guiding‐catheters in this study was mostly related to the use of aspiration catheters (ie, Export, Medtronic Inc, MN, USA) rather than inadequate back‐up support.
Preventing peripheral arterial complications after PCI in AMI
Acute coronary syndromes suggest that powerful antithrombotic and antiplatelet regimens will be used during PCI (including rescue PCI) in addition to coronary stenting (ie, platelet GpIIb/IIIa inhibitors).24 The extent of myocardial damage, risk of reinfarction and subsequent mortality rates are lowered by platelet GpIIb/IIIa inhibitors, mainly abciximab, during PCI.25,26
Surprisingly, the duration of hospital stay was similar in the two groups, despite decreased peripheral arterial complications and earlier ambulation in the radial group than in the femoral group. However, when this study was designed and when patients were included, it was not the policy of our institution to allow early discharge after PCI. Since routine discharge on day 3 is now accepted practice in many centres, the radial approach will allow rapid mobilisation and therefore earlier hospital discharge. Moreover, ambulation was achieved much later than scheduled (22 hours in the radial group instead of the expected 6 hours), which clearly shows that psychological factors and status of the patients with AMI was more significant than the vascular approach used.
The duration of hospital stay depends, therefore, on several factors, of which bleeding is only one. Despite the fact that early hospital discharge (<5 days) after PCI for AMI is now accepted practice in many centres, the medical management of cardiovascular risk factors and prevention of post‐myocardial infarction complications (ie, ventricular arrhythmias and remodelling) justifies such a duration of hospital stay.27
Conclusions
In conclusion, the FARMI trial showed that PCI for AMI has a high success rate using 5 French guiding‐catheters and radial or femoral access; the radial route lowered peripheral arterial complication rates and allowed earlier ambulation, but without subsequent significant reduction in the duration of hospitalisation. Moreover, the radial route significantly increased the length of the procedure and the cross over to femoral access during the diagnosis phase, but with no adverse consequences for the result of the PCI or biological complications.
Acknowledgements
We thank Sandy Hathaway for carefully reviewing this manuscript.
Abbreviations
AMI - acute myocardial infarction
FARMI - Five French Arterial access with Reopro in Myocardial Infarction (trial)
Gp - glycoprotein
PCI - percutaneous coronary intervention
TIMI - thrombolysis in myocardial infarction
Footnotes
Competing interest: None.
References
- 1.Weaver W D, Simes R J, Betriu A.et al Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA 19972782093–2098. [PubMed] [Google Scholar]
- 2.Grines C L, Cox D A, Stone G W, the Stent Primary Angioplasty in Myocardial Infarction Study Group et al Coronary angioplasty with or without stent implantation for acute myocardial infarction. N Engl J Med 19993411949–1956. [DOI] [PubMed] [Google Scholar]
- 3.Tcheng J E, Kandzari D E, Grines C L, CADILLAC investigators et al Benefits and risks of abxicimab use in primary angioplasty for acute myocardial infarction: the Controlled Abxicimab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial. Circulation 20031081316–1323. [DOI] [PubMed] [Google Scholar]
- 4.Brasselet C, Metz D, Perotin S.et al Direct stent implantation without predilatation through 5 French guiding catheter following transfemoral coronary angiogram: a feasibility study. Catheter Cardiovasc Interv 200360354–359. [DOI] [PubMed] [Google Scholar]
- 5.Hamon M, Sabatier R, Zhao Q.et al Mini‐invasive strategy in acute coronary syndromes: direct coronary stenting using 5Fr guiding catheters and transradial approach. Cathet Cardiovasc Interv 200255340–343. [DOI] [PubMed] [Google Scholar]
- 6.Agostoni P, Biondi‐Zoccai G G, de Benedictis M L.et al Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta‐analysis of randomised trials. J Am Coll Cardiol 200444349–356. [DOI] [PubMed] [Google Scholar]
- 7.Cox N, Resnic F S, Popma J J.et al Comparison of the risk of vascular complications associated with femoral and radial access coronary catheterization procedures in obese versus nonobese patients. Am J Cardiol 2004941174–1177. [DOI] [PubMed] [Google Scholar]
- 8.Ludman P F, Stephens N G, Harcombe A.et al Radial versus femoral approach for diagnostic coronary angiography in stable angina pectoris. Am J Cardiol 1997791239–1241. [DOI] [PubMed] [Google Scholar]
