Abstract
Objective
The aim of the study was to compare time‐trends in mortality rates and treatment patterns between patients with and without diabetes based on the Swedish register of coronary care (Register of Information and Knowledge about Swedish Heart Intensive Care Admission [RIKS‐HIA]).
Methods
Post myocardial infarction mortality rate is high in diabetic patients, who seem to receive less evidence‐based treatment. Mortality rates and treatment in 1995–1998 and 1999–2002 were studied in 70 882 patients (age <80 years), 14 873 of whom had diabetes (the first registry recorded acute myocardial infarction), following adjustments for differences in clinical and other parameters.
Results
One‐year mortality rates decreased from 1995 to 2002 from 16.6% to 12.1% in patients without diabetes and from 29.7% to 19.7%, respectively, in those with diabetes. Patients with diabetes had an adjusted relative 1‐year mortality risk of 1.44 (95% CI 1.36 to 1.52) in 1995–1998 and 1.31 (95% CI 1.24 to 1.38) in 1999–2002. Despite improved pre‐admission and in‐hospital treatment, diabetic patients were less often offered acute reperfusion therapy (adjusted OR 0.85, 95% CI 0.80 to 0.90), acute revascularisation (adjusted OR 0.78, 95% CI 0.69 to 0.87) or revascularisation within 14 days (OR 0.80, 95% CI 0.75 to 0.85), aspirin (OR 0.90, 95% CI 0.84 to 0.98) and lipid‐lowering treatment at discharge (OR 0.81, 95% CI 0.77 to 0.86).
Conclusion
Despite a clear improvement in the treatment and myocardial infarction survival rate in patients with diabetes, mortality rate remains higher than in patients without diabetes. Part of the excess mortality may be explained by co‐morbidities and diabetes itself, but a lack of application of evidence‐based treatment also contributes, underlining the importance of the improved management of diabetic patients.
Patients with diabetes have higher short‐ and long‐term mortality rates after acute myocardial infarction (MI) than those without diabetes.1,2,3,4 This pattern has remained even after the introduction of modern therapeutic principles.5,6,7 According to US mortality rate trends diabetic patients have not experienced a similar mortality rate reduction as that seen in non‐diabetic patients.8,9,10 Less use of evidence‐based treatment has been suggested as an important explanation.4,10,11,12,13,14 The systematic use of such therapy should decrease hospital mortality rate in diabetic patients so that it approaches that in those without diabetes.15
The Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (RIKS‐HIA), covering almost all Swedish patients with MI, offers detailed information on treatment patterns and prognosis in unselected patients with and without diabetes. The aim of this study is to analyse time trends in treatment patterns and prognosis in order to see whether management has improved.
Methods
Patients
The RIKS‐HIA contains information on all patients admitted to Swedish coronary care units, increasing from 19 patients registered in 1995 to 70 in 2002. Data were collected during 1995–2002. Because of an increased risk of concomitant diseases patients >80 years were not included. All 70 882 patients with a first registry recorded acute MI were included, of whom 14 873 (21%) had known diabetes mellitus.
Case record forms
Information on care of the patients is recorded into the RIKS‐HIA by means of case record forms including about 100 variables as previously explained.4 Thirty variables are recorded at admission (baseline characteristics, symptoms and ECG changes at entry). During hospital stay another 37 variables are registered, including treatments and interventional procedures. The final 33 variables are recorded at hospital discharge and include variables such as as diagnosis during hospital stay, revascularisation procedures and medications. Source data verification is continuously performed by an external monitor comparing the register information with actual hospital records in 50 patients from ten hospitals every year. In the first 1004 computer forms derived from 21 hospitals and comprising 92 368 measurements there was a 94% overall agreement between the registered information and patient records.
Follow‐up
Information on the performance on coronary procedures before and after hospital admission was obtained by matching patient data with the National Registries on coronary angiography, percutaneous revascularisation (PCI) and coronary artery bypass graft (CABG). One‐year and long‐term mortality rates were obtained by merging the RIKS‐HIA database with the National Cause of Death Register.
