Abstract
Sixteen male Long-Evans rats were tested on a modified version of Flaherty et al's (1994) anticipatory contrast paradigm to assess memory for the anticipation of reward. Prior to testing each rat received either a control or quinolinic acid induced lesion of the agranular insular cortex. In the home cage, each rat was allowed to drink a water solution containing 2% sucrose for 3 min followed by a water solution containing 32% sucrose for 3 min. Across 10 days of testing, the control rats showed significantly increased anticipatory discriminability as a function of days. In contrast, rats with agranular insular cortex lesions failed to show anticipatory discriminability. The results of a preference task revealed that both groups could perceptually discriminate between a 2% and a 32% sucrose solution. The data suggest that the agranular insular cortex may be involved in the anticipation of reward.
Keywords: agranular insular cortex, reward, anticipatory contrast
The Role of the Agranular Insular Cortex in Anticipation of Reward Contrast
It has been suggested that the orbital frontal cortex (OFC) which includes the agranular insular cortex plays an important role in the anticipation or expectancy of reward value. Support for this suggestion comes from studies with rats, monkeys and humans. Expectancy of a reward can be studied in both reference and short-term memory or working memory paradigms. On the basis of recording studies from the OFC it has been shown in rats that cells in the OFC respond when short delays are interposed between responses and reward delivery (Schoenbaum, Chiba, & Gallagher,1998). In monkeys OFC cells fire in relation to the expectancy of delivery of reward vs. no reward in a delayed reaction time task (Hikosaka & Watanabe, 2000). Similar results were reported by Tremblay & Schultz (1999) who demonstrated that selective firing of OFC cells changed when during a delay between the response and reward delivery the incentive outcome was changed. With humans it has been shown with the use of fMRI that based on the presentation of the appropriate visual cue the expectation of a pleasant taste was sufficient in producing activation in the OFC (O'Doherty, Deichmann, Critchley, & Dolan, 2002). These studies suggest that the OFC supports the anticipation of a reward as measured across a delay between a cue or a response and a reward.
Further evidence is based on lesion studies which indicate that lesions of the orbital frontal cortex produce a short-term memory or working memory deficit for odor information (Otto & Eichenbaum, 1992). Similarly temporary inactivation of the agranular insular cortex component of the OFC disrupts short-term memory or working memory for odor locations (DiPietro, Black, Green-Jordan, Eichenbaum & Kantak, 2004). Furthermore, lesions of the agranular insular cortex component of the OFC can also disrupt short-term or working memory for a reward value based anticipated motor responses (DeCoteau, Kesner, & Williams, 1997; Ragozzino & Kesner, 1999). Thus, in general, it appears that the agranular insular cortex component of the OFC may play an important role in mediating the anticipation of reward value as measured across a delay between a cue or a response and a reward as well as across a delay between reward value and a response. Since most of the data that is cited above is based on short-term or working memory, it is important to determine whether the agranular insular cortex could also play a role in acquisition of a reference memory task for food reward.
In order to examine this idea further we used a reference memory task based on the anticipation of a reward labeled as an anticipatory contrast paradigm Flaherty, Turovsky, & Krauss (1994), .Flaherty et al. (1994) demonstrated that intake of a less preferred substance such as a water solution containing 2% sucrose is suppressed when the substance is followed by a preferred substance such as a water solution containing 32% sucrose. This anticipatory contrast effect is suggested to occur because the less preferred solution is devalued by the availability of the preferred 32% solution, therefore, intake of the less preferred 2% solution will decrease in anticipation of the preferred solution. Flaherty et al. (1994) have demonstrated that this effect is dependent on the magnitude of relative hedonic difference between the two substances. .