- 9.McFadden E, Hamon M. Radial access, compression techniques, and complications in: Trans‐radial approach for radial interventions. ESM editions 2003
- 10.Montalescot G, Andersen H R, Antoniucci D.et al Summary of recommendations on percutaneous coronary intervention for the reperfusion of acute ST elevation myocardial infarction. Heart 200490676–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan T J, Faxon D P, Gunnar R M.et al Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation 198878486–502. [DOI] [PubMed] [Google Scholar]
- 12.Wallentin L, Goldstein P, Armstrong P W.et al Efficacy and safety of tenecteplase in combination with the low‐molecular‐weight heparin enoxaparin or unfractionated heparin in the prehospital setting: the Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)‐3 PLUS randomized trial in acute myocardial infarction. Circulation 2003108135–142. [DOI] [PubMed] [Google Scholar]
- 13.Cantor W J, Puley G, Natarajan M K.et al Radial versus femoral access for emergent percutaneous coronary intervention with adjunct glycoprotein IIb/IIIa inhibition in acute myocardial infarction—the RADIAL‐AMI pilot randomized trial. Am Heart J 2005150543–549. [DOI] [PubMed] [Google Scholar]
- 14.Gobeil F, Bruck M, Louvard Y.et al Comparison of 5 French versus 6 French guiding catheters for transradial coronary intervention: a prospective, randomized study. J Invasive Cardiol 200416353–355. [PubMed] [Google Scholar]
- 15.Lotan C, Hasin Y, Mosseri M.et al Transradial approach for coronary angiography and angioplasty. Am J Cardiol 199576164–167. [DOI] [PubMed] [Google Scholar]
- 16.Mann T, Cubeddu G, Bowen J.et al Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. J Am Coll Cardiol 199832572–576. [DOI] [PubMed] [Google Scholar]
- 17.Louvard Y, Benamer H, Garot P, for the OCTOPLUS study group et al Comparison of transradial and transfemoral approaches for coronary angiography and angioplasty in octogenarians (the OCTOPLUS study). Am J Cardiol 2004941177–1180. [DOI] [PubMed] [Google Scholar]
- 18.Choussat R, Black A, Bossi I.et al Vascular complications and clinical outcome after coronary angioplasty with platelet IIb/IIIa receptor blockade. Comparison of transradial and transfemoral arterial access. Eur Heart J 200021607–609. [DOI] [PubMed] [Google Scholar]
- 19.Louvard Y, Ludwig J, Lefevre T.et al Transradial approach for coronary angioplasty in the setting of acute myocardial infarction: a dual‐center registry. Cathet Cardiovasc Interv 200255206–211. [DOI] [PubMed] [Google Scholar]
- 20.Philippe F, Larrazet F, Meziane T.et al Comparison of transradial versus transfemoral approach of acute myocardial infarction with primary angioplasty and abciximab. Cathet Cardiovasc Interv 20046167–73. [DOI] [PubMed] [Google Scholar]
- 21.Ziakas A, Klinke P, Mildenberger R.et al Comparison of the radial and the femoral approaches in percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 200391598–600. [DOI] [PubMed] [Google Scholar]
- 22.Saito S, Tanaka S, Hiroe Y.et al Comparative study on transradial approach versus transfemoral approach in primary stent implantation for patients with acute myocardial infarction: results of the test for myocardial infarction by prospective unicenter randomisation for access site (TEMPURA) trial. Cathet Cardiovasc Interv 20035926–33. [DOI] [PubMed] [Google Scholar]
- 23.Gobeil F, Bruck M, Louvard Y.et al Comparison of 5 French versus 6 French guiding catheters for transradial coronary intervention: a prospective, randomized study. J Invasive Cardiol 200416353–355. [PubMed] [Google Scholar]
- 24.Petronio A S, Musumecci G, Limbruno U.et al Abxicimab improves 6‐month clinical outcome after rescue coronary angioplasty. Am Heart J 2002143334–341. [DOI] [PubMed] [Google Scholar]
- 25.Montalescot G, Barragan P, Wittenberg O, ADMIRAL Investigators et al Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction: ADMIRAL investigators. N Engl J Med 20013441895–1903. [DOI] [PubMed] [Google Scholar]
- 26.de Queiroz Fernandes Araujo J O, Veloso H H, De Paiva J M B.et al Efficacy and safety of abciximab on myocardial infarction treated with percutaneous coronary interventions: a meta‐analysis of randomised controlled trials. Am Heart J 2004148937–943. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon J T, Combs D T, Mc Lellan B A.et al Early hospital discharge after direct angioplasty for acute myocardial infarction. Cathet Cardiovasc Diagn 199535187–190. [DOI] [PubMed] [Google Scholar]