Abstract
Objective
Myocardial scintigraphy and/or conventional angiography (CA) are often performed before cardiac surgery in an attempt to identify unsuspected coronary artery disease which might result in significant cardiac morbidity and mortality. Multidetector CT coronary angiography (MDCTCA) has a recognised high negative predictive value and may provide a non‐invasive alternative in this subset of patients. The aim of this study was to evaluate the clinical value of MDCTCA as a preoperative screening test in candidates for non‐coronary cardiac surgery.
Methods
132 patients underwent MDCTCA (Somatom Sensation 16 Cardiac, Siemens) in the assessment of the cardiac risk profile before surgery. Coronary arteries were screened for ⩾50% stenosis. Patients without significant stenosis (Group 1) underwent surgery without any adjunctive screening tests while all patients with coronary lesions ⩾50% at MDCTCA (Group 2) underwent CA.
Results
16 patients (12.1%) were excluded due to poor image quality. 72 patients without significant coronary stenosis at MDCTCA were submitted to surgery. 30 out of 36 patients with significant (⩾50%) coronary stenosis at MDCTCA and CA underwent adjunctive bypass surgery or coronary angioplasty. In 8 patients, MDCTCA overestimated the severity of the coronary lesions (>50% MDCTCA, <50% CA).
No severe cardiovascular perioperative events such as myocardial ischaemia, myocardial infarction or cardiac failure occurred in any patient in Group 1.
Conclusions
MDCTCA seems to be effective as a preoperative screening test prior to non‐coronary cardiac surgery. In this era of cost containment and optimal care of patients, MDCTCA is able to provide coronary vessel and ventricular function evaluation and may become the method of choice for the assessment of a cardiovascular risk profile prior to major surgery.
Since its introduction in the 1960s,1 conventional coronary angiography (CA) has been considered the gold standard for the diagnosis of coronary artery disease because of its high contrast, temporal and spatial resolution.2,3,4 In the past few years, we have witnessed a considerable increase in diagnostic and interventional procedures. Despite the high degree of accuracy (73–89%) of non‐invasive diagnostic tests such as exercise ECG, myocardial scintigraphy and stress‐echocardiography in detecting myocardial ischaemia,5 about 20% of patients undergoing CA due to a positive result of these non‐invasive tests, had no evidence of coronary lesions.6,7
Multidetector CT (MDCT), introduced into clinical practice in 2000, has demonstrated excellent technical characteristics for coronary artery evaluation. Results in the literature show a high degree of diagnostic accuracy in detecting significant coronary lesions and, particularly, an excellent capability of excluding them, due to negative predictive values ranging from 96 to 99%.8,9,10,11,12,13,14,15,16,17,18
Patients who are candidates for major non‐coronary cardiac or vascular surgery, such as heart valve replacement, aortic aneurysm and aortic dissection, require a complete assessment of potentially dangerous co‐morbidities. There is a 5 to 10% perioperative cardiac morbidity rate during vascular surgery, even in patients at low risk for coronary disease.19 According to Paul et al.20 there is a 17% risk of severe multivessel disease in low clinical risk asymptomatic patients undergoing vascular surgery. American College of Cardiology/American Heart Association (ACC/AHA) guidelines for preoperative evaluation before major surgery recommend stratification of ischaemic heart disease with clinical and non‐invasive tests.19,20,21,22 The diagnostic accuracy is 68 to 77% for exercise ECG and 73 to 85% for stress‐echocardiography. Myocardial scintigraphy provides an accuracy of 87–89% in patients with normal resting ECG, with a radiation exposure ranging from 4.6 to 20 mSv,23 almost equivalent to MDCT coronary angiography (MDCTCA). However, for certain high‐risk patients, ACC guidelines suggest proceeding directly with coronary angiography rather than performing a non‐invasive test. Therefore, in clinical practice, CA is often performed before major vascular or cardiac surgery. Considering that millions of surgical procedures are probably performed every year worldwide (eg, 95 000 heart valve replacements/year in the USA),6,7 several hundred thousand negative CAs are still performed.
After some years of validation studies comparing MDCTCA with CA, studies on clinical utility are now warranted to demonstrate whether and how this technique can change and improve the current management of patients. The purpose of this study is to evaluate the clinical impact of MDCTCA as a preoperative screening test for cardiac risk assessment in patients who are candidates for major non‐coronary cardiac surgery and who are asymptomatic for ischaemic heart disease.
Materials and methods
Population
Between September 2004 and December 2005 143 patients (118 men and 25 women, mean age 61.3 years, range 33–81) who were candidates for non‐coronary cardiac surgery, were asymptomatic and had no history of angina or ischaemic heart disease, were enrolled in the study. Demographic data of the study population are reported in table 1. The clinical study was approved by the hospital ethical committee, and written informed consent was obtained from all subjects before inclusion. Patients with angina, known ischaemic heart disease, arrhythmias, and those having contraindications for beta‐blocker administration as well as those with serum creatinine levels of more than 1.5 mg/dl were not included in the study.
