Abstract
Objective
To assess pulmonary flow dynamics and right ventricular (RV) function in patients without significant anatomical narrowing of the pulmonary arteries late after the arterial switch operation (ASO) by using magnetic resonance imaging (MRI).
Methods
17 patients (mean (SD), 16.5 (3.6) years after ASO) and 17 matched healthy subjects were included. MRI was used to assess flow across the pulmonary trunk, RV systolic and diastolic function, and RV mass.
Results
Increased peak flow velocity (>1.5 m/s) was found across the pulmonary trunk in 14 of 17 patients. Increased RV mass was found in ASO patients: 14.9 (3.4) vs 10.0 (2.6) g/m2 in normal subjects (p<0.01). Delayed RV relaxation was found after ASO: mean tricuspid valve E/A peak flow velocity ratio = 1.60 (0.96) vs 1.92 (0.61) in normal subjects (p = 0.03), and E‐deceleration gradients = −1.69 (0.73) vs −2.66 (0.96) (p<0.01). After ASO, RV mass correlated with pulmonary trunk peak flow velocity (r = 0.49, p<0.01) and tricuspid valve E‐deceleration gradients (r = 0.35, p = 0.04). RV systolic function was well preserved in patients (ejection fraction = 53 (7)% vs 52 (8)% in normal subjects, p = 0.72).
Conclusions
Increased peak flow velocity in the pulmonary trunk was often observed late after ASO, even in the absence of significant pulmonary artery stenosis. Haemodynamic consequences were RV hypertrophy and RV relaxation abnormalities as early markers of disease, while systolic RV function was well preserved.
Keywords: arterial switch operation, pulmonary artery, right ventricle, magnetic resonance imaging, congenital heart disease
The arterial switch operation (ASO) has become the standard procedure of choice for repair of transposition of the great arteries.1,2,3 The ASO consists of translocation of the pulmonary trunk and the aorta, and subsequent relocation of the coronary arteries in the neonatal period.4 The advantages of the ASO compared with the previous intra‐atrial repair are related to creation of the left ventricle as the systemic ventricle and the maintenance of normal sinus node function.5,6,7
Supravalvar pulmonary artery stenosis is the most frequent complication after ASO.1,2,3,4,5,6,7 Reduction of the cross sectional area due to stretching of the pulmonary arteries is induced by the Lecompte manoeuvre,5,6,7 while compression of the proximal pulmonary branches may occur because of the close anatomical relation with the aorta.1,5,7,8 As a result, supravalvar pulmonary artery stenosis in ASO patients is associated with right ventricular hypertrophy and dysfunction from the increased afterload.9 These stenoses are well visualised by magnetic resonance imaging (MRI): a fixed stenosis can easily be recognised using spin echo or gradient echo sequences, and increased peak flow velocity across a significant stenosis can be detected by velocity encoded MRI.1,7,9
In patients with repaired coarctation, increased peak flow velocities across vascular segments may also be observed, because of scar formation at the site of anastomosis, even in the absence of overt narrowing.10,11 Scar tissue at the site of anastomosis may lead to decreased distensibility—thereby increasing local peak‐flow velocity—as may also be the case in ASO patients.10,11 In addition, minor degrees of stenosis or hypoplasia of the pulmonary vascular bed may lead to increased flow velocities in the pulmonary trunk,1 thereby increasing the haemodynamic burden for the right ventricle.
We hypothesised that in ASO patients without significant pulmonary artery stenosis, increased peak flow velocities are present in the pulmonary trunk, with a potential negative impact on right ventricular function. In the present study we excluded ASO patients with significant pulmonary artery stenosis as shown by anatomical imaging.
We tested these hypotheses by studying pulmonary flow dynamics and right ventricular function in patients without significant anatomical narrowing of the pulmonary trunk or pulmonary arteries late after the ASO, compared with matched healthy subjects.
