Abstract
Background
There is a strong inverse relationship between final vessel diameter and subsequent risk of treatment failure after coronary stent deployment. The aim of this study was to investigate the magnitude by which stent delivery balloon underexpansion and stent elastic recoil contributed to suboptimal final vessel geometry.
Methods
A prospective angiographic study recruiting 499 lesions (385 patients) undergoing coronary stent implantation was performed. Quantitative coronary angiography (QCA) was used to measure the minimal lumen diameters of the delivery balloon during stent deployment (MLD1) and of the stented segment following balloon deflation (MLD2). The expected balloon diameter for the deployment pressure was determined from the manufacturer's reference chart. Delivery balloon deficit was measured by subtracting the MLD1 from the expected balloon size and stent recoil was calculated by subtracting MLD2 from MLD1. Delivery balloon deficit and stent recoil were examined as a function of reference vessel diameter (RVD) and balloon–vessel (BV) ratio.
Results
The final stent MLD was a mean 27.2% (SD = 7.2) less than the predicted diameter. The mean delivery balloon deficit was 0.65 mm (SD = 0.27) and the mean stent recoil was 0.28 mm (SD = 0.17). Percentage delivery balloon deficit and stent recoil were independent of RVD. Delivery balloon deficit increased with higher BV ratios. Stent recoil was independent of BV ratio and the use of predilatation.
Conclusion
Failure to achieve predicted final stent diameter is a real problem with contribution from delivery balloon underexpansion and stent recoil. On average the final stent MLD is only 73% of the expected diameter, irrespective of vessel size.
Modern stent delivery systems use semicompliant balloons that are manufactured to have at a specific pressure, a certain nominal diameter. Inflation of the balloon at pressures greater than nominal results in a slight increase in the final diameter obtained. Manufacturers provide a compliance chart for each device, which describes the expected stent diameter for a range of deployment pressures. These values are derived from calliper or other measurements of balloons inflated in air or in a water bath. Some manufacturers report only the compliance data of the delivery balloon without the stent. These charts do not take into account the resistance encountered by compressing the atherosclerotic plaque and stretching the vessel wall during stent deployment.
Failure of the delivery balloon to reach its target size during stent deployment and subsequent elastic recoil are two mechanisms that contribute to stent under‐deployment.1 Both of these factors are a function of the vessel's elastic properties and resistance to expansion. The relative contributions of these factors may be of particular relevance in vessels of small calibre. Bakhai et al.2 reported that in vessels of small calibre, the delivery balloon size during stent deployment and the final stent minimal lumen diameter (MLD) were well below that predicted by manufacturers' compliance charts. Elastic forces in small‐calibre vessels may restrict delivery balloon expansion to a greater degree than larger vessels. This study was undertaken to investigate the magnitude by which stent delivery balloon underexpansion and elastic recoil impacts on the final stent diameter. We also examined the influence of reference vessel diameter and the performance of predilatation on these parameters.
Methods
Patient selection
We performed a prospective observational study documenting a series of native vessel percutaneous coronary intervention (PCI) cases reflecting routine clinical activity at our centre. The data collection period ran from 26 September 2003 to 12 October 2004. Three consultant cardiologists agreed to take part in this study (RHS, RAP, JLM). All data relating to the PCI procedure were collected in real time by SA who was present in the catheter laboratory during the procedures. Trial‐specific information was transcribed to a computer database system running as an adjunct to our routine clinical audit database tool. All procedures were performed according to agreed protocols with the acquisition of specific angiographic images for subsequent offline quantitative coronary angiography analysis.
A total of 499 individual lesions in 391 patients treated with coronary stent implantation were recorded during the study period. In eight cases there were no baseline reference vessel diameter (RVD) measurements and in three cases there were no images of the stent delivery balloon during deployment. The remaining 488 lesions (representing 383 patients) were included in the analysis. Postdilatation was performed in 41.2% (202/488) of cases.
Angiographic evaluation
Digital angiogram records were analysed off line by SA. Quantitative coronary angiography (QCA) was performed using an automated edge detection system, CAAS II (Cardiovascular Angiography Analysis System), Pie Medical. This system has been extensively validated in previous studies.3 Investigators were required to record paired orthogonal images of target lesion segments as part of routine PCI practice.