Gilbert and Kesner (2002) used a modified version of Flaherty et al's (1994) anticipatory contrast paradigm to examine the development of anticipatory behavior for reward value. Prior to testing each rat received either a control, hippocampal, or amygdala lesion. In the home cage, each rat was allowed to drink a water solution containing 2% sucrose for 3 min followed 15 sec later by a water solution containing 32% sucrose for 3 min. Across 10 days of testing, the rats in each lesion group showed significantly increased anticipatory discriminability as a function of days, suggesting that both the hippocampus and the amygdala can learn to anticipate reward value. However, when on transfer tests the values were changed to 2% vs 16%, in comparison to controls, the amygdala lesioned rats did not show the negative constrast effect, suggesting difficulty in separating reward value (Gilbert and Kesner,2002). The purpose of the present study was to examine the role of the agranular insular cortex as part of the OFC in the anticipatory contrast paradigm with the prediction that this brain area may play an important role in the acquisition of this task. It is, therefore predicted that an agranular insular cortex lesion might produce an impairment in the acquisition of an anticipatory discrimination.
Methods
Subjects
Sixteen male Long-Evans rats were used as test subjects. Each subject was individually housed in hanging metal cages. Each rat was slightly food-deprived to 90-95% of free-feeding weight and had continuous access to water with the exception of 15 min prior to testing. Subjects were maintained on 12-hour diurnal light-dark cycle. All testing was conducted in the home cage.
Surgery
Prior to testing, each rat was randomly assigned to receive either a quinolinic acid-induced lesion of the agranular insular cortex (n=8) or a sham operation (n=8). Prior to surgery, each animal was given atropine sulfate (0.25 mg/kg, i.p.) and anesthetized with sodium pentobarbital (Nembutal; 55 mg/kg, i.p.). Each animal was placed in a stereotaxic instrument and an incision was made in the skin covering the skull. The bone overlying the targeted brain region was removed with a small dental burr. Quinolinic acid-induced lesions of the agranular insular cortex were generated by infusing 0.125 M quinolinic acid into the brain via a 33 gauge internal cannula that was attached to a 10 μl Hamilton syringe by 0.38 mm polyethylene tubing. The injection cannula was mounted in the stereotaxic unit. Each rat received three quinolinic acid injections (0.6μl/site) into each hemisphere for a total of six injections. The solutions were slowly infused (0.2 μl/min) into each site and the cannula was left in place for 2 min following each injection to allow complete diffusion. The lesion coordinates were AP+4.9 from bregma, ML±2.6 from midline suture, DV-3.0 from dura; AP+3.9, ML±3.7, DV-4.4; AP+2.9, ML±4.5, DV-4.8. All lesion coordinates were based on Paxinos and Watson's stereotaxic atlas (1986).
Following all surgical procedures, each animal was sutured, injected with 3 cc (sc) saline in each hip to hydrate the animal, and placed on a heating pad to recover. Each animal was given a 7-10 day recovery period before testing.
Anticipatory Discrimination
Each rat was tested on a modified version of the Flaherty et al. (1994) anticipatory contrast paradigm. This paradigm was developed to measure anticipatory intake of a less preferred sucrose solution when it is followed by a preferred sucrose solution. All testing took place in the subjects' home cages. Prior to testing, each rat was deprived of water for 15 min. Each rat was given one daily trial that consisted of a sample phase followed by a test phase. On the sample phase, each rat was presented with a water solution containing 2% sucrose to drink for 3 min. The sucrose solution was presented in a 100 ml graduated cylinder attached to the front of the cage with a drinking spigot that extended into the cage similar to the daily water bottle. Following a 15 s delay where no drinking solution was present, the test phase was administered. On the test phase, each rat was given a water solution containing 32% sucrose to drink for 3 min. The amount of the solution consumed on both the sample and test phase were each recorded by the experimenter and used as the dependent measure. The procedure (2%-32%) was followed five days a week across 10 days.