Methods
Patient population
The local medical ethics committee approved the study, and informed consent was obtained from all participants before their enrolment. Twenty two ASO patients and 22 healthy subjects were studied prospectively with MRI at our institution. All patients were recruited from our local paediatric cardiology database. Inclusion criteria included transposition of the great arteries corrected by ASO (including the Lecompte manoeuvre) in the past, current age between 10 and 20 years, willingness to comply with the study procedures, and written informed consent. Exclusion criteria comprised ASO using the Jatene procedure, pulmonary stenting, general contraindications to MRI, and pulmonary artery stenosis shown by echocardiography, based on local tailoring of the pulmonary trunk of at least 50% or a peak systolic gradient of at least 60 mm Hg in the pulmonary arteries, or both.
Visual inspection of the pulmonary arteries on MRI confirmed that only ASO patients without pulmonary artery stenosis were enrolled in the study. A vessel diameter reduction of more than 50% gives rise to a reduction in blood volume flow and was considered significant.12 Two patients who had a main pulmonary artery stenosis that was visually greater than 50% were thus excluded from further analysis. Three patients failed to complete the MRI study because of problems with breath holding. In all, 17 patients and 17 healthy subjects were included for final analysis.
Age and sex matched healthy subjects were selected from our database and comprised subjects in whom congenital cardiac pathology had been excluded in the past by physical examination and echocardiography. Characteristics of the two groups (table 1) and their functional status, expressed as New York Heart Association functional class, were obtained from the patient records.
Table 1 Patient and healthy subject characteristics.
| Characteristic | Patients (n = 17) | Controls (n = 17) |
|---|---|---|
| Male/female | 13 (76%)/4 (24%) | 13 (76%)/4 (24%) |
| Age at ASO (days)* | 16 (23) | – |
| Age at MRI (years)* | 16.4 (3.2) | 16.5 (3.6) |
| Height at MRI (cm)* | 169 (13) | 169 (12) |
| Weight at MRI (kg)* | 61 (170 | 60 (13) |
| Body surface area at MRI (m2)*† | 1.7 (0.3) | 1.7 (0.2) |
| Systolic/diastolic BP at MRI (mm Hg)* | 124/74 (20/18) | 121/69 (12/10) |
| Heart rate (beats/min) | 74 (16) | 69 (9) |
*Values are mean (SD).
†According to the Mosteller formula: √ [height (cm)× weight (kg)/3600].
ASO, arterial switch operation; BP, blood pressure; MRI, magnetic resonance imaging.
Operation technique
Nine patients had undergone balloon atrial septostomy (that is, the Rashkind procedure) and 11 had received prostaglandin E1 infusions before the ASO. The ASO was carried out in patients with transposition of the great arteries (median age at surgery = 6 days) using cardiopulmonary bypass and moderate hypothermia. The operation included transection of the aorta and pulmonary trunk above their roots, transplantation of the coronary arteries into the root of the pulmonary trunk, switching of the aorta and pulmonary trunk, and subsequent reconstruction of the pulmonary trunk with a pericardial patch. The Lecompte manoeuvre was undertaken in all 17 patients.8 Associated procedures during the ASO were closure of a ventricular septal defect in five patients, closure of an atrial septal defect in seven, and closure of the ductus arteriosus in 12. No perioperative ischaemic events were recorded.
Magnetic resonance imaging
MRI studies were done using a 1.5 Tesla system (NT 15 Gyroscan Intera, Philips Medical System, Best, the Netherlands). Initial scout images were obtained in transverse, coronal, and sagittal planes using a standard multislice turbo spin echo sequence.
To visualise the pulmonary vessel anatomy a black‐blood static sequence and contrast enhanced magnetic resonance angiography were used. Black‐blood turbo spin echo imaging using sensitivity encoding13 was acquired in the double oblique transverse plane, axial to the pulmonary trunk, with the following scan parameters: field of view 350 mm, repetition time two heart beats, with actual time depending on the individual heart rate, echo time 8.6 ms, flip angle 90°, slice gap 0.8 mm, and voxel size 1.37×2.12×8.00 mm.1 Contrast enhanced magnetic resonance angiography by using Magnevist® (0.2 mmol/kg; Schering, Berlin, Germany) was carried out with the following scan parameters: field of view 400 mm, repetition time 5.1 ms, echo time 1.44 ms, and voxel size 1.56 ×3.13 ×4.00 mm.
