Children with congenital heart disease account for one in every 145 live births in the UK. Survival has improved dramatically over the past half century; in the 1950s only 20% of such patients could expect to reach adulthood, but now the number is closer to 80%. However, it is a maxim among congenital specialists that a significant proportion of their patients are “fixed, not cured”. There are currently at least 150 000 adults in the UK with congenital heart disease and this number is expected to rise by 25% by 2010. A smaller subset—roughly 12 000 patients—have complex forms of congenital heart disease and numbers are projected to rise by 50% over the same time period.w1
Imaging in congenital heart disease
A vital component to the multidisciplinary management of the congenital heart disease patient is adequate imaging of the heart and circulation. However, simple anatomical delineation alone is not sufficient. Techniques are required which can measure function, flow and pressure as well as resolve anatomical structures with a high degree of detail. Such methods should be directed by a physician with both knowledge and interest in congenital heart disease.
The major disadvantage of echocardiography has traditionally been its relatively limited field of view. Assessment of an entire congenital repair, which may include extra anatomic bypasses or systemic shunts, is often suboptimal. While central pulmonary arteries are visible there is no opportunity to evaluate the peripheral pulmonary arterial or venous structures. Similarly the tracheobronchial structures and lung parenchyma remain quite occult by this technique.
Cardiac catheterisation remained for many years the reference standard for the investigation of congenital heart disease patients. Radiation dose to this population of children and young adults is a vital consideration, however, given the non‐threshold risk of subsequent cancer induction. The European Heart Survey of congenital heart disease, which included over 4000 patients and >18 000 patient‐years, indicated that conventional angiography contributed to 42% of the overall radiation burden sustained by this population, and that patients with certain conditions (Marfan, Fontan, coarctations) bore the brunt of this exposure.w2 Biplane angiography is helpful in this regard and should be regarded as mandatory for paediatric catheterisation.
Complementary roles of cardiac magnetic resonance and computed tomography
The relative advantages of cardiac magnetic resonance (CMR) and computed tomography (CT) are listed in table 1.
Table 1 Relative advantages of cardiac magnetic resonance (CMR) vs computed tomography (CT).
| CMR | CT |
|---|---|
| • Possible to image in any plane desired | • Limited to axial acquisition |
| • No ionising radiation | • Ionising radiation ++ |
| • Relatively long breath holds for CE‐MRA | • Relatively short breath hold for CTA |
| • Overall study time long | • Overall study time very short |
| • Post‐processing tools available | • Post‐processing tools available |
| • Long axis resolution limited to 1–2 mm | • Long axis resolution as little as 0.4 mm |
| • Useful images obtainable without intravenous contrast | • Limited images available without intravenous contrast |
| • Real time imaging possible (at expense of image resolution) | • Real time imaging not possible (gating always required) |
| • Robust software for right ventricular functional assessment | • No adequate software for right ventricular function |
| • Flow measurement possible | • No measurement of flow possible |
| • Strategies available for acquiring free breathing images | • No navigated solution for free breathing |
| • Local field distortion from metal (may render exam non‐diagnostic) | • Relatively minor streak artefact from metal |
| • Poor visualisation of calcium | • Calcium readily visible |
| • Not ideal for coronary imaging | • Well adapted for coronary imaging |
| • Very low level of contrast allergy | • Low level of contrast allergy |
| • Interventional CMR developing | • Interventional CT not developed |
| • Relatively few well trained technologists | • Many well trained technologists |
| • Few dedicated cardiac magnets | • Increasing numbers of cardiac capable multislice CT machines |
CE‐MRA, contrast enhanced magnetic resonance angiography; CTA, computed tomographic angiography.
Although CMR is generally more flexible and appropriate for congenital imaging, it is not entirely free of limitations. Both cardiac and respiratory motions are problematic. Most sequences have to be gated to the cardiac cycle and acquired either as a breath hold or using multiple averages. While gating and breath holding are routine in adult patients, they present more of a challenge in a potentially uncooperative paediatric population whose faster resting heart rates are worsened with anxiety. Many centres choose to perform paediatric CMR under sedation or general anaesthesia. The latter is particularly useful since controlled ventilation can be temporarily suspended at the appropriate moment to reduce artefact where good spatial resolution is critical (for example, MR angiography). General anaesthesia in this setting appears safe, even in patients with complex congenital heart disease.w3 Technical issues specific to paediatric CMR are discussed extensively elsewhere.w4
CT is likely to become an increasingly important imaging technique for the congenital heart disease population, in part because of greater access to CT than CMR. There is some concern over this trend because CT was the second largest contributor to patient dose in the aforementioned European Heart Survey.w2 Nevertheless there are several clear indications for CT rather than CMR. Good quality images are unlikely to result from CMR in a sick or uncooperative patient, whereas the very short examination times possible using multidetector CT may still result in a clear depiction of anatomy. Spatial resolution remains superior for multidetector CT with a z‐axis resolution of approximately 0.5 mm versus 1.5–2 mm or more for MR angiography. Thus, very fine structures including small aortopulmonary collaterals and coronary arteries may be better demonstrated by CT.
The comprehensive CMR exam
Black blood anatomical imaging has been replaced to a greater or lesser extent by bright blood cine imaging.1 This latter technique results from image acquisition at multiple time points (“phases”) in the cardiac cycle with subsequent display as a beating movie loop. Although it is possible to acquire all the phases for a one beating image in a single cardiac cycle, in practice such “real time” CMR is restricted to patients with troublesome arrhythmia, since the trade‐off is reduction in spatial resolution. Most cine imaging is thus performed in a segmented manner in which the multiple phases making up a single beating image are acquired over consecutive heart beats.
