Abstract
The restriction endonuclease Type II R.NmeDI from Neisseria meningitidis 2120 (serogroup C, ST-11 complex) was characterized. The cloned nmeDIR gene was expressed in Escherichia coli cells, and the endonucleolytic and restriction activities of R.NmeDI were then observed in vitro and in vivo. The nmeDIR gene consists of 1056 bp coding 351 aa protein with a calculated molecular weight of M(r) = 39 000 ± 1000 Da. The R.NmeDI enzyme was purified to apparent homogeneity following overexpression, using metal affinity chromatography. This enzyme recognizes a palindrome sequence and cleaves double-stranded DNA upstream and downstream of its recognition sequence (12/7) RCCGGY (7/12) (R = A/G, Y = C/T) cutting out a 25-bp fragment. R.NmeDI cleaves in two steps. The enzyme cleaves the first strand randomly on either side of the recognition sequence generating an intermediate, and the second cleavage occurs more slowly and results in the production of a final reaction product. The R.NmeDI endonuclease requires two recognition sequences for effective cleavage. The tetramer is an active form of the R.NmeDI enzyme.
INTRODUCTION
Type II restriction endonucleases (REases) are a diverse and very interesting group of proteins (1). They are components of restriction-modification systems (R-M) that protect bacteria and archaea against invasion by foreign DNA. The orthodox Type II REases are homodimeric or tetrameric enzymes that specifically cleave DNA at defined sites of 4–6 bp in length within the recognition sequence and require Mg2+ ions for catalysis. DNA digested with these enzymes produces restriction fragments with 5′ or 3′ overhangs or with blunt ends.
However, it has been found that many Type II REases do not fulfill all the criteria for the orthodox Type II REases (known as Type IIP REases) and possess unique properties. Taking into account DNA substrate requirements, oligomeric status, domains per monomer and site of cleavage, few additional subtypes of Type II REases were defined according to their unique properties, e.g. IIA, IIE, IIS (2).
Type II REases do not have any obvious homology at the level of their primary sequence but they have a similar structural core, a common catalytic motif PD … D/EXK and share a common mechanism of DNA cleavage (2,3). Currently, the REBASE database includes more than 3600 Type II REases possessing more than 250 distinct DNA sequence specificities (rebase.neb.com).
Several R-M systems have been identified in the chromosome of Neisseria meningitidis using bioinformatic methods. However, only a few systems have been experimentally characterized with respect to their recognition sequence and cleavage site (rebase.neb.com). We have previously reported that cognate M.NmeDI MTase recognizes sequence 5′ RCCGGB 3′ (4). In this article, we present the characterization of the new restriction endonuclease R.NmeDI from N. meningitidis 2120. This enzyme was purified to homogeneity and its sequence specificity was then determined. R.NmeDI recognizes sequence 5′ RCCGGY 3′, cleaving double-stranded DNA on both strands upstream and downstream of the recognition sequence, releasing 25-bp fragment. We have also demonstrated that NmeDI R-M system is capable of in vivo activity.
MATERIALS AND METHODS
NmeDI REase cloning, expression and purification
Escherichia coli ER2566 [F−λ fhuA2 [lon] ompT lacZ::T7 gene 1 gal sulA11 ▵(mcrCmrr)114::IS10 R(mcr-73::miniTN10-TetS)2 R(zgb-210::Tn10) (TetS) endA1 [dcm]] (New England Biolabs) and ER1821 [F− glnV44 e14−(McrA−) rfbDI? relA1? endA1 spoT1? thi− ▵(mcrC-mrr)114::IS10 (r−m−)] (New England Biolabs) were used for cloning and expression experiments.
The gene nmeDIM (701 899–702 555 nt; 1276 bp) was amplified from chromosomal DNA of N. meningitidis 2120 obtained from Dr D.C. Stein (University of Maryland, USA) and cloned into the XbaI and HindIII sites of pBAD33, resulting in the formation of plasmid pAK10. The fragment (700 689–701744 nt) that encodes the R.NmeDI REase was amplified using the same chromosomal DNA. This amplicon of 1056 bp was cloned into the NheI and SalI sites of pET28a (Novagen), resulting in the formation of plasmid pAK11. The nmeDIR gene was cloned with the nmeDIM gene present on a compatible plasmid pAK10. The fragment (700 689–702 555 nt; 2318 bp) that encodes both the R.NmeDI REase and M.NmeDI MTase was amplified from the chromosomal DNA of N. meningitidis 2120 and cloned into the NcoI and HindIII sites of pMPMA4Ω creating plasmid pAK12.