Pulmonary valve function and flow dynamics across the pulmonary trunk were assessed using velocity encoded MRI in the proximal pulmonary trunk.14 Scan parameters were field of view 400 mm, repetition time 8.6 ms, echo time 5.3 ms, flip angle 20°, and voxel size 2.34 ×2.61 ×8.00 mm. The sequence was encoded for a through‐plane velocity up to 150 cm/s. Temporal resolution was 25.6 ms.
Systolic right ventricular function was assessed using a prospectively ECG triggered balanced gradient echo sequence. A short axis stack of 14 to 18 contiguous slices was used, covering the base of the heart to the apex,14 with the following scan parameters: field of view 350 mm, repetition time 3.2 ms, echo time 1.62 ms, flip angle 70°, and voxel size 2.19 ×1.62 ×8.00 mm.
Velocity mapping across the tricuspid valve was used for assessment of diastolic right ventricular function.14,15 Scan parameters were: field of view 300 mm, repetition time 9.4 ms, echo time 6 ms, flip angle 20°, and voxel size 2.34 ×2.61 ×8.00 mm. The sequence was encoded for a through‐plane velocity up to 100 cm/s. Temporal resolution was 25.6 ms.
Postprocessing
All images were quantitatively analysed on a workstation with an Intel® Pentium® 4 processor (Intel®, Santa Clara, USA). Contrast enhanced magnetic resonance angiography and black‐blood images of the pulmonary vessels were used to exclude significant pulmonary artery stenoses. The gradient echo right ventricular dataset was analysed with the software package MASS® (Medis®, Leiden, the Netherlands).14 Flow velocity encoded MRI data were analysed using the analytic software package Flow® (Medis®, Leiden, the Netherlands).14 All contours were manually drawn by two observers (both with one year of experience), and were subsequently checked by a radiologist (with nine years of experience), who was unaware of the patient conditions.
Vascular contours were drawn for the pulmonary trunk to generate flow curves throughout the cardiac cycle.16 The presence of substantial pulmonary valve regurgitation was assessed (>5%). Increased peak flow velocity in the pulmonary trunk was defined as maximum blood flow velocity (Vmax) exceeding 1.5 m/s.1 The duration of the forward flow wave during systole across the pulmonary trunk was divided in a first and second half, with subsequent comparison of the flow/volume ratios (flow through first half of systole divided by flow through second half of systole) as a marker of flow propagation.17
Right ventricular systolic function was assessed by drawing endocardial right ventricular contours at end diastole and end systole in all sections of the cine short axis data.16 Right ventricular end diastolic volumes (RV EDV) and right ventricular end systolic volumes (RV ESV) were obtained and indexed for body surface area according to the Mosteller formula: √ [height (cm) ×weight (kg)/3600]. Right ventricular stroke volume indexed for body surface area (RV SV) was calculated by subtracting RV ESV from RV EDV. The right ventricular ejection fraction (RV EF) was calculated by dividing RV SV by RV EDV. Right ventricular mass was calculated as previously described after drawing right ventricular endocardial and epicardial contours, with subsequent indexation for body surface area (indicated by right ventricular mass).16
For evaluation of right ventricular diastolic function, tricuspid valve contours were drawn throughout the cardiac cycle.18 Flow versus time curves of the tricuspid flow were subsequently analysed using Microsoft Excel (version 2003)19 for calculation of the following indices of diastolic right ventricular function: early filling phase (E), atrial kick phase (A) and E/A peak flow velocity ratios.18 Analysis of E slopes was done by calculation of mean deceleration gradients of E.18 Times of E, A, and diastasis were also measured.18
Statistical analysis
Statistical analysis was carried out by using SPSS for Windows (version 12.0.1; SPSS, Chicago, Illinois, USA). All data are expressed as mean (SD), unless stated otherwise. The Mann–Whitney U test was used to express differences in variables between patients and healthy subjects. Correlations between variables are expressed using Spearman's rank correlation coefficient. Statistical significance was indicated by a probability (p) value of less than 0.05.