The MLD of the stent delivery balloon at peak pressure during deployment and within the stent after deployment were measured. The RVD was calculated as an average of the proximal and distal RVDs.
QCA‐derived parameters
In vivo delivery balloon underexpansion was calculated by subtracting the MLD of the delivery balloon during stent deployment from the expected balloon diameter. The expected balloon diameter was calculated from the manufacturer's compliance chart based on the nominal balloon size and maximum inflation pressure. Percentage delivery balloon underexpansion was calculated as follows: absolute delivery balloon underexpansion/expected balloon diameter x 100.
Elastic recoil was calculated by subtracting the MLD after stent deployment from the minimal diameter (MD) of the delivery balloon during stent deployment. Percentage stent recoil was calculated as follows: absolute stent recoil/MD of the stent delivery balloon ×100.
Final stent deficit (a function of elastic recoil and stent delivery balloon underexpansion) was calculated by subtracting the MLD after stent deployment from the expected balloon diameter. Percentage final stent deficit was calculated as follows: absolute final stent deficit/expected balloon diameter ×100.
Procedural technique
All procedures were performed via the femoral or radial routes. Intravenous heparin was given at the start of the procedure to maintain an activated clotting time of 220–300 s. The choice of guide wires, balloons and stents was left to the discretion of the operators. All patients received aspirin 300 mg and clopidogrel 300–600 mg pre‐procedure. Aspirin 75 mg was continued indefinitely and clopidogrel 75 mg was continued for a minimum of 4 weeks post‐procedure.
Statistical analyses
A paired t test was used for all comparisons. For stent delivery balloon underexpansion, the MLD of the contrast‐filled delivery balloon at maximum pressure during stent deployment was compared with the expected balloon diameter. For stent recoil, the MLD of the stent delivery balloon during stent deployment was compared with the MLD after stent deployment. The expected delivery balloon diameter was also compared with the MLD after stent deployment. The study population was divided into quintiles based on the baseline RVD. The relationship between balloon underexpansion and stent recoil as a function of RVD was tested using analysis of variance. The impact of predilatation compared with direct stenting on stent delivery balloon underexpansion and stent recoil was examined using an independent samples t test. We also investigated the relationship between the balloon–vessel (BV) ratio of stent deployment and stent delivery balloon underexpansion and stent recoil. BV ratio was calculated as the expected balloon diameter divided by the RVD. Stent delivery balloon underexpansion and stent recoil were also examined for the different stents used.
Results
Baseline procedural and angiographic characteristics
The baseline characteristics are shown in table 1. The angiographic and procedural details are described in table 2.
Table 1 Baseline patient characteristics (n = 383).
| Age (years) | 62.1 (10.3) |
| Women | 109 (28.4%) |
| Diabetes | 51 (13.3%) |
| Hypertension | 209 (54.4%) |
| Hypercholesterolaemia | 361 (94.0%) |
| Smoker | |
| Current | 95 (24.7%) |
| Ex smoker | 156 (40.6%) |
| Family history of IHD | 210 (54.7%) |
| Peripheral vascular disease | 11 (2.9%) |
| Previous CABG | 12 (3.1%) |
| ACS presentation | 112 (29.1%) |
Data are presented as mean (SD) for continuous variables and number (percentage) for categorical data.
ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; IHD, ischaemic heart disease.
Table 2 Angiographic and procedural results (n = 488).
| Vessel | |
|---|---|
| LAD | 205 (42.0%) |
| LCX | 111 (22.7%) |
| RCA | 169 (34.6%) |
| LM | 3 (0.6%) |
| RVD (mm) | 2.76 (0.53) |
| MLD pre‐intervention (mm) | 0.97 (0.34) |
| MD stent deployment balloon (mm) | 2.75 (0.42) |
| MLD post stenting (mm) | 2.47 (0.41) |
| Lesion diameter stenosis (%) | |
| Before intervention | 63.28 (10.86) |
| After stenting | 9.12 (12.54) |
| Stent length (mm) | 20.65 (5.93) |
| Stent nominal diameter (mm) | 3.14 (0.43) |
| Stent deploy pressure (atm) | 14.60 (1.56) |
Data are presented as mean (SD) for continuous variables and number (percentage) for categorical data.
atm, atmospheres; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main stem; MD, minimal diameter; MLD, minimal lumen diameter; RCA, right coronary artery; RVD, reference vessel diameter.