Cine phase contrast imaging is a technique in which both the speed and direction of flowing blood are encoded as phase shifts by magnetic gradients and displayed on a grey scale.w5 Speed of flow is reflected by colour intensity. As with colour Doppler echocardiography, it is necessary to preset a range of velocities to which the grey scale is mapped. In this way the severity of valvular, conduit or chamber stenosis may be evaluated. Measurements of flow in litres per minute can be made at the level of the ascending aorta and main pulmonary artery and used for calculation of Qp:Qs, assuming no significant atrioventricular valve regurgitation is present. Left to right shunting from atrial and ventricular septal defects or anomalous pulmonary venous return, for example, is readily quantified.
Contrast enhanced magnetic resonance angiography (CE‐MRA) is the other major component of the comprehensive congenital CMR examination. This technique involves the peripheral venous injection of a bolus of gadolinium contrast agent and rapid imaging with a relatively large field of view to acquire a volume of data with high spatial resolution. Generally at least two volume acquisitions of data are made following injection in order to ensure image acquisition in both arterial and early venous phases. In some cases—for example, assessment of the Fontan circulation—it is also advisable to acquire several late venous runs to ensure adequate mixing of gadolinium with blood returning from the lower limbs (fig 1A–C).
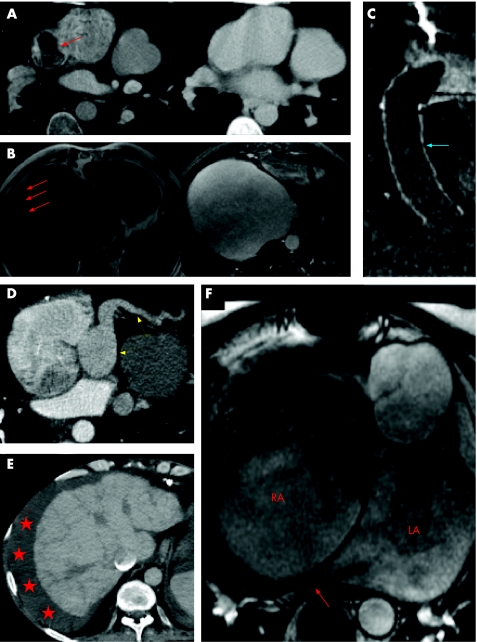
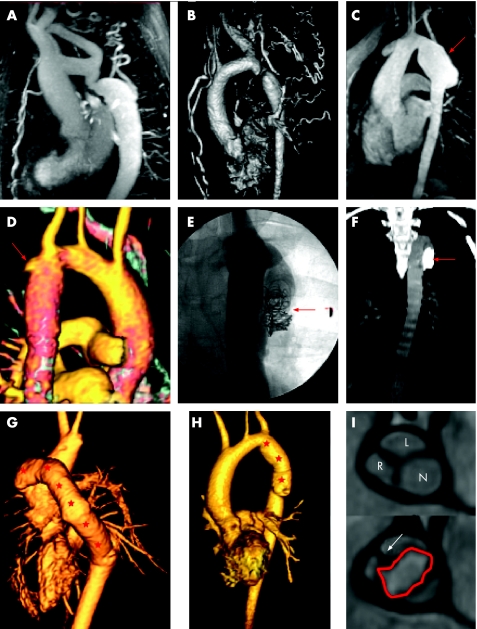
Figure 1 Real and imagined complications following Fontan conversion. (A) Axial slice from a contrast enhanced computed tomography (CT) study. Mixing of opacified and unopacified blood in the high right atrium/appendage has resulted in the appearance of a filling defect (red arrow) that is not infrequently mistaken for thrombus by the unwary (left hand panel). Delayed imaging several minutes later ensures adequate mixing of blood and contrast with total resolution of the defect (right hand panel). (B) Inversion recovery axial magnetic resonance (MR) slice. The right atrium is hugely enlarged as often happens in this condition. There appears to be material layering posteriorly (red arrows) which is highly suspicious for thrombus. However, once again delayed imaging following administration of intravenous gadolinium reveals this to have been a pseudo‐lesion (right hand panel). (C) Close up of coronal contrast enhanced CT image in a patient with an extracardiac cavopulmonary connection. The tube graft (blue arrow) between the inferior vena cava and right pulmonary artery is full of low attenuation material that fails to enhance on delayed imaging. This was a genuine thrombotic complication despite warfarin treatment. (D) Axial slice at the inferior surface of the heart from a CT angiogram. The coronary sinus and draining vein (yellow arrows) are significantly dilated as a result of raised right heart pressures. (E) Axial slice from contrast enhanced CT. This patient with a failing Fontan has ascites (asterisks) and a small rather nodular appearing liver which is cirrhotic. (F) Steady state free precession (SSFP) axial MR image in a patient with a very large right atrium which is causing significant dynamic compression of the right lower lobe pulmonary vein (red arrow). Right atrial dilatation is common as a result of largely passive forward flow of blood into the lungs.
A complete CMR exam for congenital heart disease will thus usually include most or all of these components (table 2). The importance of physician supervision cannot be overemphasised for all but the most straightforward cases.