Results
Patient and healthy subjects were matched for age and sex. Subject characteristics were comparable between both groups (table 1). All patients were in New York Heart Association functional class 1, without drug treatment.
Pulmonary artery characteristics
Cross sectional diameters of the pulmonary trunk were slightly smaller in the ASO patients than in the controls, with a mean difference of 3.5 mm between the two groups (table 2). In 14 of the 17 ASO patients (82%), the pulmonary trunk Vmax exceeded 1.5 m/s, compared with none in the healthy subject group, indicating frequently increased peak flow velocities at the level of the pulmonary trunk (table 2). In the ASO patients, the ratio of pulmonary trunk forward flow volume during the first half of systole to that in the second half was significantly smaller than in the healthy subjects, reflecting delayed propagation of pulmonary flow through the pulmonary trunk. Within the patient group, a positive correlation was found between this pulmonary trunk forward flow volume ratio and the pulmonary trunk Vmax (r = −0.42, p = 0.02), indicating that delayed flow propagation was associated with higher maximum flow velocities. No substantial pulmonary regurgitation (>5%) was present in any patient or healthy subject.
Table 2 Results in 17 ASO patients and 17 age/sex matched healthy subjects.
| Variable | Patients | Controls | p Value |
|---|---|---|---|
| PT cross sectional diameter (mm) | 25.6 (3.4) | 29.1 (3.5) | 0.01 |
| PT Vmax (m/s) | 1.88 (0.56) | 0.93 (0.19) | <0.01 |
| PT volume ratio systole1/systole2 | 1.16 (0.15) | 1.35 (0.26) | 0.01 |
| RV EF (%) | 53 (7) | 52 (8) | 0.84 |
| RV SV (ml/m?2) | 56 (8) | 50 (13) | 0.06 |
| RV EDV (ml/m2) | 108 (18) | 96 (16) | 0.06 |
| RV ESV (ml/m2) | 51 (14) | 46 (10) | 0.41 |
| RV mass (g/m2) | 14.9 (3.4) | 10.0 (2.6) | <0.01 |
| TV E/A peak flow ratio | 1.60 (0.96) | 1.92 (0.61) | 0.03 |
| TV mean DG of E phase (l/s2) | −1.69 (0.73) | −2.66 (0.96) | <0.01 |
| TV diastasis time (ms) | 19 (30) | 53 (64) | 0.04 |
Data are mean (SD).
A, atrial kick phase; ASO, arterial switch operation; DG, deceleration gradient; E, early filling phase; EDV, end diastolic volume indexed for body surface area; EF, ejection fraction; ESV, end systolic volume indexed for body surface area; PT, pulmonary trunk; RV, right ventricular; SV, stroke volume indexed for body surface area; TV, tricuspid valve; Vmax, maximum velocity.
Right ventricular function
Systolic function, expressed by RV EF and RV SV, did not differ between ASO patients and healthy subjects (table 2). Right ventricular dimensions (RV EDV and RV ESV) were also not different between the two groups (table 2).
Mean right ventricular mass in the ASO patient group was significantly greater than in the healthy subjects (table 2). In the patient group a significant correlation was found between right ventricular mass and pulmonary trunk Vmax (r = 0.49, p<0.01), indicating that right ventricular hypertrophy is associated with increased peak flow velocities in the pulmonary trunk.