Table 1 Demographics, clinical and CT findings of the entire study population*, Group 1 (patients underwent MDCT scan only) and Group 2 (patients also underwent CA).
| Study population* | Group 1 | Group 2 | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Population | 132 | 72 | 44 | |
| Men (%) | 111 (84.1) | 58 (80.6) | 38 (86.4) | 0.582 |
| Age mean, years (SD) | 60.8 (11.7) | 56.2 (13.2) | 65.5 (10.8) | 0.001 |
| Disease (%) | 0.954 | |||
| Aortic valve insufficiency/stenosis | 34 (25.7) | 20 (27.8) | 9 (20.5) | |
| Mitral valve insufficiency/stenosis | 17 (12.9) | 9 (12.5) | 7 (15.9) | |
| Ascending aorta aneurysm | 62 (47.0) | 34 (47.2) | 20 (45.4) | |
| Aortic dissection type A or B | 19 (14.4) | 9 (12.5) | 8 (18.2) | |
| Co‐morbidities (%) | ||||
| COPD | 7 (5.3) | 3 (4.2) | 3 (6.8) | 0.846 |
| Diabetes | 12 (9.1) | 6 (8.3) | 4 (9.1) | 0.842 |
| Hypertension | 88 (66.7) | 42 (58.3) | 30 (68.2) | 0.388 |
| Dyslipidosis | 37 (28.0) | 18 (25.0) | 14 (31.8) | 0.560 |
| Smoking | 47 (35.6) | 22 (30.5) | 19 (43.2) | 0.238 |
| Family history | 29 (21.9) | 15 (20.8) | 10 (22.7) | 0.994 |
| EuroSCORE (SD) | 6.2 (0.9) | 5.8 (0.9) | 6.5 (0.8) | 0.0022 |
| CT calcium scoring (Agatston score) | ||||
| Mean Agatston value (SD) | 150.3 (192.7) | 111.2 (154.7) | 214.4 (255.0) | |
| Median (min–max) | 52 (0–814) | 35.5 (0–579) | 67 (0–814) | 0.013 |
| CT angiography (quality score)* | 0.029 | |||
| Score 1 (excellent) | 47 (35.6) | 36 (50%) | 11 (25%) | |
| Score 2 (good) | 36 (27.3) | 19 (26.4%) | 17 (38.6%) | |
| Score 3 (moderate) | 33 (25.0) | 17 (23.6%) | 16 (36.4%) | |
| Score 4 (poor) | 10 (7.6) | – | – | |
| Score 5 (not assessable) | 6 (4.5) | – | – | |
| CT left ventricular function (EF)† | ||||
| EF >50% | 78 (67.2) | 51 (70.8) | 27 (61.4) | 0.395 |
| EF ⩽50% | 38 (32.8) | 21 (29.2) | 17 (38.6) | |
| Mean EF (SD) | 55.3 (27.5) | 56.5 (29.1) | 52.4 (12.4) | 0.378 |
*n = 132 Patients who underwent MDCTA scan (total number of patients = 143). †n = 116 Patients who had an MDCT scan of diagnostic quality.
COPD, chronic obstructive pulmonary disease; EF, ejection fraction.
Preoperative risk assessment
Preoperative evaluation has been focused on the analysis of cardiovascular risk factors (hypertension, dyslipidosis, smoking, diabetes, family history, obesity), co‐morbidities (chronic obstructive pulmonary disease (COPD), renal insufficiency) and surgical risk factors (re‐intervention, emergency, type of surgery). All of these clinical data have been integrated with ECG, echocardiography and CT results and combined in a grading score system for preoperative cardiac risk assessment according to the EuroSCORE.24
The clinical data of the study population are summarised in table 1.
MDCT scan protocol and image analysis
An 18–20‐Gauge cannula was positioned in an antecubital vein and patients with a heart rate (HR) greater than 65 bpm received a beta‐blocker (metoprolol, Seloken, 5–15 mg intravenously) 5 to 15 minutes before the CT scan; subjects with HR greater than 65 bpm after beta‐blocker premedication did not undergo CT evaluation.
CT scans were obtained using a 16‐row MDCT scanner (Somatom Sensation 16 Cardiac, Siemens, Forchheim, Germany). A pre‐contrast scan was made to determine the total calcium burden of the coronary tree (16×1.5 mm collimation, with a 3.8 mm/rotation table feed and 133 mAs tube current, at 120 kV). An Agatston score of more than 1000, calculated by specific software (Syngo Ca Score, Siemens Medical Solution, Forchheim, Germany) was considered an adjunctive exclusion criteria for performing MDCTCA.16 An angiographic ECG‐gated scan of the coronary arteries was then performed with the following parameters: collimation of 16×0.75 mm, gantry rotation time of 375 ms, tube voltage of 120 kV, tube current–time product of 650–750 mAs, table feed/rotation of 3.0 mm and in a cranio‐caudal direction from the carina to the diaphragm during a single breath‐hold. Ninety millilitres of contrast media (400 mgI/ml, Iomeron 400, Bracco, Milan, Italy) were administered through the 18–20 G cannula previously positioned in an antecubital vein at a rate of 4 ml/sec, followed by 40 ml saline bolus chaser using a double‐syringe power injector (Stellant, MedRad, Pittsburgh, USA). With the bolus tracking technique (CARE bolus, Siemens Medical Solution, Forchheim, Germany), the scan automatically started 5 seconds after a threshold attenuation of +100 Hounsfield units was reached in a region of interest previously positioned in the ascending aorta. Coronary angiography acquisition time ranged from 16 to 20 seconds and, during the scan, an ECG trace was obtained.