Table 2 Components of a congenital CMR exam.
| • Preparation—patient's case notes (including surgical notes) |
| • Axial steady state free precession (SSFP) cine sequence from aortic arch to diaphragm 10 mm thick, no gap |
| • Short axis oblique SSFP from ventricular apex to atrioventricular (AV) groove, 8 mm thick, no gap |
| • Fast cine phase contrast through ascending aorta, main and left and right pulmonary arteries |
| • Three dimensional contrast enhanced magnetic resonance angiography (CE‐MRA): coronal plane, sternum to spine, slice thickness 2–2.5 mm, arterial and venous phase |
| • Physician check |
| • Additional thin slices (SSFP or black blood) and cine phase contrast studies (for example, for flow through shunts, collaterals, anomalous veins or arteries, etc) as directed by monitoring physician |
| • Multiplanar reformations, maximum intensity projections and volume rendered image preparation (ideally by reporting physician) |
Angiographic post‐processing and display
There are two principal methods of post‐processing applied to contrast enhanced CMR or CT angiograms: maximum intensity projections (MIPs), and volume rendering. Three dimensional data are output as slices of minimum thickness selected by the operator at the time of acquisition. However, structures of interest such as branching pulmonary arteries rarely lie within a single plane of only a few millimetres thickness. Therefore, the MIP technique is used to create a two dimensional projection through the dataset by displaying the brightest voxel along a line projected through the three dimensional volume. The depth of the projection line is determined by the viewer at the workstation and may be anything from a few millimetres to a centimetre or more. The result is to create an image reminiscent of conventional two dimensional angiography (fig 2B,D)
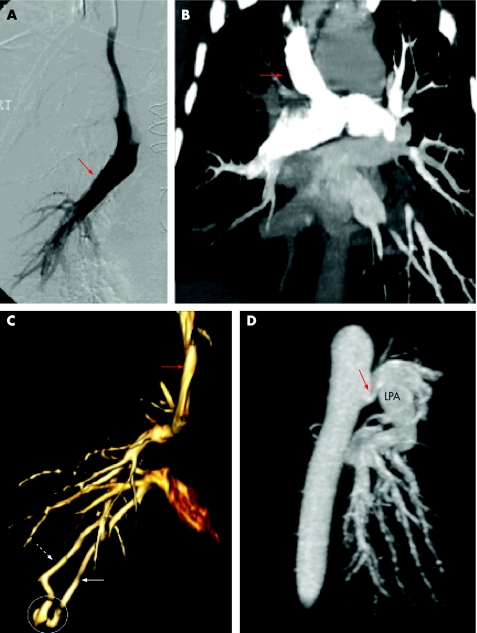
Figure 2 Surgical shunts. (A) Single frame from a conventional cine angiogram. The catheter has been placed into the superior vena cava and contrast appears to fill the right main pulmonary artery (red arrow). This was a surprise to the operator who was attempting to perform a trans‐jugular liver biopsy and was unaware that the patient had a Glenn shunt. (B) Sub‐volume maximum intensity projection (MIP) from a computed tomography angiogram (CTA) (coronal view). This CTA confirms the patency of a bidirectional Glenn shunt (red arrow) in which augmentation of pulmonary blood flow is achieved by anastomosis of the superior vena cava (SVC) to the main pulmonary artery bifurcation. (C) Volume rendered CTA in a patient with a classic Glenn shunt in which the SVC has been anastomosed to the right pulmonary artery alone (as in patient A). Such patients do not receive any blood from the inferior vena cava (IVC) on the right side and, as a consequence, are prone to develop arteriovenous malformations (AVM) in the lung because of lack of exposure to “hepatic factor” in IVC blood. This image shows one such AVM (circled) with a feeding arterial branch (dotted white arrow) and a draining vein (solid white arrow). Many AVMs are microscopic and beyond the resolution of CT, but may contribute to desaturation. The classic Glenn procedure is no longer performed partly for this reason. (D) Sub‐volume MIP from a magnetic resonance angiogram (MRA). A central surgical shunt has been created between the descending thoracic aorta and the left pulmonary artery (Potts shunt). A focal stenosis at the anastomotic site is shown (red arrow). LPA, left pulmonary artery.
Volume rendering involves the full depth of the dataset but allows for a varying degree of contrast, transparency, shading and colour which ultimately produces the impression of a three dimensional model of the structures acquired (figs 3B,C); again, this can be rotated in any desired orientation with or without further post‐processing to remove overlying structures.
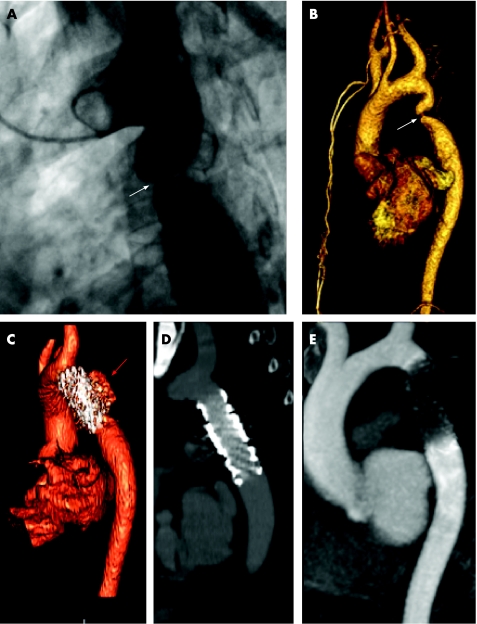
Figure 3 (A–D) Coarctation: use of computed tomography (CT) and cardiac magnetic resonance (CMR) in a single patient. (A) Conventional invasive angiography with a pigtail catheter in the transverse arch. A tight primary coarctation is visible (arrow). (B) Same patient. Volume rendered magnetic resonance angiogram (MRA) confirms the location and severity of the lesion and aids in interventional planning of size and length of stent as well as stent placement with respect to the great arch vessels, which in this case are suitably distant. There is considerable curvature of the arch just proximal to the coarctation which in retrospect might have hinted at the trouble to come. (C) Volume rendered CT angiography performed immediately after stent placement during which the patient complained of severe chest pain. The metallic stent is clearly seen and appears well centred on the lesion without evidence of waisting. However, there is an additional contrast filled structure (red arrow) dorsolateral to the stent. This represented a contained rupture of the aorta. The patient returned to the catheterisation laboratory where a second covered stent was placed to seal the leak. (D) Sub‐volume sagittal oblique maximum intensity projection (MIP). Repeat CT several days later shows resolution of the pseudoaneurysm. (E) The patient remained well and returned for follow up several months later. She underwent CMR rather than CT because of a booking error. The MIP from the MRA demonstrates the signal drop out in the region of the stent due to its metallic composition. These patients are better followed by CT for this reason.