Definitions
Myocardial infarction
From 1995 to 2000 the criteria for the diagnosis of acute MI were based on the World Health Organization criteria from 199416 combining symptoms with the increase of a biochemical marker (mainly creatine kinase‐cardiac muscle [CK‐MB]) and typical ECG changes. From late 2001 the criteria for the diagnosis of MI were changed to those of the European Society of Cardiology/American College of Cardiology/American Heart Association consensus document, using troponin T or troponin I together with typical symptoms and/or ECG‐changes.17,18
Diabetes mellitus
Diabetes was defined as a previously established diagnosis of this disease or the prescription of glucose‐lowering treatment at the time of hospital discharge (oral drugs or insulin).
Statistical analysis
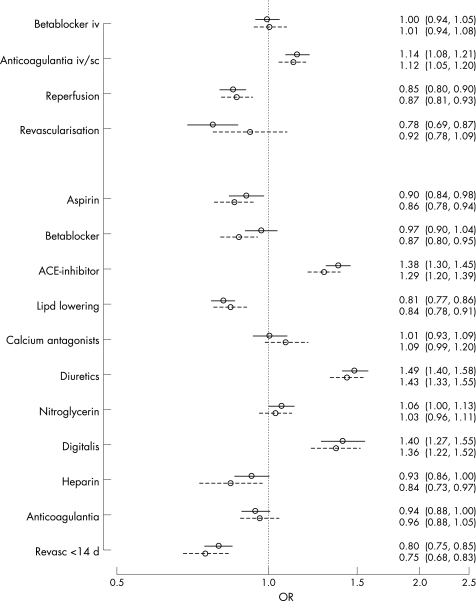
The background characteristics, treatments and complications were compared in diabetic and non‐diabetic patients. Results are presented as odds ratios (ORs) and 95% CIs. A propensity score method compensated for the non‐randomised study design. This method, described in detail elsewhere,19,20 expresses for each patient the propensity of having the same background characteristics as the average diabetic patient through a logistic regression analysis. The model includes patient characteristics (age as a third degree polynomial, sex and smoking habits), previous diseases (history of MI, heart failure, PCI or CABG, and hypertension), admission ECG characteristics and medication at admission (aspirin, beta‐blockers, ACE inhibitors, calcium antagonists, lipid‐lowering drugs, nitrates, anticoagulation therapy and diuretics). Several two‐way interactions were included in the model. To study differences between diabetic and non‐diabetic patients for in‐hospital or discharge treatment modalities, separate logistic regression models were fitted. The models included diabetes status (diabetic/non‐diabetic) as the independent variable, and the propensity score together with each respective in‐hospital or discharge treatment (see Figure 1), one for each model, as dependent variables.
Figure 1 Propensity score adjusted OR for prescription of in‐hospital (top four) and at discharge (bottom 11) treatment modalities in patients with and without diabetes and MI in 1995–1998 (dashed line) and 1999–2002 (solid line). Treatments with an OR >1.0 are given more often to patients with diabetes.
To compare the risk of mortality between diabetic and non‐diabetic patients a Cox regression analysis was performed. This model included the propensity score as well as in‐hospital treatment (beta‐blockers, anticoagulation, thrombolysis and acute revascularisation) and treatment at discharge (aspirin, beta‐blockers, ACE inhibitors, calcium antagonists, nitrates, anticoagulation, diuretics, digitalis and heparins) and revascularisation within 14 days following discharge. Estimated survival rates were calculated for the complete time period and separately for the two time periods 1995–1998 and 1999–2002. Difference in relative risk (RR) between the time periods was assessed by including an interaction term (time period × diabetes status) in the model. All analyses were done using the statistical program R (version 2.0; R Development Core Team).21
Ethical considerations
All patients were informed of their participation in the RIKS‐HIA registry and the long‐term follow‐up. The merging with other registries was approved by the National Board of Health Welfare and the Swedish Data Inspection Board.
Results
Trends in prevalence of risk factors and preadmission treatment
Diabetes versus non‐diabetes
Patients with diabetes were older (mean age: 68 versus 66 years) and more often female. They had a more frequent history of hypertension, MI, heart failure and revascularisation but smoked less. Their pre‐admission therapy more frequently included aspirin, beta‐blockers, ACE inhibitors, diuretics, nitroglycerin and lipid‐lowering drugs as shown in Table 1, which also includes changes in baseline characteristics and treatment 1995–1998 versus 1999–2002.