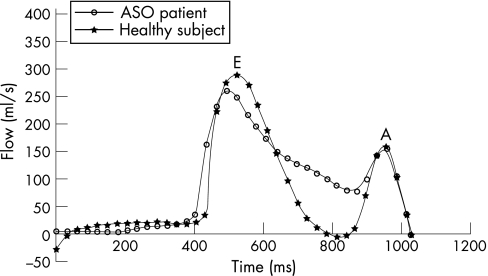
A significantly reduced mean tricuspid valve E/A peak flow velocity ratio, decreased tricuspid valve mean deceleration gradient in the E phase, and loss of diastasis time were all present in our ASO patient group, indicating delayed right ventricular relaxation during diastole (table 2, fig 1). In addition, the tricuspid valve mean deceleration gradient in the E phase and loss of diastasis were positively correlated with right ventricular mass (r = 0.35, p = 0.04 and r = 0.45, p<0.01, respectively), as would be expected because delayed relaxation is associated with hypertrophy accompanying stiffening of the right ventricular myocardium.
Figure 1 Right ventricular inflow curves across the tricuspid valve in an ASO patient and a matched healthy subject. The biphasic inflow pattern consists of two peaks, representing the early inflow (E) and the late atrial kick (A). Note the decreased mean deceleration gradient after the E peak in the ASO patient compared with the matched healthy subject.
Discussion
Cardiac MRI was used to assess pulmonary flow dynamics and right ventricular function in patients late after the arterial switch operation without significant anatomical narrowing of the pulmonary arteries, as compared with matched healthy subjects. This study revealed frequently increased peak flow velocities in the pulmonary trunk, even in the absence of significant anatomical narrowing of the pulmonary arteries. Further haemodynamic consequences were delayed pulmonary flow propagation, right ventricular hypertrophy, and right ventricular relaxation abnormalities as early markers of disease, whereas systolic right ventricular function remained well preserved.
Pulmonary artery characteristics
ASO patients often have increased peak flow velocities at the level of the pulmonary trunk compared with healthy subjects. Various reasons for this have been postulated. Reduction of cross sectional area is induced by stretching of the pulmonary arteries following the Lecompte manoeuvre.2,5,6,7 The non‐spiral configuration of the pulmonary arteries after this manoeuvre has been reported to promote the formation of stenotic plaques through an altered wall shear stress distribution,2 while scarring at the anastomosis site often results in circumferential narrowing.5,6,7,9 Recent reports of patients with repaired coarctation also indicate that, even in the absence of significant stenosis at the site of anastomosis, loss of distensibility because of stiff scar tissue is a major contributor to an increased peak flow velocity.10,11 It is likely that local scar tissue with loss of distensibility made a major contribution to increased peak flow velocities in our ASO patients, in addition to possible minor degrees of narrowing at the site of anastomosis or peripheral pulmonary branches. In this study the pulmonary trunk was slightly smaller in the ASO patient group than in the matched healthy subjects. Previous studies indicated normal dimensions of the pulmonary trunk and peripheral pulmonary branches before the ASO, excluding a congenital deformity.5,6
Right ventricular function
In this study, systolic right ventricular function was well preserved late after the ASO. These findings are supported by previous reports.1,20
The right ventricular mass was significantly greater in the ASO patients than in the healthy controls, indicating right ventricular hypertrophy. This often occurs as a compensatory mechanism for increased right ventricular afterload,9,21 which in this study was reflected by increased peak flow velocities across the pulmonary trunk. Increased right ventricular afterload was also indicated by the delayed propagation of the systolic pulmonary flow.17 Ischaemic damage caused by coronary insufficiency has been suggested as another explanation for compensatory right ventricular hypertrophy after ASO.20 However, none of our patients had perioperative ischaemic events according to the available patient records, so ventricular hypertrophy from ischaemic damage was not suspected.