Acquired volume was then reconstructed with an effective slice thickness of 1 mm, a reconstruction interval of 0.5 mm and a field of view of 200 mm, normally using a medium Kernel convolution filter (B31, soft tissue). Images were reconstructed by means of retrospective synchronisation based on the previously acquired ECG. The standard reconstruction temporal windows used were 60, 65, 70 and 75% of the R–R interval. A multiphase reconstruction for left ventricular function evaluation was also carried out (10 phases, from 0 to 90% of the R–R interval, in a short axis plane, covering the whole left ventricle from the valvular plane to the apex, with 8–10 mm thickness and 2–4 mm interval depending on heart size.
Images were sent to a dedicated workstation (Wizard, Siemens, Forchheim, Germany) and all the axial images of the reconstructed datasets (60, 65, 70 and 75% of R–R interval) were simultaneously visualised and examined by two expert cardiovascular radiologists in consensus and the dataset having the best image quality for each coronary vessel was sought; if all of the standard reconstruction intervals were considered suboptimal in quality, adjunctive datasets were reconstructed (eg, 25, 30 or 35% of R–R interval).
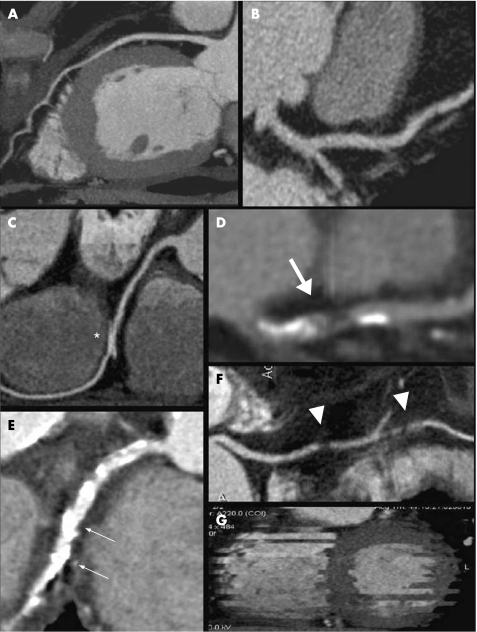
Axial images, multiplanar reconstructions, curved multiplanar reformation and 3D volume rendering were used for coronary evaluation, as well as specific software for vascular stenosis quantification (Syngo Vessel View, Siemens Medical Solution, Forchheim, Germany). Two expert cardiovascular radiologists, blinded to clinical data (risk factors and co‐morbidities), in consensus, assessed all of the MDCTCA scans. Left main (LM), left anterior descending (LAD), left circumflex (LCX) and right coronary artery (RCA) including 1.5 mm side branches were evaluated for stenosis equal to or greater than 50%, using the coronary segmentation proposed by the AHA as a model.25 In case of disagreement between the observers regarding the percentage of stenosis, results of the “Syngo Vessel View” analysis were utilised as a reference. For each vessel, the image quality was scored in terms of artefacts and visibility as follows: 1, excellent; 2, good (minor artefacts); 3, moderate (moderate artefacts such as blurring and stair‐step owing to motion or blooming because of mild calcifications); 4, poor (severe artefacts due to heavy calcifications or cardiac motion); 5, not assessable (fig 1). To establish a global “examination” quality score, the highest coronary vessel score was used as the reference for any patient.
Figure 1 MDCT multiplanar reconstruction images showing samples of the image quality score. (A) Score 1 (excellent quality). (B) Score 2 (good quality) due to a slight blurring of vessel edges. (C) Score 3 (moderate quality) due to cardiac motion (*). (D) Score 3 (moderate quality) due to blooming artefacts derived from calcifications (large arrow). (E) Score 4 (poor quality) due to severe calcifications (blooming, arrows). (F) Score 4 (poor quality) due to extensive blurring of the vessel edge with gaps (arrowheads). (G) Score 5 (not assessable) due to arrhythmias during the CT scan. The vessel is not visualised.
The left ventricular function (left ventricular end‐systolic volume (ESV), end‐diastolic volume (EDV), stroke volume (SV = EDV–ESV), ejection fraction (EF = SV/EDV x 100) and myocardial mass) was also evaluated by another cardiovascular radiologist using specific software (Syngo Argus, Siemens Medical Solution, Forchheim, Germany). In all patients, EF results of CT and echocardiography were compared: in case of disagreement between these two techniques, CT results were used as the reference. In view of the aim of the study and considering the high negative predictive value (96–99%) of coronary CT angiography reported in the literature,8,9,10,11,12,13,14,15,16,17,18 all patients without significant stenosis at CT scan underwent surgery without CA, while CA was performed before surgery in all patients with coronary stenosis ⩾50%, in order to confirm the CT data and proceed with revascularisation (percutaneous transluminal coronary angioplasty, PTCA ).
The CA procedures were performed under normal routine conditions at an interval of 1–6 days after the CT scan. The angiograms were assessed with QCA software (ACA, Philips Medical Systems) by an expert cardiologist unaware of the MDCTA results. Any treatment decisions such as PTCA, stent placement or coronary artery bypass grafting were based only on CA results.