Ventricular function
Accurate and reproducible measurement of left and right ventricular function is vital in congenital heart disease. Late outcome can often be predicted from these data both in patients with poorly functioning ventricles in the normal anatomical position, such as in tetralogy of Fallot,2 as well as in those with ventricular inversions as seen in surgically or congenitally corrected transposition of the great arteries (TGA).3w6 While echocardiography is reasonable for function assessment of the left ventricle (LV), the right ventricle (RV) is often incompletely visualised with the result that size and function are frequently visually overestimated. Several groups have published reference ranges for normal RV and LV function adjusted for age, sex and ethnicity.4,5
There can be up to a 10% increase in end diastolic volume when moving from an older gradient echo sequence to the steady state free precession sequences in contemporary use.6 While most groups make LV measurements from a stack of cine images acquired in the short axis, there is no such consensus for RV assessment. The axial orientation may permit a more reproducible assessment with lower inter‐ and intra‐observer variability.w7 Others have suggested that a modified short axis acquisition avoids the anatomical constraints of either of the standard approaches.w8
RV volume measurement is particularly important in patients followed up post‐repair of tetralogy of Fallot. Severe pulmonary incompetence is a common consequence of outflow tract enlargement in infancy and leads to progressive dilatation and failure of the RV. The main clinical dilemma is at what stage to perform pulmonary valve replacement. Serial CMR studies with RV volume and ejection fraction measurements are helpful in identifying the progressively enlarging ventricle which places the patient at risk of both arrhythmias and eventual pump failure.7
CT has been used to make measurements of both LV and RV function. The temporal resolution of multidetector systems is determined partly by the rotation speed of the gantry and partly by the segmentation algorithm employed. Multidetector CT datasets appear accurate for ejection fraction and volume measurements of the LV compared to MRI and outperform the same measurements made by either echo or gated single photon emission computed tomography.w9
Scar detection
LV myocardial scar has been known to reflect prognosis in ischaemic heart disease for many years. CMR is ideally suited to detecting small amounts of scar due to its excellent spatial and contrast resolution. The delayed enhancement technique—in which scar appears white compared to black normal myocardium8—is relatively robust and is capable of detecting even very small amounts of myocardial fibrosis.
Several investigators have applied this technique to congenital heart disease populations. One study of repaired tetralogy of Fallot patients demonstrated strong correlations between extent of RV delayed enhancement and clinical markers of worse prognosis, including decreased exercise tolerance and ejection fraction, as well as more clinically documented arrhythmia.9 The same investigators had earlier shown similar findings in a cross sectional study of patients with repaired TGA.10 Concordant results were published by another group studying patients with TGA demonstrating reduced peak oxygen consumption in patients with the greatest amounts of RV delayed enhancement.11 Interestingly, although cardiopulmonary bypass is often invoked as the insult responsible for scar formation, this latter study measured similar scar mass in both the post‐atrial switch patients and the (never operated) congenitally corrected transposition (CC‐TGA) group, implying that myocardial hypertrophy outstripping myocardial perfusion reserve may be a more credible hypothesis.
In contrast, another group of workers who studied atrial switch and unoperated CC‐TGA found almost no evidence of scar either with delayed enhancement imaging or by resting perfusion as measured by positron emission tomography.12 These authors suggest that age at time of operative repair might explain the discrepancies between their findings and those of other groups. However, while increased myocardial vulnerability at a later age of repair might explain differences between atrial switch patients, it clearly cannot account for the apparent variability in the never operated CC‐TGA population. The true prevalence and significance of scar in these patients therefore remain to be determined.
Pulmonary vasculature and flow
Assessment of pulmonary arterial anatomy is vitally important, particularly so in the variant of tetralogy of Fallot characterised by ventricular septal defect and pulmonary atresia. The spectrum of pulmonary malformation includes hypoplasia or atresia of the main or segmental pulmonary arteries, non‐confluence of the left and right pulmonary arteries, and focal narrowings at any point in the branch pulmonary arteries. In the majority of severe cases, multiple collateral vessels may arise from the descending thoracic aorta (fig 4C), brachiocephalic and subclavian arteries, and occasionally from the abdominal aorta or coronary circulation. Surgical attempts to gather these multiple vessels and anastomose them directly to the distal pulmonary arteries require meticulous preoperative planning based on individual patient anatomy.