To express R.NmeDI REase, the E. coli ER2566 strain was transformed with pAK10 and pAK11. The single colony of the fresh transformants carrying both plasmids was used to inoculate 5 ml LB broth (5) supplemented with kanamycin (30 µg/ml) and chloramphenicol (34 µg/ml) for overnight incubation at 37°C. Five milliliters of the overnight culture was used to inoculate 1l of LB broth containing the appropriate antibiotics and 1% arabinose. When the OD600 of the culture reached 0.6, IPTG was added to a final concentration of 1 mM and the incubation was continued at 30°C for an additional 12 h.
The culture was centrifuged and the bacterial pellet was resuspended in 10 ml buffer containing 50 mM NaHPO4, 500 mM NaCl, 20 mM imidazole, 10 mM β-mercaptoethanol, 0.1% Tween 20 and 100 µM PMSF. After sonication the cellular debris was removed by centrifugation at 40 000g for 1 h and the supernatant was then applied to a 3-ml Ni-NTA Agarose column previously equilibrated with 100 ml of the above buffer. The column was washed with 100 ml of buffer containing 50 mM NaHPO4, 500 mM NaCl, 40 mM imidazole and 10% glycerol. The proteins were then eluted with increased concentration of imidazole (50 mM to 0.3 M) in the same buffer. R.NmeDI REase was eluted at 0.2–0.25 M imidazole. The homogeneity of the enzyme was determined by 10% SDS–PAGE. The total yield of R.NmeDI protein was 2.5 mg/l of induced culture.
Endonuclease activity assay
Endonucleolytic activity in vitro was assayed (6) by incubation of 0.3 pmol DNA with 3.0 pmol of purified R.NmeDI in a final volume of 20 µl containing 10 mM Tris–HCl (pH 7.5), 10 mM MgCl2 and 0.1 mg/ml bovine serum albumin for 2 h at 37°C. The cleavage products were analyzed using 0.7–1.2% TBE agarose, and 5% or 20% neutral polyacrylamide gels. DNA was visualized by staining with ethidium bromide.
Determination of the recognition sequence and the cleavage site
The recognition sequence was determined by mapping the location of cleavage sites on different substrate DNAs. This was achieved by comparison of the profiles of R.NmeDI and R.Cfr10I cleavage restriction fragments, as well as by the double digestion profile of different plasmid DNAs using both these enzymes.
To determine the cleavage site, phagemid DNA pBluescript KS II (+) was digested with R.NmeDI and was then sequenced using pKSRDIf (5′ CCACTACGTGAACCATCACC 3′) (221–240 nt) and pKSRDIr (5′ AGCGTGACCGCTACACTTG 3′) (408–389 nt) primers. Additionally, an extension reaction was carried out in the presence of four deoxynucleotides, [γ-32P] ATP labeled primer pKSRDIf or pKSRDIr and pBluescript KS II (+) as the template. The labeled product of this reaction was digested with R.NmeDI. Following the inactivation of the enzyme, the reaction mixture was divided into half: one-half was stopped immediately, and the other was treated with the Klenow Fragment at 37°C for 10 min in the presence of dNTPs. Both cleavage products were loaded onto 8% denaturing polyacrylamide gel along with the products of the sequencing reaction based on the dideoxynucleotide chain termination method using pBluescript KS II (+) as the template and the same primers as for the extension reaction.
Gel filtration
The enzyme samples (250 μg) were loaded onto a Sepharose G200 column (1 × 50 cm) which was equilibrated with a buffer containing 10 mM Tris–HCl, pH 7.5, 50 mM NaCl and 1 mM EDTA. Separation was carried out at a flow rate of 0.2 ml/min. The column was calibrated with dextran blue (2 MDa), thyroglobulin (670 kDa), R.Cfr10I [calculated tetramer molecular mass 128 kDa (7)], bovine serum albumin (66.2 kDa), restriction endonuclease EcoRV [calculated dimer molecular mass 57.2 kDa (8)] and lysozyme (14.4 kDa). Elution profiles were monitored by measuring absorbance at 280 nm and the specific λ DNA digestion profile for R.Cfr10I, R.EcoRV and R.NmeDI. For the interpolation of unknown molecular mass, a linear dependence of the logarithm of the molecular mass on the elution time was assumed.