Perioperative patient management
Patients were strictly monitored from the operative room until hospital discharge. Patients have been monitored by continuous intra‐arterial blood pressure and pulse oximetry and continuous 12‐lead ECG recording, echocardiography and chest x ray immediately after intervention and during the intensive‐care stay (approximately 72 h). During this period, simultaneous measurement of cardiac biomarkers with higher sensitivity in early (CK‐MB at 1, 3, 6 and 9 h) and late (cardiac troponin‐I, every 6 h) phases of myocardial infarction have been performed. Once out of intensive care until discharge, ECG and blood pressure were monitored routinely twice daily.
Standard pharmacological therapies, according to baseline diseases, were not discontinued. In patients with coronary lesions (even if <50% of stenosis) depicted by MDCT, aspirin was administered (100 mg daily), together with statin therapy (plaque stabilisation) and beta‐blockers, if allowed by clinical condition and/or pharmacological interaction.
Identification of adverse events
Any perioperative (within 30 days) adverse events were analysed from the medical records, with particular attention paid to myocardial infarction, myocardial ischaemia and cardiac failure.
Cardiac biomarker measurement
A cardiac troponin‐I value higher than 1.2 ng/ml (10 times higher than the cut‐off value of 99th percentile in our laboratory) and/or a CK‐MB value 10% more than the CK value were considered indicative for myocardial infarction.
Definition of perioperatve myocardial ischaemia
Myocardial ischaemia was defined as an ST‐segment elevation (⩾2 mm in V1–V2 or V3 leads and ⩾1 mm in other leads), or ST‐segment depression (⩾1 mm) in at least two contiguous leads or symmetric inversion of T‐waves (⩾1 mm) in at least two continuous leads.
Definition of perioperative myocardial infarction
Myocardial infarction was defined as a significant biomarker alteration together with ischaemic ECG changes, new Q‐wave changes (⩾30 ms in two continuous leads) or new wall motion abnormalities depicted by echocardiography. This definition conforms to the newly formulated ESC/ACC consensus for the redefinition of myocardial infarction.26
Definition of cardiac death
Cardiac death was defined as death secondary to myocardial infarction, arrhythmias or cardiac failure.
Statistical analysis
Statistical analysis was performed using SPSS for Windows, version 13.00 (SPSS Inc, Chicago, Illinois, USA); continuous variables were expressed as mean ±SD or median and range where appropriate; categorical data were expressed as percentages.
Student t test with Levene test for assessing homoscedasticity or Mann–Whitney U test and Χ2 test or Fisher exact test were used to compare continuous and categorical variables between groups, respectively.
All p values refer to two‐tailed tests of significance. A value of p<0.05 was considered significant.
A positive predictive value (PPV) of MDCTCA was also obtained from patients who underwent both diagnostic tests (Group 2).
Bland‐Altman analysis was performed to calculate limits of agreement between CT and echocardiography in left ventricular function assessment.
Results
Before MDCTCA pre‐medication with beta‐blockers was necessary in 64.3% (92/143) of the patients because of a HR>65 bpm. Six patients (4.2%) were excluded from the study due to persistent high HR after pre‐medication.
Five patients were excluded from the study due to a calcium score >1000 and all patients with an image quality score of 4–5 (poor–not assessable, n = 16, 12.2%) were excluded from coronary CT evaluation. In 6 patients, the examination quality was “not assessable” (score 5) due to arrhythmias (n = 4) or breathing (n = 2), while 10 patients presented “poor” image quality (score 4) due to extensive calcifications (blooming artefacts, n = 7), cardiac motion (blurring artefacts, n = 2) or a contrast‐filled superior vena cava (beam hardening artefacts, n = 1).
Left ventricular EF was >50% in 78/116 (67.2%). and <50% in 38/116 (32.8%) patients. Left ventricular global function (EF) results were used as an additional parameter for a better definition of cardiovascular risk: patients with decreased function were considered at high risk for perioperative heart failure and were strictly monitored by transoesophageal echocardiography perioperatively and also in the postoperative period. EF values correlated well with MDCT and echocardiography. At Bland‐Altman analysis 95% confidence intervals ranged from –8.3 to 8.9%. In case of significant disagreement between these two techniques (9/116 patients), CT values were used for clinical assessment.
Patients with an exam quality score of 1 to 3 and without stenosis or with stenosis <50% (72/116, 62.1%) underwent surgery without CA (Group 1, fig 2) while patients with a quality score of 1 to 3 and stenosis ⩾50% (44/116, 37.9%) underwent CA before surgery (Group 2, fig 3). No significant differences (p>0.05) regarding demographics and clinical data were found between the two groups, except for age, Agatston score and EuroSCORE, which were significantly (p<0.05) higher in Group 2 (stenosis >50% at MDCTCA) (table 1).
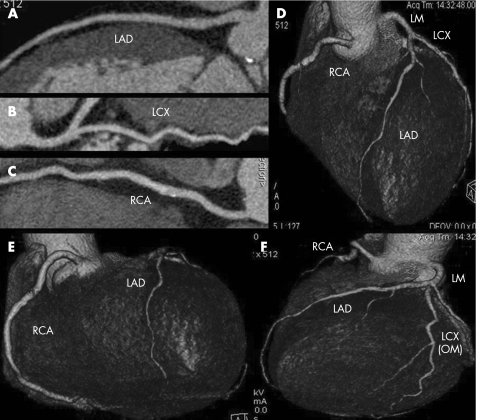
Figure 2 CT coronary angiography of a 63‐year‐old man with an aortic aneurysm. Multiplanar curved reconstructions (A,B,C) and volume rendering images (D,E,F) show the absence of any significant lesions in the coronary tree. This patient underwent surgery without conventional angiography.