Discrimination Preference Task
A preference task was implemented to test whether animals in each lesion group could perceptually discriminate between two simultaneously presented water solutions containing different amounts of sucrose. Following testing on the anticipatory discrimination task, each rat was tested for five days on a preference discrimination task. Testing was again conducted in the home cage. On discrimination trials, two graduated cylinders similar to those used in the prior experiment were presented simultaneously to each rat for 15 min daily across five consecutive days. One cylinder contained a water solution with 2% sucrose and the other contained water solution with 32% sucrose. Each day one cylinder was presented on the right-hand side of the face of the cage and the other was present on the left-hand side of the cage. The position of each solution was randomly determined each day such that each solution was randomly presented on both the left and right side. The amount consumed of each solution was recorded by the experimenter and used as the dependent measure.
Histology
At the conclusion of all testing, each animal was deeply anesthetized with an injection of 1.5 ml sodium pentobarbital (60 mg/kg; i.p.), and perfused intracardially with saline followed by a 10% formalin solution. The brain was removed from the skull and stored in a 10% formalin/30% sucrose solution. Each brain was frozen and cut at 24 μm sections throughout the lesion area. Every third section was mounted on a glass slide, stained with cresyl violet, and examined for histological verification of the lesion placement.
Results
Histology
Representative example of the smallest and largest agranular insular cortex lesions are shown in Figure 1. All the rats received bilateral removal along the entire anterior-posterior axis of the agranular insular cortex. The anterior components of the larger lesions contained some damage to the ventral portions of the orbital frontal cortex. The posterior aspect of the larger lesions included damage to the ventral parietal cortex and the dysgranular insular cortex. Granular insular cortex was spared in all of the agranular insular cortex lesions.
Figure 1.
Illustrations of the smallest (black) and largest (stippled) agranular insular cortex lesions.
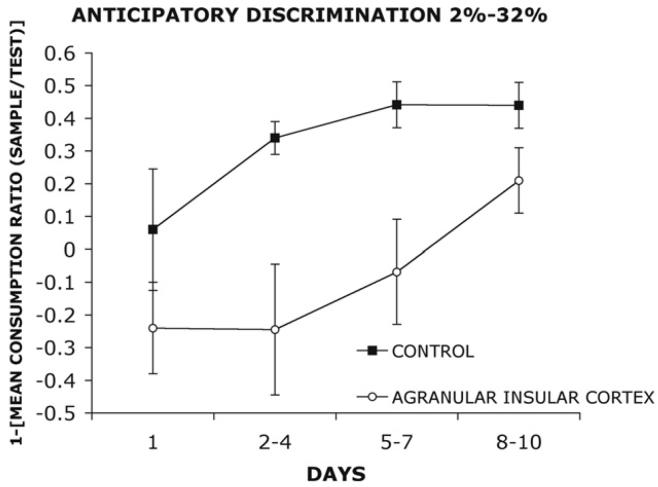
Anticipatory Discriminability
Figure 2 shows the anticipatory discriminability of control and agranular insular lesioned rats. As previously described by Gilbert and Kesner (2002) using this paradigm, the data were converted to ratio scores (amount consumed [mL] on the sample phase/amount consumed [mL] on the test phase) to normalize the scores, control for individual differences, and account for differences in intake across the groups. This intake ratio score was then subtracted from “1” so that scores near “1” were indicative of increased anticipatory discriminability and scores approaching “0” were indicative of impaired performance or low anticipatory discriminability. Increased anticipatory discriminability, therefore, is defined as a high intake ratio. The data show that control rats initially show low mean intake ratio scores on Day 1 but show increased intake ratio scores across Days 2-4, 5-7, and 8-10. Therefore, the intake ratio scores increase as a function of days of testing, indicating increased anticipatory discriminability. However, rats with agranular insular cortex lesions show low mean intake ratio scores across Days 1, 2-4, and 5-7. The intake ratio scores do increase on Days 8-10 but do not reach the level of control rats. The data indicate that compared to controls the anticipatory discriminability is impaired in rats with agranular insular cortex lesions. The data were grouped into one block of baseline trials (Day 1) and three blocks of three trials (Day 2-4, Day 5-7, Day 8-10) for analysis. A repeated-measures two-way analysis of variance with lesion (control, agranular insular cortex) as a between group factor and block (Days 1, 2-4, 5-7, 8-10) as a within factor revealed a significant main effect of group F(1, 14) = 8.12, p < .01 and a significant main effect of block F(3, 42) = 5.28, p < .01, but there was no significant interaction F(3, 42) = .99, p = .41. A Newman Keuls comparison test of the main effect of block indicated that mean intake ratio scores were significantly higher on Days 8-10 compared to Day 1 and Days 2-4 (p < .05). One possibility is that the agranular insular lesioned rats drank more than controls on the combined sample and test phase of the experiment across blocks of trials (1, 2-4, 5-7, and 8-10). The means and standard errors are shown in Table 1 and indicate that there are no reliable differences between the two groups within a day and across days. A subsequent repeated measures analysis of variance with group (control, agranular insular cortex) as a between group factor and block (1, 2-4, 5-7, and 8-10) as a within group factor with total amount consumed on sample and test phase as the dependent variable did not reveal a significant main effect of group F(1,14)= .17, p=.69, a main effect of block F(3,42)= 1.66, p=.19, or a group × block interaction F(3,42)= .57, p=.64.