Table 1 Baseline characteristics and treatment in patients with and without diabetes during the two time periods.
| Time period 1995–1998 | Time period 1999–2002 | |||||
|---|---|---|---|---|---|---|
| Diabetes | Diabetes | |||||
| No (n = 22 582) | Yes (n = 5679) | OR for diabetes (95% CI) | No (n = 33427) | Yes (n = 9134) | OR for diabetes (95% CI) | |
| Baseline characteristics | ||||||
| Age (years, mean)* | 66.4 | 68.1 | 1.19 (1.15–1.23) | 66.5 | 68.4 | 1.21 (1.19–1.24) |
| Male (%) | 70.8 | 63.4 | 0.72 (0.67–0.76) | 69.0 | 63.2 | 0.77 (0.73–0.81) |
| Previous diseases | ||||||
| Hypertension | 29.2 | 45.4 | 2.02 (1.90–2.14) | 30.7 | 50.1 | 2.26 (2.16–2.37) |
| Smoker | 27.6 | 17.3 | 0.55 (0.51–0.59) | 29.5 | 19.3 | 0.57 (0.54–0.61) |
| MI | 21.2 | 33.0 | 1.83 (1.71–1.95) | 16.7 | 27.8 | 1.93 (1.82–2.03) |
| Heart failure | 7.0 | 16.8 | 2.68 (2.46–2.92) | 6.6 | 17.2 | 2.92 (2.73–3.13) |
| PCI | 3.1 | 4.4 | 1.43 (1.23–1.66) | 3.7 | 6.0 | 1.66 (1.49–1.84) |
| CABG | 4.3 | 6.0 | 1.45 (1.27–1.64) | 5.3 | 7.7 | 1.73 (1.58–1.88) |
| PCI/CABG | 6.8 | 9.5 | 1.45 (1.31–1.61) | 8.1 | 13.1 | 1.72 (1.60–1.85) |
| Treatment on admission | ||||||
| Aspirin | 29.7 | 42.7 | 1.76 (1.66––1.87) | 30.2 | 46.8 | 2.04 (1.94–2.13) |
| Beta‐blocker | 29.3 | 38.6 | 1.51 (1.42–1.61) | 29.7 | 44.0 | 1.86 (1.77–1.95) |
| ACE inhibitor | 10.7 | 26.9 | 3.06 (2.84–3.29) | 12.9 | 32.8 | 3.29 (3.12–3.48) |
| Calcium antagonist | 15.6 | 23.7 | 1.68 (1.56–1.81) | 13.9 | 22.1 | 1.76 (1.66–1.86) |
| Lipid‐lowering drugs | 6.7 | 10.6 | 1.64 (1.48–1.81) | 12.1 | 24.9 | 2.42 (2.28–2.56) |
| Diuretics | 19.9 | 38.8 | 2.55 (2.39–2.72) | 17.9 | 37.5 | 2.75 (2.61–2.89) |
| Oral anticoagulant | 5.8 | 8.1 | 1.45 (1.30–1.62) | 5.6 | 9.2 | 1.71 (1.57–1.86) |
| Nitroglycerin | 16.5 | 27.5 | 1.91 (1.79–2.05) | 12.7 | 22.8 | 2.03 (1.92–2.15) |
| Treatment in hospital | ||||||
| Anticoagulants (subcutaneous, intravenous) | 57.7 | 62.7 | 1.23 (1.16–1.31) | 37.5 | 42.6 | 1.24 (1.18–1.30) |
| Beta‐blocker (intravenous) | 31.9 | 28.1 | 0.83 (0.78–0.89) | 40.4 | 35.7 | 0.82 (0.78–0.86) |
| Reperfusion† | 41.1 | 31.0 | 0.64 (0.61–0.69) | 36.8 | 26.1 | 0.60 (0.57–0.64) |
| Revascularisation‡ | 5.1 | 3.9 | 0.75 (0.65–0.88) | 8.2 | 5.4 | 0.64 (0.58–0.71) |
| Complications at hospital | ||||||
| Atrial fibrillation | 13.9 | 18.5 | 1.42 (1.32–1.54) | 12.5 | 17.5 | 1.50 (1.41–1.60) |
| Heart failure | 37.6 | 50.7 | 1.71 (1.61–1.81) | 30.1 | 45.4 | 1.93 (1.84–2.03) |
| Treatment at discharge | ||||||
| Aspirin | 84.2 | 80.1 | 0.76 (0.70–0.82) | 87.7 | 84.1 | 0.74 (0.69–0.79) |
| Beta blocker | 79.7 | 74.6 | 0.75 (0.70–0.81) | 84.5 | 82.4 | 0.85 (0.80–0.91) |
| ACE inhibitor | 34.4 | 49.7 | 1.88 (1.77–2.00) | 40.5 | 57.6 | 1.99 (1.90–2.09) |
| Calcium antagonist | 13.3 | 19.3 | 1.56 (1.44–1.69) | 11.8 | 17.7 | 1.61 (1.51–1.72) |