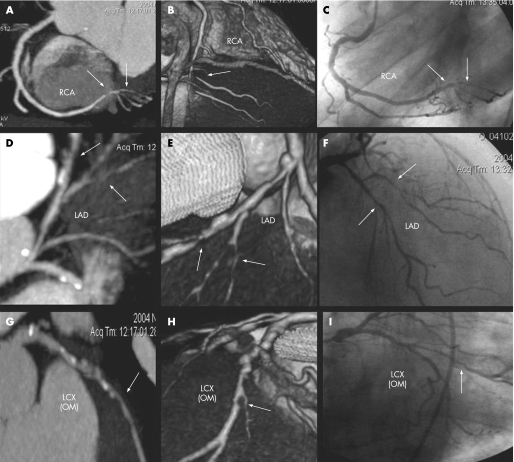
Figure 3 A 71‐year‐old‐man with mitral insufficiency. CT multiplanar curved reconstructions (maximum intensity projection thin, A,D,G), CT volume rendering images (B,E,H) and conventional angiography images (C,F,I) showing significant lesions (arrows) in right coronary artery (RCA), left anterior descending artery (LAD) and the obtuse marginal (OM) branch of the left circumflex artery (LCX). In this case, coronary artery bypass graft was added to the surgery (mitral valve replacement).
Group 1 (exam quality score 1–3, coronary lesions <50%, 72 patients)
Thirty‐nine patients did not have any coronary lesions while 33 patients presented with subcritical (<50%) coronary lesions. Of the 59 total subcritical stenoses, 29 were located in the proximal LAD, CX or RCA, 19 in the middle and 11 in distal segments.
Group 2 (exam quality score 1–3 and coronary lesion >50%, 44 patients)
Significant coronary lesions identified by CT scan in patients with a diagnostic image quality score (1–3) were confirmed by CA in 36/44 (81.8%) cases, with a positive predictive value of 80.3%. In 8/44 patients, MDCTCA overestimated a subcritical stenosis (30–40%) with respect to angiography: due to calcifications (blooming artefacts) or motion (blurring artefacts), the quality score was 3, providing false positive results. Thirty of the 36 true positive patients were treated with PTCA (n = 26) or surgical revascularisation (n = 4), while six patients with borderline stenosis in the small coronary vessels (four obtuse marginal, two diagonal branches) underwent surgery without any adjunctive treatment.
A summary of the CT scan results for coronary artery stenosis in both groups is shown in tables 2 and 3.
Table 2 Distribution of coronary lesions in Group 1 patients identified by MDCTCA in the proximal, middle and distal portions of the coronary arteries.
| Group 1 (MDCTCA only) | Patients (n) | Lesions (n) | Lesions <50% |
|---|---|---|---|
| Proximal | |||
| LM | 72 | 72/72 | 0/72 |
| LAD | 72 | 55/72 | 17/72 |
| LCX | 72 | 65/72 | 7/72 |
| RCA | 72 | 67/72 | 5/72 |
| Total | 288 | 259/288 | 29/288 |
| Middle | |||
| LAD | 72 | 63/72 | 9/72 |
| LCX | 72 | 65/72 | 7/72 |
| RCA | 72 | 69/72 | 3/72 |
| Total | 216 | 197/216 | 19/216 |
| Distal | |||
| LAD | 72 | 66/72 | 6/72 |
| LCX | 72 | 69/72 | 3/72 |
| RCA | 72 | 70/72 | 2/72 |
| Total | 216 | 205/216 | 11/216 |
| Total | 720 | 661/720 | 59/720 |
| Per patient analysis | 72 | 39/72 | 33/72 |
LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main artery; RCA, right coronary artery.
Table 3 Distribution of coronary lesions in Group 2 patients identified by MDCTCA and CA in the proximal, middle and distal portions of the coronary arteries. CT positive predictive value (PPV) is also reported.
| Group 2 (MDCTCA + CA) | Patients (n) | MDCTCA no lesions or lesions <50% | CA no lesions or lesions <50% | MDCTCA lesions ⩾50% | CA lesions ⩾50% | MDCTCA PPV (%) |
|---|---|---|---|---|---|---|
| Proximal | ||||||
| LM | 44 | 44/44 | 44/44 | 0/44 | 0/44 | |
| LAD | 44 | 27/44 | 29/44 | 17/44 | 15/44 | |
| LCX | 44 | 41/44 | 41/44 | 3/44 | 3/44 | |
| RCA | 44 | 39/44 | 40/44 | 5/44 | 4/44 | |
| Total | 176 | 151/176 | 154/176 | 25/176 | 22/176 | 88 |
| Middle | ||||||
| LAD | 44 | 26/44 | 29/44 | 18/44 | 15/44 | |
| LCX | 44 | 39/44 | 40/44 | 5/44 | 4/44 | |
| RCA | 44 | 40/44 | 41/44 | 4/44 | 3/44 | |
| Total | 132 | 105/132 | 110/132 | 27/132 | 22/132 | 81.5 |
| Distal | ||||||
| LAD | 44 | 34/44 | 37/44 | 10/44 | 7/44 | |
| LCX | 44 | 38/44 | 40/44 | 6/44 | 4/44 | |
| RCA | 44 | 41/44 | 42/44 | 3/44 | 2/44 | |
| Total | 132 | 113/132 | 119/132 | 19/132 | 13/132 | 68.4 |
| Total | 440 | 369/440 | 383/440 | 71/440 | 57/440 | 80.3 |
| Per patient analysis | 44 | 0 | 8 | 44 | 36 | 81.8 |
LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main artery; RCA, right coronary artery.