Figure 4 Arterial and venous anomalies. (A) Volume rendered computed tomography (CT) angiogram in a patient with congenitally corrected transposition of the great arteries. There is a single coronary artery (red arrow) supplying both ventricles. The dominant branch courses directly in front of the aorta (itself anterior in this condition) and thus immediately posterior to the sternum. This is important information before surgical intervention. This image also demonstrates the ability of CT to render metal and demonstrates both the valved pulmonary conduit (black arrow) as well as the biventricular pacing system (white arrow). (B) Volume rendered magnetic resonance angiogram (MRA). Example of Scimitar syndrome with partial anomalous pulmonary venous return from the entire right lung via a single large graining vein (white arrow) which enters the inferior vena cava (arrowhead). A common associated finding is a degree of systemic arterial supply to the lung parenchyma seen here as a thin vessel (red arrows) originating from the abdominal aorta close to the celiac axis and coursing towards the right lung base. (C) Volume rendered MRA of the thoracic aorta in a patient with ventricular septal defect and pulmonary atresia. A pseudo‐ductus (red arrow) gives origin to a stenosed hypoplastic vessel which then branches (white arrows) to supply the right middle and lower lobes. (D) Sub‐volume maximum intensity projection from MRA. Coronal view of the thorax to demonstrate the presence of both left and right sided superior vena cavae (SVC, white arrows) in this patient with transposition of the great arteries. It is useful for the surgeon to be aware of this preoperatively since it may be possible to baffle both SVC into the systemic venous atrium.
Choe et al studied 10 patients in whom the central pulmonary arteries had not been visualised at catheter angiography and showed that angiography had failed to demonstrate the main pulmonary artery in seven cases, the proximal left pulmonary artery in two cases, and the entire pulmonary arterial tree in one case.w10 The success of CMR was despite a protocol that employed only black blood static images in nine cases and gradient echo cine images in one case, techniques which would certainly be considered suboptimal today.
Several more recent reports have emphasised the value of CE‐MRA for vascular assessment. In one large study of 73 patients comparing CE‐MRA to conventional angiography, the former had an accuracy of 95% for the detection of branch pulmonary artery stenosis, with lower inter‐ and intra‐observer variability for measurements compared to angiography.w11 This technique is also accurate for the detection of aortopulmonary collaterals.13
Venous phase angiography is an integral part of most MRA acquisitions and is possible because of the persistence of gadolinium in the blood pool beyond the first pass. Since the central and pulmonary venous structures are in close proximity to the arterial vessels, the same volume acquisition can be performed twice with a breath hold for each. One prospective study comparing MRA to catheter findings in patients with partial anomalous pulmonary venous drainage (fig 4B) found the former more accurate against a surgical gold standard.14
Accurate measurement of pulmonary blood flow is important in many congenital heart disease patients. In the recent past, lung perfusion scintigraphy has been used for this purpose, but it incurs a radiation dose and may need to be performed many times—for example, in the follow up to pulmonary intervention. CMR is able to measure flow accurately and quickly using phase contrast imaging. This has been shown to be accurate in normalw12 and stenotic pulmonary arteries15 as well as in patients with classic, partial or total cavopulmonary connections.w13
Aorta
One of the most common referrals for CMR in the congenital heart disease population is for assessment of the aorta. There exists a large population of young adults with repaired coarctation, of whom a proportion will have restenosis or late complications of surgery (figs 3 and 5). Clinically significant coarctation can be detected by CMR with a high degree of reliability compared to invasive catheter angiography (fig 3A,B).16 Likewise both CT and CMR demonstrate the entirety of the aorta which allows for comment on aortopathy, valve morphology, arch hypoplasia and patch aneurysm (fig 5C–F).
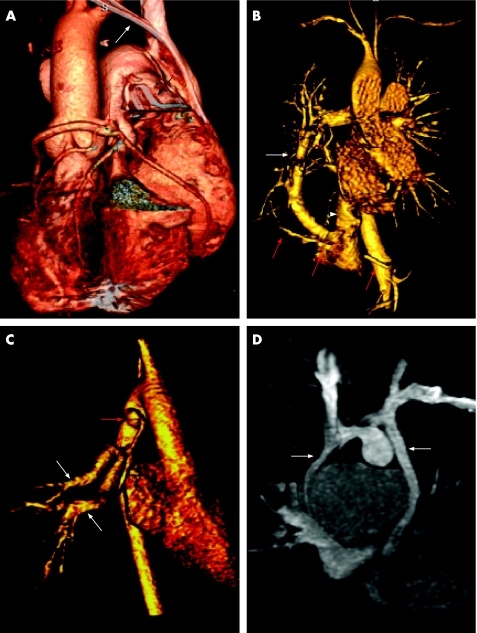
Figure 5 Spectrum of disease in aortic coarctation. (A) Maximum intensity projection (MIP) from magnetic resonance angiogram (MRA) demonstrating a tight primary coarctation of the aorta with numerous collateral vessels and enlarged internal mammary arteries. (B) Volume rendered dataset from an MRA dataset in a patient with an aortic interruption undiscovered until adult life. It was not possible to pass a wire from the distal to proximal segment of the aorta at the time of catheterisation. Ultra thin slice cardiac computed tomography (CT) confirmed that there was no direct communication between the two segments. (C) Sub‐volume MIP from an MRA. A prior coarctation repair has occurred. Dilatation of the synthetic material used has over time resulted in a large patch aneurysm (red arrow) requiring further repair. (D–F) Small aneurysm at the anastomotic line (red arrow) in another patient with previous patch repair. Four years later this had enlarged considerably and was embolised with coils at catheter angiography (E, red arrow). The aneurysm and foreign material are easily followed up for change on CT examination (F, red arrow). (G) Volume rendered MRA. An unusual form of coarctation repair in which an “elephant trunk” graft is used to bypass an interrupted or severely hypoplastic arch in addition to a coarctation. The synthetic tube graft extends from the ascending to the descending thoracic aorta (asterisks). (H) Volume rendered MRA showing a more conventional tube graft repair (asterisks). Resection with end‐to‐end anastomosis is more common today than use of synthetic materials. (I) Bright blood cine images to show the closed (upper) and open appearances of the aortic valve in a coarctation patient. The right, left and non‐coronary cusps are labelled. On the bottom image there is evidence of a partial fusion creating a raphe (white arrow) between the right and left coronary cusps, resulting in a functionally bicuspid valve (fish mouth appearance outlined in red).