Delayed right ventricular relaxation was indicated by decreased tricuspid valve E/A peak flow velocity ratio, reduced tricuspid valve mean deceleration gradient of the E phase, and loss of diastasis time in our ASO patient group. In contrast to mild to moderate degrees of isolated pulmonary stenosis, which have little impact on right ventricular function,9 a supravalvar increase in peak flow velocity proved to have negative repercussions for right ventricular function in this study. Delayed right ventricular relaxation is related to hypertrophy accompanying stiffening of the myocardium,20 and can be seen as an early marker of diastolic dysfunction which may precede right ventricular systolic dysfunction.20,22 The impact of right ventricular diastolic dysfunction is considerable, as published reports suggest that its prognostic value is equal to impaired left ventricular diastolic function.20,22,23 Impaired left ventricular diastolic function is a major contributor in one third of patients with congestive heart failure16,22,24 and causes diminished exercise performance.24,25 Aging contributes to diastolic dysfunction by an increase in ventricular mass and a loss of elastic myocardial properties over time.16,24 Thus impaired right ventricular relaxation might pose a future risk for right ventricular dysfunction in our patient group, with negative implications for prognosis.
Conclusions
The evaluation by MRI of right ventricular function and pulmonary flow dynamics late after the ASO revealed an increased peak flow velocity across the pulmonary trunk, even in the absence of significant pulmonary stenosis at the surgical anastomosis. Further haemodynamic consequences were right ventricular hypertrophy and right ventricular relaxation abnormalities as early markers of disease, though systolic right ventricular function was still well preserved.
Acknowledgements
We thank Jan Verwoerd from Philips Medical Systems, Best, the Netherlands, for his help with the MRI sequences. We also thank Annette A van den Berg‐Huysmans for her statistical support.
Abbreviations
A - atrial kick phase
ASO - arterial switch operation
E - early filling phase
EDV - end diastolic volume
EF - ejection fraction
ESV - end systolic volume
MRI - magnetic resonance imaging
PT - pulmonary trunk
RV - right ventricular
SV - stroke volume
Vmax - maximum blood flow velocity
Footnotes
Competing interests: None declared.
References
- 1.Gutberlet M, Boeckel T, Hosten N.et al Arterial switch procedure for D‐transposition of the great arteries: quantitative midterm evaluation of hemodynamic changes with cine MR imaging and phase‐shift velocity mapping‐initial experience. Radiology 2000214467–475. [DOI] [PubMed] [Google Scholar]
- 2.Tang T, Chiu I S, Chen H C.et al Comparison of pulmonary arterial flow phenomena in spiral and Lecompte models by computational fluid dynamics. J Thorac Cardiovasc Surg 2001122529–534. [DOI] [PubMed] [Google Scholar]
- 3.Formigari R, Santoro G, Guccione P.et al Treatment of pulmonary artery stenosis after arterial switch operation: stent implantation vs. balloon angioplasty. Catheter Cardiovasc Interv 200050207–211. [DOI] [PubMed] [Google Scholar]
- 4.McMahon C J, Ravekes W J, Smith E O.et al Risk factors for neo‐aortic root enlargement and aortic regurgitation following arterial switch operation. Pediatr Cardiol 200424329–335. [DOI] [PubMed] [Google Scholar]
- 5.Massin M M, Nitsch G B, Dabritz S.et al Growth of pulmonary artery after arterial switch operation for simple transposition of the great arteries. Eur J Pediatr 199815795–100. [DOI] [PubMed] [Google Scholar]
- 6.Santoro G, Di Carlo D, Formigari R.et al Late onset pulmonary valvar stenosis after arterial switch operation for transposition of the great arteries. Heart 199879311–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss F, Habermann C R, Lilje C.et al MRI of pulmonary arteries in follow‐up after arterial‐switch‐operation (ASO) for transposition of great arteries (d‐TGA). Rofo 2005177849–855. [DOI] [PubMed] [Google Scholar]
- 8.Lecompte Y, Neveux J Y, Leca F.et al Reconstruction of the pulmonary outflow tract without prosthetic conduit. J Thorac Cardiovasc Surg 198284727–733. [PubMed] [Google Scholar]
- 9.Beek F J, Beekman R P, Dillon E.et al MRI of the pulmonary artery after arterial switch operation for transposition of the great arteries. Pediatr Radiol 199323335–340. [DOI] [PubMed] [Google Scholar]
- 10.Verhaaren H, De Mey S, Coomans I.et al Fixed region of nondistensibility after coarctation repair: in vitro validation of its influence on Doppler peak velocities. J Am Soc Echocardiogr 200114580–587. [DOI] [PubMed] [Google Scholar]
- 11.Seifert B L, DesRochers K, Ta M.et al Accuracy of Doppler methods for estimating peak‐to‐peak and peak instantaneous gradients across coarctation of the aorta: An In vitro study. J Am Soc Echocardiogr 199912744–753. [DOI] [PubMed] [Google Scholar]
- 12.Meire H B, Cosgrove D. Vascular ultrasound. In: Grainger and Allison's diagnostic radiology. New York: Churchill Livingstone, 19972459–2481.