All patients underwent planned surgical procedures: replacement or repair of the aortic/mitral valve, ascending aorta replacement ± aortic valve repair/replacement (David or Bentall technique), and aortic arch or descending aorta replacement, at a mean time of 19 days (SD±17.4, range 1–44) from CT and/or CA.
Thirty out of 116 patients (25.8%) presented with systemic complications and 6/116 (5.2%) died from non‐cardiac causes. Cardiac events, where present, had mostly been minimal, such as transient atrial fibrillation (n = 33), transient myocardial ischaemia (n = 11) or tachycardia; two patients in Group 2 had severe cardiac complications. The first patient, with an aortic insufficiency and an EF of 26%, had cardiac failure on the second postoperative day. The second patient had an acute myocardial infarction on the first postoperative day; the patient had undergone replacement of the ascending aorta and CABG for type A dissection and three diseased coronary vessels. A summary of complications is shown in table 4; no significant differences were found between the two groups in systemic (p = 0.633) or cardiac (p = 0.746) events.
Table 4 In‐hospital mortality and complications of the entire study group.
| Perioperative and postoperative mortality and complications | Group 1 (n = 72) | Group 2 (n = 44) | p Value |
|---|---|---|---|
| Mortality | 4 | 2 | 0.81 |
| Complications | |||
| Systemic | 0.633 | ||
| Paraplegia | 1 | 0 | |
| Transient respiratory insufficiency | 5 | 2 | |
| Prolonged respiratory insufficiency | 2 | 1 | |
| Re‐intervention for bleeding | 3 | 2 | |
| Hyperpyrexia/fever>38° | 9 | 5 | |
| Death due to ventilated assisted pneumonia | 2 | 1 | |
| Other causes of death (haemorrhage, infection) | 2 | 1 | |
| Cardiac | 0.746 | ||
| Transient atrial fibrillation | 23 | 10 | |
| Heart failure | 0 | 1 | |
| Transient myocardial ischaemia | 7 | 4 | |
| Acute myocardial infarction | 0 | 1 |
None of the patients in Group 1 had any cardiac complications such as myocardial infarction, myocardial ischaemia or heart failure during surgery or during the hospital stay.
Discussion
In the past few years, CT technology has shown the most relevant technical evolution as compared to other imaging modalities. Increasing spatial and temporal resolution have given excellent results in the detection of coronary lesions. The advent of 16‐row and 64‐row technology improved diagnostic capability in the detection of coronary lesions, reaching a positive predictive value of up to 97%.11 However, motion artefacts and heavy calcifications that often characterise coronary vessels in high‐risk populations may affect diagnostic accuracy.17,18 On the other hand an excellent negative predictive value of 99%11,13,15,16 encourages its clinical use as a screening test in a low–intermediate risk population.27,28 Gilard et al.28 recently confirmed the high capability of MDCTCA to rule out significant coronary stenoses in patients before aortic valve replacement, with a negative predictive value of 100%; CA was performed in all patients as a gold standard reference. No previous studies have been performed assuming as clinical reality the high negative predictive value of MDCT.
Patients with recognised myocardial ischaemia do not gain any particular advantage from CT coronary angiography; due to the need for prompt interventional or surgical revascularisation, they should undergo CA directly and, eventually, coronary angioplasty or bypass surgery. In the past few years, there has been a considerable increase in interventional coronary procedures5: the use of drug‐eluting stents has significantly reduced the incidence of re‐stenosis to treat multivessel disease. Moreover, the establishment of efficient systems with acute myocardial infarction means primary angioplasty has become the most effective form of revascularisation in many centres. Therefore, it could be of clinical value to eliminate from catheterisation laboratories a still considerable number of negative coronary angiographies, allowing availability to the increasing requests of interventional procedures.
Advances in surgical techniques and postoperative care allow cardiac surgery in older patients with severe co‐morbidities, but accurate preoperative cardiac risk stratification is mandatory. High‐risk patients constitute a special challenge for cardiologists, surgeons and anaesthesiologists in an attempt to identify subclinical co‐morbidities without an excessive cost increase. MDCTCA has been widely tested,8,9,10,11,12,13,14,15,16,17,18 is more accurate than other non‐invasive tests because it can provide a direct visualisation of coronary arteries and is less expensive and dangerous with respect to invasive CA. Furthermore, other vascular areas, such as the supra‐aortic vessels, and ascending aorta or visceral vessels, can be evaluated during the same examination with a small adjunctive amount of contrast medium. MDCT is also able to provide a reliable assessment of left ventricular global function, using the same acquired datasets of CTCA. The improved temporal resolution of the latest generation of scanners has resulted in good accuracy for cardiac function evaluation as has emerged from comparison with other imaging modalities such as echocardiography and MRI.29,30,31,32,33
Severe perioperative cardiac complications such as myocardial ischaemia and myocardial infarction could be caused either by prolonged ischaemia and coronary plaque rupture. Prolonged myocardial ischaemia in the perioperative setting may arise from increased myocardial oxygen demand (tachycardia, hypertension, pain, drugs) or reduced supply (hypotension, vasospasm, anaemia, hypoxia, plaque rupture). Prolonged ischaemia has been recognised as the major cause of perioperative myocardial infarction or death, which peak during the first three perioperative days.34 A strategy with an accurate preoperative cardiac risk assessment with the use of MDCTCA and a prophylactic medical therapy (beta‐blockers, aspirin and statins) may reduce the risk of severe cardiac complications.