| Lipid‐lowering drugs | 27.5 | 25.1 | 0.89 (0.82–0.95) | 58.9 | 54.8 | 0.85 (0.81–0.89) |
| Diuretics | 32.8 | 53.6 | 2.37 (2.23–2.53) | 29.2 | 51.6 | 2.59 (2.47–2.72) |
| Digitalis | 7.7 | 14.2 | 1.99 (1.81–2.18) | 5.3 | 10.8 | 2.16 (1.99–2.35) |
| Nitroglycerin | 36.3 | 45.2 | 1.45 (1.36–1.54) | 28.6 | 37.3 | 1.48(1.41–1.56) |
| Oral anticoagulant | 20.0 | 20.7 | 1.04 (0.97–1.13) | 22.4 | 23.8 | 1.08 (1.02–1.14) |
| Heparin | 6.5 | 6.0 | 0.91 (0.80–1.04) | 13.8 | 13.2 | 0.96 (0.89–1.02) |
| Glucose lowering therapy (oral) | 39.1 | 37.9 | ||||
| Insulin | 30.6 | 30.4 | ||||
| Combination | 7.7 | 11.6 | ||||
| Revascularisation <14 days | 13.4 | 10.2 | 0.74 (0.67–0.81) | 27.5 | 20.5 | 0.68 (0.64–0.72) |
Values are percentages if not otherwise stated.
*OR for age represents the increase in relative odds of being a diabetic patient for an increase of 1 SD of age (10.2 years) in the whole sample.
†Reperfusion; acute thrombolysis or acute PCI.
‡Revascularisation; acute PCI or CABG or both.
Changes over time
During the time period studied, the prevalence of known diabetes changed from 20.9% in 1995 to 22.5% in 2001. Previous hypertension, revascularisation and smoking became more common and previous MI less common within the diabetic cohort. Non‐diabetic patients showed a similar but less pronounced change causing diabetic patients to present with a more serious disease history at admission over time (Table 1). Comparing the first and second time period, pre‐admission use of aspirin, beta‐blockers, ACE inhibitors and lipid‐lowering drugs increased among diabetic patients, while the use of nitroglycerin, diuretics and calcium‐channel‐blockers became less frequent. Similar but less pronounced changes were observed in non‐diabetic patients (Table 1). In patients without diabetes admission ECG showed ST elevation in 47% of patients during the first time period and in 43% during the second time period. The corresponding values for patients with diabetes were 40% and 33%.
In‐hospital treatment trends
Comparing the first time period with the second, there was an increased use of intravenous beta‐blockers and acute revascularisation in hospital and at‐discharge use of aspirin, beta‐blockers, ACE inhibitors, lipid‐lowering treatments, heparins, anticoagulation and revascularisation within 14 days; the use of diuretics, digitalis and nitroglycerin decreased in patients both with and without diabetes. Diabetic patients less often received aspirin (84% versus 88%), beta‐blockers (82% versus 84%), lipid‐lowering drugs (55% versus 59%), and revascularisation within 14 days (20% versus 28%). For glucose‐lowering therapy there was no change from the first to the second time period in the use of insulin or oral agents (Table 1).