CMR phase contrast studies can provide direct information regarding stenosis severity by measurement of peak velocity across the narrowed area; this converts to pressure drop through use of the Bernouilli equation. Aortic flow measurements made just distal to a coarctation and again at the level of the diaphragm may demonstrate a step up in bulk flow consistent with a significant collateral network.
Measurements of aortic diameter and proximity of the head and neck vessels to the stenosis allow for planning when a percutaneous intervention is envisaged. CMR fails, however, in the setting of stented coarctation repair where the presence of stainless steel results in local magnetic field distortion such that the stented portion is totally obscured (fig 3E).
Aneurysmal dilatation of the aorta is equally well suited to CMR assessment. Marfan syndrome patients not only suffer a risk of sudden death from aortic root dissection but may subsequently develop aneurysm or dissection of other major vessels. Large field‐of‐view MRA enables a fast screen of the entire thoracic and abdominal aorta in a single breath hold and should be offered to this patient group every 3–5 years. CMR also facilitates detection of many of the secondary signs of Marfan syndrome, including thoracic cage deformities, vertebral anomalies and dural ectasia.
One of the major advantages of CMR in the longitudinal follow up of aortic conditions is the lack of ionising radiation. These patients are invariably young and require serial imaging over a lifetime. CT should be reserved for cases where a thoracic aortic stent graft has been placed (fig 3D), or where there are other standard contraindications to CMR. This ideal situation may not always pertain, depending on local availability of equipment and expertise. An argument can be made for consistency in choice of modality for follow up. It has been reported that swapping between CT and CMR may increase inter‐technique variability by up to 11 mmw14—this degree of variability can be substantially reduced by use of an ECG gated technique for either modality. Such measurement discrepancies make an argument for congenital imaging to occur in a single expert centre where a consistent approach is taken.
Coronary arteries
Coronary angiography by CMR remains in evolution. Numerous different strategies have been applied in an attempt to compensate for artefact from respiratory and cardiac motion, with variable success. High heart rates in children, and the need for very high spatial resolution, further limit the applicability of CMR, although one group has published encouraging results in children followed up after arterial switch (Jatene repair) for TGA.17
CT may be a reasonable alternative, particularly if dose modulating techniques are used. One large Chinese study compared electron beam CT to catheterisation in over 200 children with congenital diagnosesw15 and showed an overall diagnostic accuracy of 83%. Tellingly, most of the failures were in younger children with small vessels and rapid heart rates.
Coronary anomalies are relatively frequent in the congenital heart disease population. In many cases they are of little or no significance and may be “normal” for a particular condition. The one major exception to this is an anomalous left coronary artery or large conus branch passing directly in front of an enlarged RV, and thus running immediately posterior to the sternum (fig 4A). Unsuspected, there may be serious consequences at the time of sternotomy.
Coronary anomalies in adults are generally readily detectable with CMR using standard techniques (fig 6A–D). Even so there remain occasions when the very high spatial resolution of CT is advantageous for defining the exact course of the vessel. This is particularly true when the anomalous artery has an abnormal fistulous communication to another vessel or chamber (fig 6E).
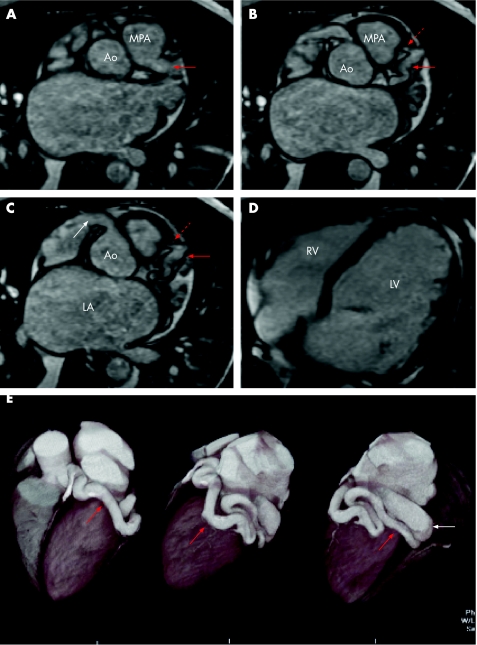
Figure 6 Axial steady state free precession (SSFP) sequential magnetic resonance (MR) images showing two unusual coronary anomalies. (A) A large tubular structure (red arrow) extends from the posterolateral aspect of the main pulmonary artery (MPA). Ao, aorta. (B) The vessel is seen to run in the distribution of the left anterior descending artery (solid red arrow). A smaller side branch is visible heading back towards the right ventricular outflow tract (dotted red arrow). This is the ALCAPA syndrome (anomalous left coronary artery from the pulmonary artery), which may result in coronary steal and ischaemia. The usual treatment is disconnection from the pulmonary artery and subsequent left internal mammary artery graft. (C) At a slightly lower level than B the right coronary artery (RCA) origin is visible (white arrow). This is much larger than normal and implies that there is increased volume flow through this vessel as a result of a fistulous connection (not shown) between it and the abnormally low pressure left coronary circulation. (D) Blood flows from high to low pressure—that is, from the aorta via the RCA into the left coronary circulation retrogradely up the left anterior descending and circumflex arteries and into the right ventricular outflow tract. As a consequence there is a large left to left shunt with considerable left ventricular dilatation. Left atrial (LA) dilatation is also apparent on images A–D. LV, left ventricle; RV, right ventricle. (E) Volume rendered computed tomography (CT) angiogram images from a different patient. This patient with a dilated right heart underwent CT after MRI had demonstrated an enlarged circumflex artery suggestive of a fistula. It was not possible to be certain on the MRI where the point of drainage was. High resolution gated cardiac CT, however, beautifully and unequivocally showed the vessel (red arrow) to be draining into the coronary sinus (white arrow).