Results of surgical outcomes in our population showed that none of the patients without coronary lesions or stenosis <50% at the MDCT scan (72/116, 62.1%) had any severe cardiac complications such as myocardial ischaemia, myocardial infarction or heart failure during surgery or while in hospital. Analysing the cardiac events of both groups (table 4), MDCTCA demonstrates the same capability of CA to avoid severe perioperative cardiac complications (p = 0.746).
Most of the exclusions (15/21, 71.4%) from the study were caused by extensive coronary calcifications (five patients with an Agatston score >1000 did not undergo MDCTCA and 10 patients with a mean Agatston score of 647.2±156.4 had poor image quality). These patients underwent calcium before surgery: 2/5 patients with an Agatston score >1000 and 4/10 patients with poor image quality presented with significant coronary lesions (6/15 or 40% in total). The clinical utility of the calcium score is still a matter of discussion in the literature; in this subset of patients, the CT scan for total calcium burden assessment showed a greater utility in patient selection (exclusion criteria) before CT coronary angiography rather than a clear prognostic value.
Study limitations
This is a non‐randomised, purely observational study. Coronary angiography was not performed (voluntarily, for the clinical purpose) in patients with a negative CT scan result, and an excellent negative predictive value could only be hypothesised (and supported) by the absence of any perioperative cardiovascular events within 30 days of follow‐up, but not from a direct imaging comparison.
The estimated radiation dose during MDCTCA (10 to 18 mSv depending on scan length and sex) is a cause for concern; the radiation dose is higher than the dose of a conventional coronary angiography (2–15.8 mSv, average 7.3 mSv) but it is not so different from the radiation exposure to a myocardial rest–stress scintigraphy (4.6 to 20 mSv, average 10–12 mSv).23 The CT dose can be reduced by 30–40% with technical adjustments such as prospective x ray or body mass index tube current modulation. Another limit is the small number of patients with cardiovascular events; further studies with larger populations are necessary to confirm these preliminary results.
The relatively high number of indeterminate exams (16/132, 12.1%) could also be considered as a limitation of the study. Artefacts from cardiac and respiratory motion could be reduced with faster scanners, as well as artefacts generated by coronary calcifications which could be reduced in the future with thinner collimations and special kernel convolution filters.
Conclusions
MDCT could be considered a complete screening test before non‐coronary cardiac surgery (coronary artery anatomy and stenosis as well as ventricular function). MDCT seems to be feasible and reliable in cardiac risk stratification and has the potential of becoming the sole screening test before high‐risk surgery.
Abbreviations
CA - conventional angiography
LAD - left anterior descending artery
LCX - left circumflex artery
LM - left main artery
MDCTCA - multidetector CT coronary angiography
RCA - right coronary artery
Footnotes
Competing interests: None declared.
References
- 1.Sones F M., Jr Cine‐coronary arteriography. Ohio Med 1962581018–1019. [PubMed] [Google Scholar]
- 2.Ricketts H J, Abrams H L. Percutaneous selective coronary cine arteriography. JAMA 1962181620–624. [PubMed] [Google Scholar]
- 3.Judkins M P. Selective coronary arteriography. A percutaneous transfemoral technic. Radiology 196789815–824. [DOI] [PubMed] [Google Scholar]
- 4.Noto T J, Jr, Johnson L W, Krone R.et al Cardiac catheterization 1990: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn 19912475–83. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke R A, Brundage B H, Froelicher V F.et al American College of Cardiology/American Heart Association Expert Consensus document on electron‐beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 2000102126–140. [DOI] [PubMed] [Google Scholar]
- 6.American Heart Association Statistical fact sheep: miscellaneous. http://www.americanheart.org/downloadable/heart/1103834461175FS19CVP5.pdf (accessed 8 Sept 2004)
- 7.AHA Statistical Updates Heart disease and stroke statistics: 2006 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. http://circ.ahajournals.org/cgi/reprint/CIRCULATIONAHA.105.171600v1 (accessed 11 Apr 2006) [DOI] [PubMed]
- 8.Achenbach S, Giesler T, Ropers D.et al Detection of coronary artery stenoses by contrast‐enhanced, retrospectively electrocardiographically‐gated, multislice spiral computed tomography. Circulation 20011032535–2538. [DOI] [PubMed] [Google Scholar]
- 9.Nieman K, Cademartiri F, Lemos P A.et al Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation 20021062051–2054. [DOI] [PubMed] [Google Scholar]
- 10.Kopp A F, Schroeder S, Kuettner A.et al Non‐invasive coronary angiography with high resolution multidetector‐row computed tomography. Results in 102 patients. Eur Heart J 2002231714–1725. [DOI] [PubMed] [Google Scholar]