Multiple adjustments
The propensity score adjusted ORs for in‐hospital treatments during the two time periods are shown in Figure 1. Reperfusion therapy (thrombolysis or acute PCI) (OR 0.85, 95% CI 0.80 to 0.90), acute revascularisation (acute PCI or CABG) (OR 0.78, 95% CI 0.69 to 0.87), aspirin (OR 0.90, 95% CI 0.84 to 0.98), lipid‐lowering treatment at discharge (OR 0.81, 95% CI 0.77 to 0.86) and revascularisation within 14 days (OR 0.80, 95% CI 0.75 to 0.85) remained significantly less frequent among diabetic patients during the second time period, while beta‐blockers, heparins and anticoagulation therapy were prescribed to an equal proportion of patients with and without diabetes at discharge.
Mortality
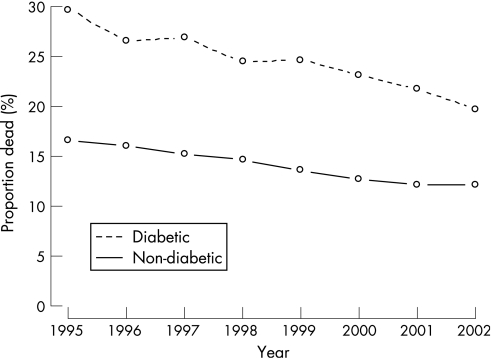
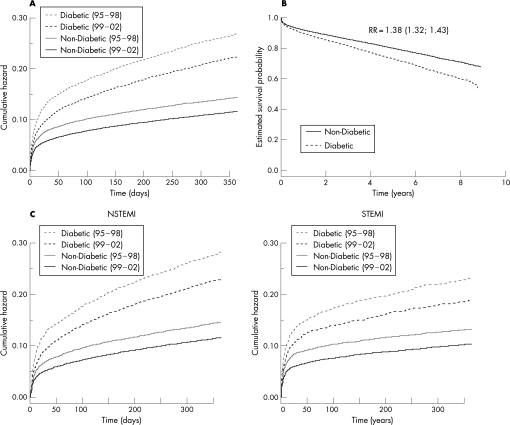
The crude 1‐year mortality rate decreased from 1995 to 2002 from 16.6% to 12.1% in patients without diabetes compared with 29.7 to 19.7%, respectively, in those with diabetes. An improvement in mortality rate was seen in both groups, but a fatal outcome remained higher among diabetic patients (Figure 2). Mortality rate within 30 days decreased from 18.3% in 1995 to 10.6% in 2002 for patients with diabetes and from 10.2% to 6.7% in those without diabetes. One‐year survival rate curves are shown in Figure 3A and long‐term survival rate in Figure 3B. The unadjusted estimated RR for diabetic patients during the complete time period was 1.95 (95% CI 1.89 to 2.01). Following adjustments (propensity score and in‐hospital and discharge treatment) the RR was 1.38 (95% CI 1.32 to 1.43). The adjusted RR decreased from 1.44 (95% CI 1.36 to 1.52) in 1995–1998 to 1.31 (95% CI 1.24 to 1.38) in 1999–2002 (p = 0.012). Long‐term survival rate curves separate over time to the disadvantage of patients with diabetes, especially in the youngest age group. During the whole time period diabetic patients <65 years had an adjusted RR of 1.66 (95% CI 1.50 to 1.84), 65–74 years 1.42 (1.33 to 1.51) and >74 years 1.34 (1.26–1.42). The decreasing relative risk per age group was statistically significant (p for trend <0.001). The decrease in the 1‐year mortality rate was of the same magnitude in different levels of hospitals (from university to local) both for patients with and without diabetes. One‐year mortality rate in the whole cohort was 14% after admission for ST elevation MI (STEMI) and 17% after non‐STEMI (NSTEMI). The pattern of higher mortality rate in NSTEMI patients was similar for patients with and without diabetes during both time periods. This pattern remained after exclusion of patients admitted with left ventricular bundle block (LBBB; Figure 3C). After adjusting for difference in treatment and other risk factors by the propensity score, diabetic NSTEMI patients had a RR from mortality rate during the whole study‐period of 1.42 (95% CI 1.35 to 1.50) while the corresponding RR for diabetic STEMI patients was 1.36 (95% CI 1.26 to 1.45).
Figure 2 Crude one‐year mortality rates in patients with and without diabetes with MI in 1995–2002.