Stent delivery balloon underexpansion
There was a significant difference between the minimal diameter of the stent delivery balloon during stent deployment and the predicted stent delivery balloon diameter (mean 2.75 (SD = 0.42) mm vs mean 3.40 (SD = 0.48), respectively; p<0.001).
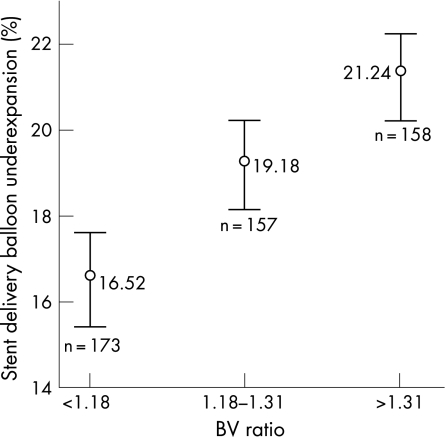
Absolute delivery balloon underexpansion was mean 0.65 (SD = 0.27) mm, 95% CI 0.63 to 0.67 mm. In all cases, the delivery balloon failed to achieve the expected size. The delivery balloon was mean 18.93% (SD = 7.00) smaller than the expected size, 95% CI 18.31% to 19.55% (fig 1).
Figure 1 Percentage stent delivery balloon underexpansion for the different balloon–vessel (BV) ratio groups, mean±95% confidence intervals (p<0.001).
Stent elastic recoil
There was a significant difference between the minimal diameter of the stent delivery balloon during stent deployment and the MLD within the stent following deployment (mean 2.75 mm (SD = 0.42) vs mean 2.47 mm (SD = 0.41), respectively; p<0.001].
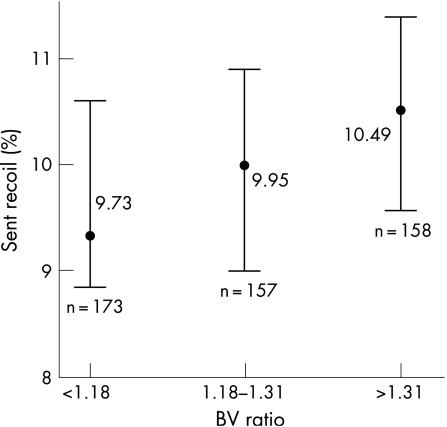
Absolute stent elastic recoil was mean 0.28 mm (SD = 0.17), 95% CI 0.26 to 0.29 mm. Percentage stent recoil was mean 10.02% (SD = 5.87), 95% CI 9.50% to 10.53%.
Comparison of final stent MLD with expected stent delivery balloon diameter
There was a significant difference between the MLD after stent deployment and the predicted stent delivery balloon diameter (mean 2.47 mm (SD = 0.41) vs 3.40 mm (SD = 0.48), respectively; p<0.001).
The absolute difference between the MLD after stent deployment and the expected stent delivery balloon diameter (final stent deficit) was mean 0.93 mm (SD = 0.29), 95% CI 0.90 to 0.95 mm. In all cases, the stent MLD was less than that expected from the manufacturer's compliance chart. The final stent MLD was a mean 27.17% (SD = 7.21) less than the expected diameter (95% CI 26.53% to 27.80%).
Impact of reference vessel diameter on delivery balloon underexpansion, stent recoil and final stent deficit
The study population was divided into five groups based on the baseline RVD (<2.35, 2.35–2.59, 2.60–2.81, 2.82–3.18, >3.18). Percentage delivery balloon deficit was similar across the different RVD groups, p = 0.630 (table 3). Similar results were obtained when comparing percentage stent recoil and percentage final stent deficit with RVD, p = 0.224 and p = 0.114, respectively (tables 4 and 5).