In vivo restriction activity analysis
The restriction activity of E. coli cells carrying the nmeDIR gene was measured by determination of the plating efficiency of phage λvir. The efficiency of plaquing (eop) of λvir was calculated as the ratio of plaques formed on E. coli ER1821-containing plasmid encoding the NmeDI R-M system (pAK12) to those formed on the E. coli ER1821 containing pMPMA4Ω plasmid. The expression of the genes nmeDIM and nmeDIR was induced by 1% arabinose.
Other methods
Protein concentrations were measured according to the method of Lowry (9) using the Bicinchoninic Acid Kit (Sigma-Aldrich) with bovine serum albumin as a protein standard. All other methods used routine procedures (5).
Enzymes and chemicals
Restriction enzymes, T4 DNA ligase, Pfu DNA polymerase, IPTG (isopropyl-β-d-thiogalactopyranoside) and DNA and protein markers were purchased from Fermentas, and used under the conditions recommended by the manufacturer. Kits for DNA clean-up and plasmid DNA preparation were purchased from A&A Biotechnology, Gdansk, Poland. Ni-NTA Agarose was purchased from Qiagen. All the chemicals were purchased from Sigma, unless otherwise stated.
Computer analysis
DNA and protein sequences were compared with GenBank and SWISS-PROT databases on the BLAST server hosted by the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/blast). We used the genomic sequence of the N. meningitidis strain FAM 18 (www.sanger.ac.uk/Projects/N_meningitidis), which like strain 2120 belongs to serogroup C (complex ST-11).
RESULTS
Genetic organization of the NmeDI R-M system and comparison of R.NmeDI REase with other proteins
The nmeDIR gene (1056 bp) (accession number AJ 238948) encodes a protein consisting of 351 amino acids. The nmeDIR gene is associated with the nmeDIM gene, which encodes M.NmeDI MTase (4). The nmeDIR and nmeDIM have the same orientation but the first nine codons of the nmeDIR gene overlap with the 3′ end of the nmeDIM gene. The amino acid sequence of R.NmeDI was compared to protein sequences in GenBank and SWISS-PROT databases, using the BLAST suite of programs (10). No significant homology was observed with R.Cfr10I or R.BsrFI, which also recognize the 5′ RCCGGY 3′ sequence. However, the amino acid sequence of the R.NmeDI shows: (i) 72% similarity to a putative type II restriction endonuclease SusEORF1689P (acc. no. YP_822963.1), whose predicted recognition sequence is 5′ RCCGGY 3′ but whose cleavage site is unknown and (ii) 61% similarity to PatTORF727P (acc. no YP_660308.1), which is cognate to M.PatTORF727P MTase, whose predicted recognition sequence is 5′ RCCGGB 3′.
Purification of the restriction endonuclease R.NmeDI
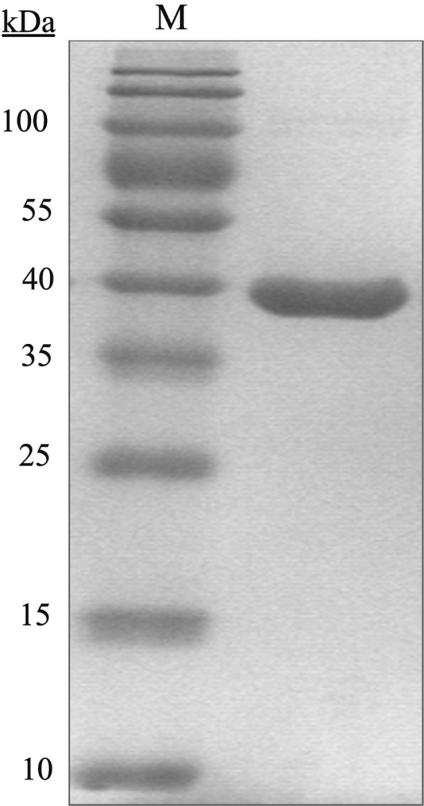
The R.NmeDI REase was purified to apparent homogeneity from E. coli ER2566 (pAK10 and pAK11) cells using metal affinity chromatography and a Ni-NTA Agarose column. The molecular mass of R.NmeDI was M(r) = 39 000 ± 1000 Da as estimated from SDS–PAGE (Figure 1) and was consistent with that predicted on the basis of the amino acid sequence.