Figure 3 (A) Estimated 1‐year cumulative HR of death for diabetic (dashed line) and non‐diabetic (solid line) patients during the two time periods: 1995–1998 (grey line) and 1999–2002 (black line). (B) Estimated long‐term survival rate curves in patients with and without diabetes and MI in 1995–2002. RR represents the estimated RR with corresponding 95% CI for mortality rate over the complete time period after adjustments by propensity score and in‐hospital/discharge treatment in a Cox regression model. (C) Estimated 1‐year cumulative HR of death after STEMI and NSTEMI for diabetic (dashed line) and non‐diabetic (solid line) patients during the two time periods 1995–1998 (grey line) and 1999–2002 (black line) after excluding ECG with LBBB.
Discussion
In this time‐trend analysis there is a clear‐cut improvement in survival rate after an acute MI over time, more apparent in patients with diabetes (33%) than in those without non‐diabetes (25%). The observed 10% absolute reduction in mortality rate over 8 years corresponds to one life saved for ten patients treated. Diabetes still remains an independent predictor for mortality rate, particularly in younger patients. Although patient management improved, reasons for the excess mortality rate may include shortcomings in the delivery of important treatment modalities and unsatisfactory secondary prevention in diabetic patients.
The Swedish register of coronary care (RIKS‐HIA) undergoes regular external monitoring ascertaining high data reliability. It offers excellent opportunities to mirror the everyday management of patients with MI with a sample size sufficient to compare two time periods within a total time‐frame of 8 years. This may seem a fairly short time‐span, but knowledge was gained rapidly and great efforts were made to create and distribute guidelines in this field. It is important to study whether such efforts are successful. A comparison of patients with and without diabetes is of particular interest since diabetic patients have been neglected over the years, not benefiting from therapeutic progress to the same extent as non‐diabetic patients.4,8
The propensity score method was applied in an attempt to overcome the problem of non‐randomised allocation of treatment in the diabetic and non‐diabetic patient. This score adjusts for factors that may have influenced how treatments were chosen. Accordingly, observed discrepancies in patient management should represent a true difference and not just reflect chance.
Treatment patterns improved substantially for all patients. Early revascularisation and the use of lipid‐lowering drugs became twice as common in patients with and without diabetes during the second time period, but the rate of interventions was still less common among diabetic patients, despite recent reports on their efficacy in this particular group.4,6,15,22 Revascularisation was offered to diabetic patients less often, even after adjustment for other risk factors. In the Fragmin and Fast Revascularisation During Instability in Coronary Artery Disease (FRISC) 2 study early revascularisation reduced mortality rate and reinfarction by almost 40% in diabetic patients with unstable coronary artery disease.6 Reperfusion treatment was used less frequently in diabetic patients. This might be because of a misinterpretation of symptoms leading to longer admission times, or to problems in diagnosing diabetic patients because of atypical symptoms at the onset of a MI.23 Another misconception may be that thrombolytic drugs cause bleeding complications in diabetic patients.24
Lipid‐lowering treatment was prescribed less often to diabetic patients despite their need for especially aggressive lipid‐lowering treatment.22
The use of ACE inhibitors was higher in diabetic patients, probably reflecting a more aggressive treatment of hypertension and prevention of renal disease in diabetic patients, but also their higher prevalence of heart failure. However, in the light of the Heart Outcomes Prevention Evaluation (HOPE) and the European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease (EUROPA) trials, there still seems to be a need for more extensive use, especially in diabetic patients.
The importance of an aggressive glucose‐lowering treatment for outcome in diabetic patients with MI was highlighted by the Diabetes and Insulin–Glucose Infusion in Acute Myocardial Infarction (DIGAMI) trials.25,26,27 Benefits of strict glucose control have also been reported in other intensive care populations.28 However, the use of oral glucose‐lowering drugs and insulin was unchanged over time. Unfortunately no details on metabolic control, in hospital or during follow‐up, or on the type of diabetes are available in the RIKS‐HIA.