Figure 2.
Mean intake (ml) ratio scores as a function of days of testing of a water solution containing 2% sucrose followed by a solution containing 32% sucrose (2%-32%) in a group of control and agranular insular cortex lesioned rats. The data were converted to ratio scores (amount consumed on the sample phase/amount consumed on the test phase). This ratio score was then subtracted from “1”, so that high intake ratio scores were indicative of increased anticipatory discriminability and low ratio scores were indicative of low anticipatory discriminability.
Table 1.
Mean (± standard error) total intake (ml) of a water solution containing 2% sucrose and a solution containing 32% sucrose as a function of days of testing in a group of control and agranular insular cortex lesioned rats.
| Day 1 | Days 2-4 | Days 5-7 | Days 8-10 | |
|---|---|---|---|---|
| Control | 9.63 (.82) | 10.04 (.64) | 10.92 (.56) | 10.75 (.65) |
| Agranular Insular Cortex | 10.63 (1.18) | 9.38 (.91) | 10.88 (1.17) | 11.75 (.82) |
Discrimination Preference Task
Figure 3 shows mean intake (ml) across 15 min of simultaneously presented water solutions containing either 2% sucrose or 32% sucrose by control and agranular insular cortex lesioned rats. Rats in both lesion groups tended to prefer the 32% sucrose solution over the 2% sucrose solution as indicated by the increased intake of 32% sucrose solution relative to intake of the 2% sucrose solution when the two were presented simultaneously. Therefore, this preference indicates that both groups can perceptually discriminate between a 2% and 32% sucrose solution.
Figure 3.
Mean intake (ml) across 15 min of simultaneously presented water solutions containing either 2% sucrose or 32% sucrose by control and agranular insular cortex lesioned rats.
The data were grouped into one block of five trials for analysis. A two-way repeated-measures analysis of variance with lesion (control, agranular insular cortex) as the between factor and concentration (2%, 32%) as the within factor revealed a significant main effect of concentration F(1,14) = 72.28, p< .0001, indicating that animals tended to prefer the 32% sucrose solution over the 2% solution. However, the analysis did not detect a significant main effect of lesion F(1,14) = 1.56, p= .23 or a concentration × lesion interaction F(1,14) = 0.54, p= .48. These results indicate that there were no significant differences between control and agranular insular cortex lesioned rats on the preference task suggesting that agranular insular cortex lesions do not impair the ability to discriminate 2% and 32% sucrose solutions.