Table 3 Percentage delivery balloon underexpansion (DBU) depending on baseline RVD, p = 0.630.
| RVD range (mm) | Number | Mean DBU (%) | SD | 95% CI for mean | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| <2.35 | 101 | 19.51 | 7.22 | 18.08 | 20.93 |
| 2.35–2.59 | 97 | 19.42 | 7.34 | 17.94 | 20.90 |
| 2.60–2.81 | 95 | 18.33 | 7.17 | 16.87 | 19.79 |
| 2.82–3.18 | 100 | 18.93 | 6.01 | 17.74 | 20.12 |
| >3.18 | 95 | 18.30 | 7.45 | 16.78 | 19.82 |
| Total | 488 | 18.91 | 7.04 | 18.28 | 19.53 |
Table 4 Percentage stent recoil depending on baseline RVD, p = 0.224.
| RVD range (mm) | Number | Mean recoil (%) | SD | 95% CI for mean | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| <2.35 | 101 | 11.28 | 5.89 | 10.11 | 12.44 |
| 2.35–2.59 | 97 | 9.82 | 5.69 | 8.68 | 10.97 |
| 2.60–2.81 | 95 | 9.58 | 5.86 | 8.39 | 10.78 |
| 2.82–3.18 | 100 | 9.82 | 5.68 | 8.70 | 10.95 |
| >3.18 | 95 | 9.66 | 6.23 | 8.39 | 10.93 |
| Total | 488 | 10.05 | 5.88 | 9.52 | 10.57 |
Table 5 Percentage final stent deficit (FSD) depending on baseline RVD, p = 0.114.
| RVD range (mm) | Number | Mean FSD (%) | SD | 95% CI for mean | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| <2.35 | 101 | 28.68 | 7.07 | 27.28 | 30.08 |
| 2.35–2.59 | 97 | 27.41 | 7.42 | 25.92 | 28.91 |
| 2.60–2.81 | 95 | 26.29 | 6.87 | 24.89 | 27.69 |
| 2.82–3.18 | 100 | 26.90 | 7.02 | 25.51 | 28.29 |
| >3.18 | 95 | 26.31 | 7.58 | 24.76 | 27.85 |
| Total | 488 | 27.13 | 7.22 | 26.49 | 27.78 |
Impact of BV ratio on stent delivery balloon underexpansion and stent recoil
The mean BV ratio for stent implantation was 1.25 (SD = 0.17). The study population was divided into three groups based on the BV ratio (<1.18, 1.18–1.31 and >1.31). Percentage stent delivery balloon underexpansion increased with increasing BV ratio as shown in fig 1 (p<0.001 by analysis of variance). Percentage stent recoil was similar across the BV ratio groups (p = 0.261 by analysis of variance) (fig 2).
Figure 2 Percentage stent recoil for the different balloon–vessel (BV) ratio groups, mean±95% confidence intervals (p = 0.261).
Influence of stent type on stent delivery balloon underexpansion and stent recoil
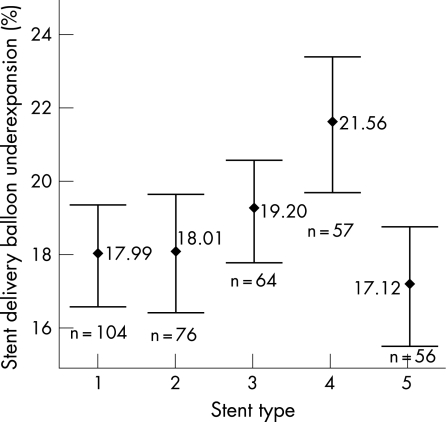
We investigated the influence of stent type on stent delivery balloon underexpansion. The five commonest stents used were included in the analysis. A greater degree of stent delivery balloon underexpansion was recorded for the Taxus stent compared with the rest (p = 0.004) (fig 3).
Figure 3 Percentage stent delivery balloon underexpansion for the different stent types, mean±95% confidence intervals (p = 0.004). Stent type: 1 = Cypher select (Cordis corporation), 2 = Zeta (Guidant corporation), 3 = Amazonia (Minvasys), 4 = Taxus (Boston scientific), 5 = Driver (Medtronic corporation).
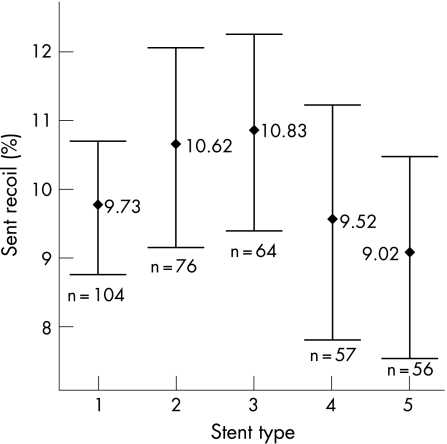
The degree of stent recoil was similar for the different stent types used (p = 0.349) (fig 4).