Importantly, the extended follow‐up time revealed a successively increasing difference in adjusted mortality rate, to the disadvantage of diabetic patients. Factors such as cardiac dysfunction and less intense treatment of the MI may expose them to an increased risk for worse long‐term outcome compared with those without diabetes. It may also be an expression of the demand for a more aggressive secondary prevention combined with treatment aiming for normoglycaemia.27,29,30
Despite these concerns the encouraging significant decrease over time in the RR of mortality (1.44 to 1.31) for a diabetic patient should be acknowledged. Improved pre‐infarction management of cardiovascular risk factors together with increased in‐hospital use of aspirin, anticoagulants, beta‐blockers, statins, ACE inhibitors and early revascularisation during the second time period are probable contributors. Factors not covered by the registry may also make a contribution, such as diabetes duration and glucose control before, during and after the infarction. Future research should be directed towards improved understanding and management of these factors in diabetic patients with MI. It could be argued that a trend towards more NSTEMI and less STEMI could be an explanation of the findings of improved mortality rate. However, as presented in the results, during both time periods there was a higher mortality rate after NSTEMI (with or without LBBB) compared with STEMI for patients both with and without diabetes, and the RR of mortality after NSTEMI/STEMI between the time periods did not change. However, the absolute risk was improved the later time periods, with improved mortality rates after STEMI and NSTEMI for patients both with and without diabetes. Thus our results, with a difference in mortality rates between patients with and without diabetes and the improvement seen among patients with diabetes, could not be explained by a change to more NSTEMI during the later time period.
Study limitations
A confounding factor is the introduction of new and widened diagnostic criteria for MI.17,18 These criteria were not introduced until late 2001, thereby affecting only a limited part of the study population. Moreover the related increase in the incidence of MI was, when checked for Sweden, mostly seen in patients >80 years of age. Patients >80 years old were not included in the present investigation, because of the risk of co‐morbidities not accounted for by the registry. As previously discussed some important factors that might have influenced both the selection of treatment and outcome were not recorded in the database, ie, serum creatinine, left ventricle ejection fraction and glucose control.
Conclusions
Survival rate after acute MI in patients with diabetes has improved over the last few years. As recorded in the RIKS‐HIA, the most important contributing factors are improvements in the use of evidence‐based pharmacological and interventional treatments and an expanded use of secondary prevention. There are, however, ample opportunities for further improvements in the care of diabetic patients with MI. Diabetes is as an important risk factor for short‐ and long‐term mortality rate, partly relating to underlying co‐morbidities but also to a less than optimal use of established treatment modalities, especially lipid‐lowering therapy and early revascularisation.
Abbreviations
CABG - coronary artery bypass graft
LBBB - left ventricular bundle block
MI - myocardial infarction
NSTEMI - non‐ST elevation myocardial infarction
OR - odds ratio
PCI - percutaneous revascularisation
RIKS‐HIA - Swedish Register of Information and Knowledge about Swedish Heart Care Admission
STEMI - ST elevation myocardial infarction
Footnotes
Funding: The Swedish Heart‐Lung Foundation, The Swedish Association of Local Authorities, The Swedish AFA, the Swedish National Board of Health and Welfare and the Swedish Society of Cardiology supported this study.
Competing interests: None.
References
- 1.Malmberg K, Rydén L. Myocardial infarction in patients with diabetes mellitus. Eur Heart J 19889259–264. [DOI] [PubMed] [Google Scholar]
- 2.Granger C B, Califf R M, Young S.et al Outcome of patients with diabetes mellitus and acute myocardial infarction treated with thrombolytic agents. J Am Coll Cardiol 199322920–925. [DOI] [PubMed] [Google Scholar]
- 3.Woodfield SL, Lundergan CF, Reiner JS, et al. for the Gusto‐1 Angiographic Investigators. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO‐1 experience. J Am Coll Cardiol 1996281661–1669. [DOI] [PubMed] [Google Scholar]
- 4.Norhammar A, Malmberg K, Rydén L, Tornvall P, Stenestrand U, Wallentin L, for the Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (RIKS‐HIA) Under utilisation of evidence‐based treatment partial explanation for the unfavorable prognosis in diabetic patients with acute myocardial infarction. Eur Heart J 200324838–844. [DOI] [PubMed] [Google Scholar]