- 13.Pruessmann K P, Weiger M, Boesiger P. Sensitivity encoded cardiac MRI. J Cardiovasc Magn Reson 200131–9. [DOI] [PubMed] [Google Scholar]
- 14.van der Geest R J, Reiber J H. Quantification in cardiac MRI. J Magn Reson Imaging 199910602–608. [DOI] [PubMed] [Google Scholar]
- 15.Paelinck B P, Lamb H J, Bax J J.et al Assessment of diastolic function by cardiovascular magnetic resonance. Am Heart J 2002144198–205. [DOI] [PubMed] [Google Scholar]
- 16.Van Straten A, Vliegen H W, Hazekamp M G.et al Right ventricular function after pulmonary valve replacement in patients with tetralogy of Fallot. Radiology 2004233824–829. [DOI] [PubMed] [Google Scholar]
- 17.Grotenhuis H B, Kroft L J M, Vliegen H W.et al Magnetic resonance imaging of function and flow in post‐operative congenital heart disease. In: Higgins CB, de Roos A, editors. MRI and CT of the cardiovascular system. Philadelphia: Lippincott, Williams & Wilkins, 2006411–428.
- 18.Pluim B M, Lamb H J, Kayser H W.et al Functional and metabolic evaluation of the athlete's heart by magnetic resonance imaging and dobutamine stress magnetic resonance spectroscopy. Circulation 199897666–672. [DOI] [PubMed] [Google Scholar]
- 19.Van Straten A, Vliegen H W, Lamb H J.et al Time course of diastolic and systolic function improvement after pulmonary valve replacement in adult patients with tetralogy of Fallot. J Am Coll Cardiol 2005461559–1564. [DOI] [PubMed] [Google Scholar]
- 20.Yu C M, Sanderson J E, Chan S.et al Right ventricular diastolic dysfunction in heart failure. Circulation 1996931509–1514. [DOI] [PubMed] [Google Scholar]
- 21.Taylor A M, Dymarkowski S, Hamaekers P.et al MR coronary angiography and late‐enhancement myocardial MR in children who underwent arterial switch surgery for transposition of great arteries. Radiology 2005234542–547. [DOI] [PubMed] [Google Scholar]
- 22.Pepi M, Agostoni P, Marenzi G.et al The influence of diastolic and systolic function on exercise performance in heart failure due to dilated cardiomyopathy or ischemic heart disease. Eur J Heart Fail 19991161–167. [DOI] [PubMed] [Google Scholar]
- 23.Meluzin J, Spinarova L, Hude P.et al Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J Am Soc Echocardiogr 200518435–444. [DOI] [PubMed] [Google Scholar]
- 24.Mandinov L, Eberli F R, Seiler C.et al Diastolic heart failure. Cardiovasc Res 200045813–825. [DOI] [PubMed] [Google Scholar]
- 25.Singh G K, Greenberg S B, Yap Y S.et al Right ventricular function and exercise performance late after primary repair of tetralogy of Fallot with the transannular patch in infancy. Am J Cardiol 1998811378–1382. [DOI] [PubMed] [Google Scholar]