Figure 4 Percentage stent recoil for the different stent types, mean±95% confidence intervals (p = 0.349). Stent type: 1 = Cypher select (Cordis corporation), 2 = Zeta (Guidant corporation), 3 = Amazonia (Minvasys), 4 = Taxus (Boston scientific), 5 = Driver (Medtronic corporation).
Impact of predilatation on stent delivery balloon underexpansion and stent recoil
Predilatation was performed in 59% (290/488) of cases. The mean stent delivery balloon underexpansion was similar for cases with predilatation and direct stenting (19.09% (SD = 7.26) vs 18.82% (SD = 6.82), respectively; p = 0.884). The mean stent recoil was also similar between cases with predilatation and direct stenting (10.36% (SD = 6.14) vs 9.51% (SD = 5.43), respectively; p = 0.116).
Discussion
Principal findings of this study
The major finding of this study is that the post‐deployment stent MLD is on average 27% less than that predicted by the manufacturer's compliance chart. Despite the use of high inflation pressures, stent delivery balloon underexpansion (mean 19%) and stent elastic recoil (mean 10%) resulted in stent under‐deployment. Previous studies using intravascular ultrasound have reported that the mean MLD after stent deployment was 72% of the expected stent diameter and the mean minimum cross‐sectional area (CSA) was 62% of the expected stent area.4
Stent delivery balloon underexpansion
We found that the percentage by which the stent delivery balloon failed to reach the target size and the percentage stent deficit after deployment were independent of RVD. Smaller and larger vessels exhibited the same proportionate degree of resistance to stent expansion.
An important relationship between BV ratio of stent deployment and stent delivery balloon underexpansion was observed. Stent delivery balloon underexpansion was greater in those cases with a BV ratio >1.31 compared to cases with a BV ratio <1.18. Stent delivery balloon expansion is under the natural constraints of reference vessel size. Oversizing the stent delivery balloon relative to the RVD resulted in greater restriction of balloon expansion compared to cases where the balloon size was closely matched to the RVD.
There was a small but statistically significant greater magnitude of stent delivery balloon underexpansion for the Taxus Express stent delivery system compared to the other stent types. This subgroup analysis is hypothesis‐generating and further studies are required to determine if a true difference does exist with the Taxus Express stent delivery system.
Bermejo et al.1 showed that the in vivo size of the stent delivery balloon during stent deployment was not uniform along its entire length. The mean maximal balloon CSA was 30% larger and the mean minimal balloon CSA 12% smaller than the nominal balloon size. The diameter of the stent delivery balloon during deployment will vary depending on the site at which measurements are made. Hehrlein et al.5 measured the MD of the stent delivery balloon during peak pressure, as in our study. They reported that the delivery balloon diameter of the Duet and the NIR stent delivery system were on average 14% and 18% smaller than predicted by the manufacturers. Gunn et al.6 reported that the in vivo diameter of the stent delivery balloon during deployment was on average 4% smaller than the expected diameter. However, they did not measure the minimal diameter of the stent delivery balloon but instead recorded the diameter one‐quarter and one‐half distance from the balloon ends.
The characteristics of the vessel wall such as elasticity, plaque composition, fibrosis and calcification are likely to be the main factors that restrict stent expansion. Due to the complexities and variable pattern of coronary disease, it would not be possible for manufacturers to produce charts that take into account vessel compliance. The charts provided by the manufacturers represent the relative compliance of the stent delivery balloon system. They do not accurately predict the stent dimensions achieved during deployment at the site of atherosclerotic lesions.
When hard resistant lesions are stented, semi‐compliant balloons used for the stent delivery system may fail to expand fully at the lesion site. Inflating the balloon to higher pressures can result in supranominal balloon expansion at the margins with a portion remaining constrained at the lesion site.7 This potentially hazardous stretch of the arterial wall at the balloon margins could increase the risk of vessel dissection or perforation. Postdilatation with non‐compliant balloons has been shown to improve stent expansion without the risk of vessel perforation.8 Additional postdilatation with non‐compliant balloons may be required to overcome stent delivery balloon underexpansion.