- 5.Otter W, Kleybrink S, Doering W.et al Hospital outcome of acute myocardial infarction in patients with and without diabetes mellitus. Diabet Med 200421183–187. [DOI] [PubMed] [Google Scholar]
- 6.Norhammar A, Malmberg K, Diderholm E.et al Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol 20044385–91. [DOI] [PubMed] [Google Scholar]
- 7.Bertrand M E, Simoons M L, Fox K A A.et al The Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology. Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J 2002231809–1840. [DOI] [PubMed] [Google Scholar]
- 8.Gu K, Cowie C C, Harris M I. Diabetes and decline in heart disease mortality in US adults. JAMA 19992811291–1297. [DOI] [PubMed] [Google Scholar]
- 9.Malmberg K, Yusuf S, Gerstein H C.et al Impact of diabetes on long‐term prognosis in patients with unstable angina and non‐Q‐wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 20001021014–1019. [DOI] [PubMed] [Google Scholar]
- 10.McGiure D K, Emanuelsson H, Granger C B, et al., for the GUSTO II‐b Investigators Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO II‐b study. Eur Heart J 2000211750–1758. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson I, Hildebrandt P, Seibaek M, et al., for the TRACE Study Group Long‐term prognosis of diabetic patients with myocardial infarction. Relation to antidiabetic treatment. Eur Heart J 2002211937–1943. [DOI] [PubMed] [Google Scholar]
- 12.Löwel H, Koenig W, Engel S.et al The impact of diabetes mellitus on survival after myocardial infarction. Can it be modified by drug treatment? Diabetologia 200043218–226. [DOI] [PubMed] [Google Scholar]
- 13.Eagle K A, Goodman S G, Avezum A, Budaj A, Sullivan C M, López‐Sendón J, for the Grace Investigators Practice variation and missed opportunities for reperfusion in ST‐segment‐elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE). Lancet 2002359373–377. [DOI] [PubMed] [Google Scholar]
- 14.Gitt A, Schiele R, Wienbergen H et al., for the MITRA study Intensive treatment of coronary artery disease in diabetic patients in clinical practice: results of the MITRA study. Acta Diabetol 200340S343–S347. [DOI] [PubMed] [Google Scholar]
- 15.Schnell O, Schafer O, Kleybrink S.et al Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry. Diabetes Care 200427455–460. [DOI] [PubMed] [Google Scholar]
- 16.Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P.et al Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Circulation 199490583–612. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000211502–1513. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 200036959–969. [DOI] [PubMed] [Google Scholar]
- 19.Joffe M M, Rosenbaum P R. Invited commentary: propensity scores. Am J Epidemiol 1999150327–333. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum P R, Rubin D B. The central role of the propensity score in observational studies for causal effects. Biometrika 198341–55.
- 21.R Development Core Team R: A language and environment for statistical computing (manual). R Foundation for Statistical Computing, Vienna, Austria, www.R‐project.org 2004
- 22.Colhoun H M, Betteridge D J, Durrington P N, Hitman G A, Neli H A, Livingstone S J, Thomason M J, Mackness M I, Charlton‐Menys V, Fuller JH; CARDS investigators Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet 2004364685–696. [DOI] [PubMed] [Google Scholar]
- 23.Janand‐Delenne B, Savin B, Habib G.et al Silent myocardial ischemia in patients with diabetes. Who to screen. Diabetes Care 1999221396–1400. [DOI] [PubMed] [Google Scholar]
- 24.McGuire D K, Emanuelsson H, Granger CB et al., for the GUSTO II‐b Investigators Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO II‐b study. Eur Heart J 2000211750–1758 [DOI] [PubMed] [Google Scholar]
- 25.Malmberg K, Rydén L, Efendic S et al., on behalf of the DIGAMI study group Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 19952657–65. [DOI] [PubMed] [Google Scholar]
- 26.Malmberg K, Norhammar A, Wedel H.et al Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results form the Diabetes and Insulin–Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999992626–2632. [DOI] [PubMed] [Google Scholar]
- 27.Malmberg, L Rydén, H. Wedel et al., for the DIGAMI 2 Investigators. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 200526650–661. [DOI] [PubMed] [Google Scholar]
- 28.Van den Berghe G, Wouters P J.et al Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Cri Care Med 200331359–366. [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study (UKPDS) Group Intense blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998352103–117. [PubMed] [Google Scholar]
- 30.Gaede P, Vedel P, Larsen N.et al Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 200330348, 383–348, 393. [DOI] [PubMed] [Google Scholar]