Interventional CMR: the future
Hybrid angiography and CMR suites—so called XMR labs—comprise not only the standard CMR and digital angiographic equipment which are placed side by side in two adjacent rooms, but also a “floating” table top designed to move from one piece of imaging equipment to the other in a smooth movement. Thus, it is possible to begin a procedure under one form of image guidance and complete it under the other, or even to move the patient back and forth as required at various points in the procedure. Several groups have demonstrated the feasibility of this arrangement and claim that using partial CMR guidance results in a lower overall radiation dose to the patient.18
MR guided intervention is attractive because its three dimensional nature allows accurate positioning of catheters and devices in a way that is difficult with two dimensional angiography. However, there are currently limitations which relate to catheter tracking in the CMR environment. Standard angiographic catheters are generally unsuitable since they contain ferromagnetic elements and require non‐CMR compatible guidewires. Specially designed catheters are employed which are tracked under CMR either passively—that is, they contain a substance which either generates a signal or causes local signal loss in the magnetic field—or actively, in which case the catheter itself is designed to act as a CMR coil generating signal. Unfortunately, local tissue heating remains a cause for concern with active catheters and may limit the duration of CMR fluoroscopy. Passive tracking employing a balloon catheter filled with an iron oxide compound was used in an exciting study published last year in which five adult patients with coarctation of the aorta underwent stent placement under CMR guidance with a high degree of success.19
Another mooted advantage of MR guided catheterisation in adult congenital heart disease is the ability to measure flow directly using the cine phase contrast technique outlined earlier. Response to various pulmonary vasodilators may be promptly evaluated without the underlying assumptions made by conventional methods. Nonetheless, calculation of pulmonary vascular resistance still requires knowledge of pulmonary artery pressure which cannot yet be measured reliably by CMR.w16 Muthurangu et al demonstrated the feasibility of the combined XMR approach in 24 paediatric patients and showed not only that cine phase contrast measurements were more accurate than flow measurements made using the Fick principle, but that there was a highly significant reduction in mean radiation dose to the patients compared to historical controls.20 However, pulmonary artery catheter placement still required fluoroscopic x ray guidance in over half the cases, and mean procedure time was reported as in excess of 2 h in these anaesthetised children. Thus, although interventional CMR appears promising, it is some years away from being a genuine standalone alternative to catheterisation.
Conclusion
CMR and CT imaging have revolutionised the management of children and adults with congenital heart disease. CMR in particular is ideally suited to post‐surgical follow up examinations in this relatively radiosensitive population. CT provides excellent anatomical information but little functional data and is best reserved for those for whom CMR is not possible. This type of imaging should be performed by experienced professionals with proper training in the pathophysiology of congenital heart disease as well as training in radiological techniques, equipment and post‐processing tools. A cardio‐radiological alliance is likely to result in the best possible service for the end user—the patient.
Additional references appear on the Heart website— http://heart.bmj.com/supplemental
INTERACTIVE MULTIPLE CHOICE QUESTIONS
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs).
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
Copyright © 2007 BMJ Publishing Group and British Cardiovascular Society.
Supplementary Material
Acknowledgements
Dr Naeem Merchant, Dr Narinder Paul and Dr Yves Provost (cardiac imaging) and the adult congenital heart disease physicians and surgeons at Toronto General Hospital, Canada. Dr John Thomson and Dr Kate English (ACHD) Leeds General Infirmary, UK.
Footnotes
Funding: Salaried by University of Leeds. No industry support to declare.