Discussion
The results of this study indicate that in contrast to controls rats with agranular insular cortex lesions do not display a negative contrast effect, suggesting that they have difficulty in anticipating a higher reward value across a 3 min delay between the 2% and 32% sucrose solutions. This effect cannot be due an increase in the amount of liquid consumption and cannot be due to the inability to discriminate betweeen a 2% and 32 % sucrose solution, because they preferred the 32% in comparison with the 2% sucrose solution in a simultaneous taste preference discrimination situation. In additional research we were able to show that these rats had no difficulty in discrimination between a 2% and an 8% sucrose solution. Thus, the agranular insular cortical lesions are not likely to affect taste discrimination. Even though this task is a reference memory task, the results are consistent with the findings that lesions or temporary inactivation of the agranular insular cortex disrupt short-term memory or working memory for food reward value and for odor locations (DeCoteau, Kesner, & Williams, 1997; DiPietro, Black, Green-Jordan, Eichenbaum, & Kantak, 2004, Ragozzino & Kesner, 1999). The exact contribution made by the agranular insular cortex to learning the negative contrast effect is not clear. It is possible that the difficulty in learning the task is due to the inability to generate the appropriate rule. This idea would be consistent with the proposal made by Wise, Murray and Gerfen (1996) and Kesner (2000) that the different subregions of the prefrontal cortex operate on different levels of rule complexity with the simplest rules assigned to the orbital frontal cortex including the agranular insular cortex. Yet, the agranular insular cortex lesioned rats can learn a simple taste discrimination, but have difficulty when the rule involves prospective or anticipatory coding. Perhaps the agranular insular cortex does process information at a somewhat higher level of complexity.
It is of interest to compare these results with a previous study in which lesions were made of the amygdala, an area that is highly interconnected with the OFC and agranular insular cortex. In this case rats with amygdala lesions had no difficulty in displaying a reliable increased anticipatory discriminability as a function of days, suggesting that the amygdala can learn to anticipate reward value. However, when on transfer tests the values were changed to 2% vs 16%, in comparison to controls, the amygdala lesioned rats did not show the negative constrast effect, suggesting difficulty in separating reward value (Gilbert and Kesner,2002). This suggests that the amygdala is sensitive to differences in magnitude of reward, whereas the agranular insular cortex may play a more important role in utilizing a rule to differentiate between magnitude of reward differences. Both brain regions are likely to play a role in short-term or working memory for magnitude of food reward (Kesner & Williams, 1995).
Thus, in conclusion it appears that the OFC and specifically the agranular insular cortex play an important role in the anticipation of reward when anticipation of an award of one magnitude with a reward of a different magnitude. Furthermore, this region may also play a role across a delay between differential reward value and a response.
Acknowledgments
This research was supported by NSF Grant BNS 892-1532 and Human Frontier Science Program Grant RG0110/1998B. The authors would like to thank Dr. Rose Roeloffs, Amra Peterson, Heather Luker, Kelly Symes, and Angela Jackson for their assistance in data collection. We would also like to thank Robert Schaffer and Benjamin Kunz for their capable histological work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- DeCoteau WE, Kesner RP, Williams JM. Short-term memory for food reward magnitude: The role of the prefrontal cortex. Behavioural Brain Research. 1997;88:239–249. doi: 10.1016/s0166-4328(97)00044-2. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behavioral Neuroscience. 2004;118:1042–1051. doi: 10.1037/0735-7044.118.5.1042. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Involvement of the amygdala but not the hippocampus in pattern separation based on reward value. Neurobiology of Learning and Memory. 2002;77:338–353. doi: 10.1006/nlme.2001.4033. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Turovsky J, Krauss KL. Relative hedonic value modulates anticipatory contrast. Physiology and Behavior. 1994;55:1047–1054. doi: 10.1016/0031-9384(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000;28:219–228. [Google Scholar]
- Kesner RP, Williams JM. Memory for magnitude of reinforcement: Dissociation between the amygdala and hippocampus. Neurobiology of Learning and Memory. 1995;64:237–244. doi: 10.1006/nlme.1995.0006. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behavioral Neuroscience. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1986. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Kesner RP. The role of the agranular insular cortex in working memory for food reward value and allocentric space in rats. Behavioural Brain Research. 1999;98:103–112. doi: 10.1016/s0166-4328(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Critical Reviews in Neurobiology. 1996;103:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]