- 11.Leschka S, Alkadhi H, Plass A.et al Accuracy of MSCT coronary angiography with 64‐slice technology: first experience. Eur Heart J 2005151482–1487. [DOI] [PubMed] [Google Scholar]
- 12.Leber A W, Knez A, von Ziegler F.et al Quantification of obstructive and nonobstructive coronary lesions by 64‐slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol 200546147–154. [DOI] [PubMed] [Google Scholar]
- 13.Achenbach S, Ropers D, Pohle F K.et al Detection of coronary artery stenoses using multi‐detector CT with 16 x 0.75 collimation and 375 ms rotation. Eur Heart J 2005261978–1986. [DOI] [PubMed] [Google Scholar]
- 14.Kuettner A, Beck T, Drosch T.et al Diagnostic accuracy of noninvasive coronary imaging using 16‐detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol 200545123–127. [DOI] [PubMed] [Google Scholar]
- 15.Mollet N R, Cademartiri F, Krestin G P.et al Improved diagnostic accuracy with 16‐row multi‐slice computed tomography coronary angiography. J Am Coll Cardiol 200545128–132. [DOI] [PubMed] [Google Scholar]
- 16.Heuschmid M, Kuettner A, Schroeder S.et al ECG‐gated 16‐MDCT of the coronary arteries: assessment of image quality and accuracy in detecting stenoses. AJR Am J Roentgenol 20051841413–1419. [DOI] [PubMed] [Google Scholar]
- 17.Dirksen M S, Jukema J W, Bax J J.et al Cardiac multidetector‐row computed tomography in patients with unstable angina. Am J Cardiol 200595457–461. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann U, Moselewski F, Cury R C.et al Predictive value of 16‐slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: patient‐versus segment‐based analysis. Circulation 20041102638–2643. [DOI] [PubMed] [Google Scholar]
- 19.Bartels C, Bechtel J F, Hossmann V.et al Cardiac risk stratification for high‐risk vascular surgery. Circulation 1997952473–2475. [DOI] [PubMed] [Google Scholar]
- 20.Paul S D, Eagle K A, Kuntz K M.et al Concordance of preoperative clinical risk with angiographic severity of coronary artery disease in patients undergoing vascular surgery. Circulation 1996941561–1566. [DOI] [PubMed] [Google Scholar]
- 21.Eagle K A, Berger P B, Calkins H.et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 20021051257–1267. [PubMed] [Google Scholar]
- 22.Mangano D T, Goldman L. Preoperative assessment of patients with known or suspected coronary disease. N Engl J Med 19953331750–1756. [DOI] [PubMed] [Google Scholar]
- 23.United Nations Scientific Committee on the Effects of Atomic Radiation 2000 Report. Sources and effects of ionizing radiation. Volume I: Sources, Annex D: Medical radiation exposures. http://www.unscear.org/docs/reports/annexd.pdf (accessed 13 Oct 2005)
- 24.Nashef S A, Roques F, Michel P.et al European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999169–13. [DOI] [PubMed] [Google Scholar]
- 25.Austen W G, Edwards J E, Frye R L.et al A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975515–40. [DOI] [PubMed] [Google Scholar]
- 26.Alpert J S, Thygesen K, Antman E.et al Myocardial infarction redefined: a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 200036959–969. [DOI] [PubMed] [Google Scholar]
- 27.Reant P, Brunot S, Lafitte S.et al Predictive value of noninvasive coronary angiography with multidetector computed tomography to detect significant coronary stenosis before valve surgery. Am J Cardiol 2006971506–1510. [DOI] [PubMed] [Google Scholar]
- 28.Gilard M, Cornily J C, Pennec P Y.et al Accuracy of multislice computed tomography in the preoperative assessment of coronary disease in patients with aortic valve stenosis. J Am Coll Cardiol 2006472020–2024. [DOI] [PubMed] [Google Scholar]
- 29.Juergens K U, Grude M, Maintz D.et al Multi‐detector row CT of left ventricular function with dedicated analysis software versus MR imaging: initial experience. Radiology 2004230403–410. [DOI] [PubMed] [Google Scholar]
- 30.Hundt W, Siebert K, Wintersperger B J.et al Assessment of global left ventricular function: comparison of cardiac multidetector‐row computed tomography with angiocardiography. J Comput Assist Tomogr 200529373–381. [DOI] [PubMed] [Google Scholar]
- 31.Schlosser T, Pagonidis K, Herborn C U.et al Assessment of left ventricular parameters using 16‐MDCT and new software for endocardial and epicardial border delineation. AJR Am J Roentgenol 2005184765–773. [DOI] [PubMed] [Google Scholar]
- 32.Heuschmid M, Rothfuss J, Schroder S.et al Assessment of left ventricular myocardial function using 16‐slice multidetector‐row computed tomography: comparison with magnetic resonance imaging and echocardiography. Eur Radiol 200616551–559. [DOI] [PubMed] [Google Scholar]
- 33.Salm L P, Schuijf J D, de Roos A.et al Global and regional left ventricular function assessment with 16‐detector row CT: comparison with echocardiography and cardiovascular magnetic resonance. Eur J Echocardiogr 20067308–314. [DOI] [PubMed] [Google Scholar]
- 34.Landesberg G, Mosseri M, Zahger D.et al Myocardial infarction after vascular surgery: the role of prolonged stress‐induced, ST depression‐type ischemia. J Am Coll Cardiol 2001371839–1845. [DOI] [PubMed] [Google Scholar]