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article. The author declares that the answer to the questions on your competing interest form are all No and therefore has nothing to declare
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
References
- 1.Pereles F S, Kapoor V, Carr J C.et al Usefulness of segmented trueFISP cardiac pulse sequence in evaluation of congenital and acquired adult cardiac abnormalities. AJR Am J Roentgenol 20011771155–1160.Study evaluating older and newer forms of bright blood imaging, emphasising the particular strengths of steady state free precession imaging which remains the central part of any congenital CMR examination. [DOI] [PubMed] [Google Scholar]
- 2.Knauth A L, Gauvreau K, Powell A J.et al Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart 2006 Nov 29; [Epub ahead of print]Demonstrated that both increased RV size and decreased ejection fraction were independent predictors of poor outcome in tetralogy of Fallot patients. [DOI] [PubMed]
- 3.Warnes A. Transposition of the great arteries. Circulation 20061142699–2709.Detailed review of the pathophysiology and long term complications in transposition patients. [DOI] [PubMed] [Google Scholar]
- 4.Alfakih K, Plein S, Thiele H.et al Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady‐state free precession imaging sequences. J Magn Reson Imaging 200317323–329. [DOI] [PubMed] [Google Scholar]
- 5.Tandri H, Daya S K, Nasir K.et al Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol 2006981660–1664. [DOI] [PubMed] [Google Scholar]
- 6.Alfakih K, Thiele H, Plein S.et al Comparison of right ventricular volume measurement between segmented k‐space gradient‐echo and steady‐state free precession magnetic resonance imaging. J Magn Reson Imaging 200216253–258.Important demonstration of the importance of allowing for changing methodologies when performing serial measurements of ventricular volume and function. [DOI] [PubMed] [Google Scholar]
- 7.Therrien J, Provost Y, Merchant N.et al Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol 2005956779–782.Small observational study from the Toronto group suggesting that although favourable remodelling of the RV may occur post‐pulmonary valve replacement, the RV fails to normalise if the preoperative end diastolic volume is too great. [DOI] [PubMed] [Google Scholar]
- 8.Kim R J, Fieno D S, Parrish T B.et al Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 19991001992–2002.Classic paper demonstrating the extraordinarily high degree of correlation between infarct size as measured with the delayed enhancement technique compared to the extent of myonecrosis demonstrated by histopathology. [DOI] [PubMed] [Google Scholar]
- 9.Babu‐Narayan S V, Kilner P J, Li W.et al Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome. Circulation 2006113405–413. [DOI] [PubMed] [Google Scholar]
- 10.Babu‐Narayan S V, Goktekin O, Moon J C.et al Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation 20051112091–2098. [DOI] [PubMed] [Google Scholar]
- 11.Giardini A, Lovato L, Donti A.et al Relation between right ventricular structural alterations and markers of adverse clinical outcome in adults with systemic right ventricle and either congenital complete (after Senning operation) or congenitally corrected transposition of the great arteries. Am J Cardiol 2006981277–1282. [DOI] [PubMed] [Google Scholar]
- 12.Fratz S, Hauser M, Bengel F M.et al Myocardial scars determined by delayed‐enhancement magnetic resonance imaging and positron emission tomography are not common in right ventricles with systemic function in long‐term follow up. Heart 2006921673–1677.One of the few published papers to suggest that scar is an uncommon finding in the systemic RV; as such it stands in direct contradiction to references 10 and 11 above. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geva T, Greil G F, Marshall A C.et al Gadolinium‐enhanced 3‐dimensional magnetic resonance angiography of pulmonary blood supply in patients with complex pulmonary stenosis or atresia: comparison with x‐ray angiography. Circulation 2002106473–478. [DOI] [PubMed] [Google Scholar]
- 14.Prasad S K, Soukias N, Hornung T.et al Role of magnetic resonance angiography in the diagnosis of major aortopulmonary collateral arteries and partial anomalous pulmonary venous drainage. Circulation 2004109207–214.Study showing that three dimensional MRA techniques are accurate for the diagnosis and assessment of pulmonary venous anomalies and major aortopulmonary collateral arteries compared to conventional cardiac catheterisation. [DOI] [PubMed] [Google Scholar]
- 15.Sridharan S, Derrick G, Deanfield J.et al Assessment of differential branch pulmonary blood flow: a comparative study of phase contrast magnetic resonance imaging and radionuclide lung perfusion imaging. Heart 200692963–968.Showed excellent agreement between cine MR phase contrast and the previous reference standard—nuclear imaging—for measurements of pulmonary blood flow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen J C, Powell A J, Gauvreau K.et al Magnetic resonance imaging predictors of coarctation severity. Circulation 2005111622–628. [DOI] [PubMed] [Google Scholar]
- 17.Taylor A M, Dymarkowski S, Hamaekers P.et al MR coronary angiography and late‐enhancement myocardial MR in children who underwent arterial switch surgery for transposition of great arteries. Radiology 2005234542–547.Demonstrated the feasibility of free breathing MR coronary angiography in a young population with reasonable overall results and (in some cases) exceptionally good image quality. [DOI] [PubMed] [Google Scholar]
- 18.Razavi R, Hill D L, Keevil S F.et al Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet 20033621877–1882.Pilot study exploring the feasibility of MRI guided catheterisation for a range of indications and demonstrated a lower radiation dose for these patients compared to controls. [DOI] [PubMed] [Google Scholar]
- 19.Krueger J J, Ewert P, Yilmaz S.et al Magnetic resonance imaging‐guided balloon angioplasty of coarctation of the aorta: a pilot study. Circulation 20061131093–1100.Fascinating description of MR guided procedures in five coarctation patients with generally good results. In this paper very little of the procedure occurred under conventional fluoroscopy. [DOI] [PubMed] [Google Scholar]
- 20.Muthurangu V, Taylor A, Andriantsimiavona R.et al Novel method of quantifying pulmonary vascular resistance by use of simultaneous invasive pressure monitoring and phase‐contrast magnetic resonance flow. Circulation 2004110826–834.An example of the XMR concept being put into practice in order to generate pulmonary vascular resistance measurements from a combination of data acquired by both CMR and conventional catheterisation. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.