Figure 1.
Determination of the molecular mass of R.NmeDI. Purified R.NmeDI separated on a 10% SDS–PAGE gel and stained with Coomassie Brilliant Blue R250. M, The PageRuler™ Prestained Protein Ladder (10, 15, 25, 35, 40, 55, 70, 100, 130 and 170 kDa) was used as the protein molecular mass marker.
Determination of the optimal conditions for the in vitro endonucleolytic activity of R.NmeDI REase
To determine the optimal conditions for the in vitro endonucleolytic activity of R.NmeDI REase, different buffers were tested, taking into account ionic strength, pH, Mg2+ ion concentrations and cofactors (ATP, AdoMet). The maximum activity of the enzyme was observed at 37°C in the presence of 10 mM Tris–HCl (pH 7.5), 10 mM MgCl2 and 0.1 mg/ml BSA in the absence of any salt or cofactors (data not shown).
Determination of the recognition sequence
R.NmeDI is associated with the cognate M.NmeDI MTase, which recognizes sequence 5′ RCCGGB 3′ (4) and this suggested that R.NmeDI recognizes the same sequence.
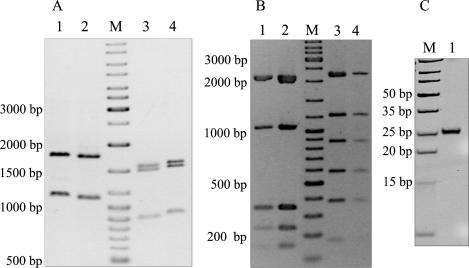
Comparison of the DNA fragments resulting from the cleavage of pBluescript KS II (+) and pBADHisA by R.NmeDI and R.Cfr10I both of which recognize the sequence 5′ RCCGGY 3′, showed the same banding pattern except that R.NmeDI fragments were smaller by ∼20–30 nt (Figure 2A). Double digestion of the pET28a and pBR322 plasmid DNAs with R.NmeDI and R.Cfr10I showed the presence of the same fragments as for the single digestion with R.Cfr10I (Figure 2B). The smaller size of the DNA fragments of pBluescript KS II (+) and pBADHisA obtained after cleavage with R.NmeDI compared to the size of those resulting from digestion with R.Cfr10I suggested that R.NmeDI cleavage takes place on either side of the recognition sequence to excise fragments of 20–50 nt. This was confirmed when the reaction products were separated on 20% PAGE (Figure 2C).
Figure 2.
Determination of the recognition sequence of R.NmeDI. (A) Comparison of the plasmid DNA cleavage by R.NmeDI and R.Cfr10I. Lane 1, pBluescript KS II (+) cleaved with R.Cfr10I; lane 2, pBluescript KS II (+) cleaved with R.NmeDI; lane 3, pBADHisA cleaved with R.NmeDI; lane 4, pBADHisA cleaved with R.Cfr10I. (B) Double digestion of the plasmid DNA with R.Cfr10I and R.NmeDI does not change the banding profile. Lane 1, pBR322 cleaved with R.Cfr10I and R.NmeDI; lane 2, pBR322 cleaved with R.NmeDI; lane 3, pET28a cleaved with R.NmeDI; lane 4, pET28a cleaved with R.Cfr10I and R.NmeDI. When double digestion was carried out the DNA was first digested with R.Cfr10I for 180 min and then with R.NmeDI for 180 min. M, DNA molecular weight marker: 10 000, 8000, 6000, 5000, 4000, 3500, 3000, 2500, 2000, 1500, 1200, 1000, 900, 800, 700, 600, 500, 400, 300, 200 and 100 bp. (C) R.NmeDI cleaves double-stranded DNA on both sides of its recognition sequence. Lane M, DNA Ladder, Ultra Low Range: 10, 15, 20, 25, 35, 50, 75, 100 and 150 bp; lane 1, pBluescript KS II (+) DNA digested with R.NmeDI and electrophoresed on 20% polyacrylamide gel in TBE buffer.