Residual plaque burden after coronary stent implantation has been shown to be important in limiting stent expansion and predicting in‐stent re‐stenosis.9,10 Lesion preparation prior to coronary stenting may modify vessel compliance and improve stent expansion. In our observational study, we found that predilatation had no impact on stent delivery balloon underexpansion compared to direct stenting. This may have been due to case selection and a randomised study would be required to evaluate further the impact of lesion preparation on stent expansion. Plaque debulking techniques such as directional coronary atherectomy or rotational atherectomy may be more effective in reducing lesion resistance and improving stent expansion. Directional coronary atherectomy followed by stenting has been shown to be associated with a greater acute gain in vessel diameter and a lower angiographic re‐stenosis rate at 6 months compared to stenting alone.11,12 Rotational atherectomy has been used to facilitate stent expansion in severely calcified coronary lesions and cases where balloon angioplasty has been unsuccessful.13
Stent elastic recoil
IVUS studies have shown that the principal mechanism by which coronary stents enlarge the lumen is through vessel stretching and increasing the area bound by the external elastic membrane.9 Coronary stents are affected by a number of external compressive forces during deployment. These include the elastic recoil of the atheromatous lesion and non‐diseased sections of the arterial wall. Stents are designed with a high radial force to prevent recoil following deployment.
Acute elastic recoil that occurs after balloon deflation accounts for up to 50% of the loss in acute gain achieved with balloon angioplasty.14 Initial observation with the Palmaz‐Schatz coronary stent suggested that stenting largely eliminated acute elastic recoil after coronary intervention.15 However, in this study stenting was used for the treatment of re‐stenotic lesions and high‐pressure stent deployment was not used. In normal porcine coronary arteries, acute stent recoil measured using a 0.018″ imaging IVUS core wire positioned in the guide wire lumen of the delivery balloon was found to be the predominant mechanism by which stents failed to achieve the nominal CSA.16
The present study has shown that immediately after coronary stent deployment there is a reduction in the luminal diameter due to elastic recoil. The mean percentage elastic recoil was independent of RVD, stent type, BV ratio and predilatation. Our findings are consistent with the results of previously published studies. Danzi et al.17 measured the elastic recoil of 406 tubular stents using videodensitrometry QCA. Elastic recoil was defined as the difference between the mean delivery balloon CSA at the peak pressure and the mean CSA after stent deployment. They showed that the mean CSA stent recoil was 13%. Bermejo et al.1 defined elastic recoil similar to the present study, as the difference between the MLD of the delivery balloon during peak inflation and the MLD after stent deployment. They showed that in 57 stented lesions, the mean elastic recoil was 12%.
In vitro studies using mechanical bench testing have shown a considerable variation in stent elastic recoil, ranging from 2% to 18% depending on stent design and construction.18 Greater elastic recoil has been observed with coil stents compared with slotted tube stents.19 However, these studies have not taken into account the effects of stent implantation into atheromatous coronary lesions. In our study, we found no difference in elastic recoil between the four commonest stents used.
The observation that immediate stent elastic recoil occurs with modern stents when deployed at high pressure has implications for stent design. Modification of stent or strut geometry, use of new chromium/cobalt alloys with greater radial force may allow maximisation of hoop strength while maintaining stent flexibility.
Conclusion
Interventional cardiologists need to be aware that compliance charts provided by manufacturers do not accurately predict in‐vivo stent diameters. A combination of stent delivery balloon underexpansion and elastic recoil limits the final stent size independent of RVD. Further studies examining the impact of stent design, lesion preparation and the use of postdilatation on stent expansion are required.
Abbreviations
BV - balloon–vessel ratio
CSA - cross‐sectional area
MD - minimal diameter
MLD - minimal lumen diameter
QCA - quantitative coronary angiography
RVD - reference vessel diameter
Footnotes
Competing interests: None.
References
- 1.Bermejo J, Botas J, Garcia E.et al Mechanisms of residual lumen stenosis after high‐pressure stent implantation: a quantitative coronary angiography and intravascular ultrasound study. Circulation 199898112–118. [DOI] [PubMed] [Google Scholar]
- 2.Bakhai A, Booth J, Delahunty N.et al The SV stent study: a prospective, multicentre, angiographic evaluation of the BiodivYsio phosphorylcholine coated small vessel stent in small coronary vessels. Int J Cardiol 200510295–102. [DOI] [PubMed] [Google Scholar]
- 3.Gronenschild E, Janssen J, Tijdens F. CAAS. II: A second generation system for off‐line and on‐line quantitative coronary angiography. Cathet Cardiovasc Diagn 19943361–75. [DOI] [PubMed] [Google Scholar]
- 4.Takano Y, Yeatman L A, Higgins J R.et al Optimizing stent expansion with new stent delivery systems. J Am Coll Cardiol 2001381622–1627. [DOI] [PubMed] [Google Scholar]
- 5.Hehrlein C, DeVries J J, Wood T A.et al Overestimation of stent delivery balloon diameters by manufacturers' compliance tables: a quantitative coronary analysis of Duet and NIR stent implantation. Catheter Cardiovasc Interv 200153474–478. [DOI] [PubMed] [Google Scholar]
- 6.Gunn J, Beacock D, Morton A.et al Immediate stent recoil: a forgotten phenomenon. Br J Cardiol (Acute Interv Cardiol) 20051215–19. [Google Scholar]
- 7.Hehrlein C, DeVries J J, Arab A.et al Role of the “dogbone” effect of balloon‐expandable stents: quantitative coronary analysis of DUET and NIR stent implantation introducing a novel indexing system. J Invasive Cardiol 20021459–65. [PubMed] [Google Scholar]
- 8.Brodie B R, Cooper C, Jones M.et al Is adjunctive balloon postdilatation necessary after coronary stent deployment? Final results from the POSTIT trial. Catheter Cardiovasc Interv 200359184–192. [DOI] [PubMed] [Google Scholar]
- 9.Maehara A, Takagi A, Okura H.et al Longitudinal plaque redistribution during stent expansion. Am J Cardiol 2000861069–1072. [DOI] [PubMed] [Google Scholar]
- 10.Prati F, Di Mario C, Moussa I.et al In‐stent neointimal proliferation correlates with the amount of residual plaque burden outside the stent: an intravascular ultrasound study. Circulation 1999991011–1014. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Moussa I, Akiyama T.et al Low restenosis rate in lesions of the left anterior descending coronary artery with stenting following directional coronary atherectomy. Cathet Cardiovasc Diagn 199845131–138. [DOI] [PubMed] [Google Scholar]
- 12.Airoldi F, Di Mario C, Stankovic G.et al Clinical and angiographic outcome of directional atherectomy followed by stent implantation in de novo lesions located at the ostium of the left anterior descending coronary artery. Heart 2003891050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moussa I, Di Mario C, Moses J.et al Coronary stenting after rotational atherectomy in calcified and complex lesions. Angiographic and clinical follow‐up results. Circulation 199796128–136. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez A E, Palacios I F, Fernandez M A.et al Time course and mechanism of early luminal diameter loss after percutaneous transluminal coronary angioplasty. Am J Cardiol 1995761131–1134. [DOI] [PubMed] [Google Scholar]
- 15.Haude M, Erbel R, Issa H, Meyer J. Quantitative analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon‐expandable Palmaz‐Schatz stents. J Am Coll Cardiol 19932126–34. [DOI] [PubMed] [Google Scholar]
- 16.Carrozza J P, Jr, Hermiller J B, Jr, Linnemeier T J.et al Quantitative coronary angiographic and intravascular ultrasound assessment of a new nonarticulated stent: report from the Advanced Cardiovascular Systems MultiLink stent pilot study. J Am Coll Cardiol 19983150–56. [DOI] [PubMed] [Google Scholar]
- 17.Danzi G, Fiocca L, Capuano C.et al Acute stent recoil: in vivo evaluation of different stent designs. Catheter Cardiovasc Interv 200152147–153. [DOI] [PubMed] [Google Scholar]
- 18.Barragan P, Rieu R, Garitey V.et al Elastic recoil of coronary stents: a comparative analysis. Catheter Cardiovasc Interv 200050112–119. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Brown D L, Ischinger T A.et al Effect of stent design on reduction of elastic recoil: a comparison via quantitative intravascular ultrasound. Catéter Cardiovasc Interv 199947251–257. [DOI] [PubMed] [Google Scholar]