Based on the above results we conclude that R.NmeDI recognizes sequence 5′ RCCGGY 3′ and cleaves double-stranded DNA on both strands upstream and downstream of its recognition sequence to generate the 25-nt fragment. It can be concluded that R.NmeDI does not recognize sequence 5′ RCCGGB 3′ since the digestion of pBADHisA or pBR322 should generate 15 and 23 fragments, respectively.
Identification of the cleavage site of R.NmeDI
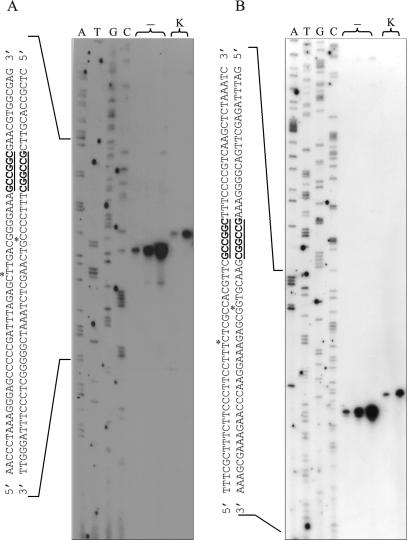
The cleavage site of R.NmeDI was determined by comparing dideoxynucleotide sequencing ladders with: (i) fragments generated by R.NmeDI cleavage of the extension reaction products and (ii) the action of the Klenow Fragment on these digestion products (Figure 3). To determine the precise cleavage site upstream of the recognition sequence, [γ-32P] ATP labeled primer pKSRDIf was used in the extension reaction. The fragment generated by R.NmeDI digestion of the labeled product co-migrates with a sequencing ladder band to a position 13 nt before the recognition sequence. This band corresponds to the nucleotide after which the cleavage occurs [Figure 3A, lanes (–)]. These results indicate that the cleavage point upstream of the recognition sequence occurs 12 nt before the 5′ RCCGGY 3′ sequence. Lane K in Figure 3A shows the result when the fragment generated by R.NmeDI digestion of the extension reaction product was further treated with the Klenow Fragment. The single band obtained by the action of the Klenow Fragment co-migrates with the sequencing ladder to a position 8 nt before the recognition sequence. From this result it can be inferred that the cleavage point is 7 nt before the 5′ RCCGGY 3′ sequence on the complementary DNA strand upstream of the recognition sequence.
Figure 3.
Determination of the cleavage site of R.NmeDI. DNA sequencing reactions were performed on pBluescript KS II (+) DNA containing the R.NmeDI recognition site using pKSRDIf or pKSRDIr primers. Labeled test DNA was generated using the same primers. The labeled substrate was then digested with R.NmeDI. After inactivating R.NmeDI, the reaction mixture was divided into two aliquots: one was mixed with stop solution immediately [lanes (−)]; the other was treated with Klenow Fragment at room temperature for 10 min and then mixed with stop solution (lane K). The R.NmeDI cleavage products were loaded onto 8% denatured polyacrylamide gel along with the products of the sequencing reaction based on the dideoxynucleotide chain termination method using pBluescript KS II (+) as the template and the same primers as for the extension reaction. (A) Determination of the cleavage site of R.NmeDI upstream of the recognition sequence. (B) Determination of the cleavage site of R.NmeDI downstream of the recognition sequence.
To determine the precise cleavage site downstream of the recognition sequence, [γ-32P] ATP labeled primer pKSRDIr was used in the extension reaction. As previously described, an analogous analysis of the results obtained was carried out. The position of the fragment resulting from R.NmeDI digestion of the labeled product in relation to the dideoxynucleotide sequencing ladder indicates that the cleavage site downstream of the recognition sequence is located 7 nt after sequence 5′ RCCGGY 3′. Analysis of the position of the fragment generated by R.NmeDI cleavage and further treated with the Klenow Fragment indicates that the cleavage site downstream of 5′ RCCGGY 3′ sequence on the complementary DNA strand occurs 12 nt after the recognition sequence (Figure 3B).
Both reaction products obtained from both extension reactions after cleavage with R.NmeDI and treatment with the Klenow Fragment were extended by 5 nt, indicating that R.NmeDI cleaves DNA to generate 5-nt 5′ extensions.
Summarizing, the data presented in Figure 3 indicate that R.NmeDI cleaves both DNA strands symmetrically upstream and downstream of the recognition sequence generating 5 nt 5′ overhangs, thus:
Determination of the quaternary structure of R.NmeDI
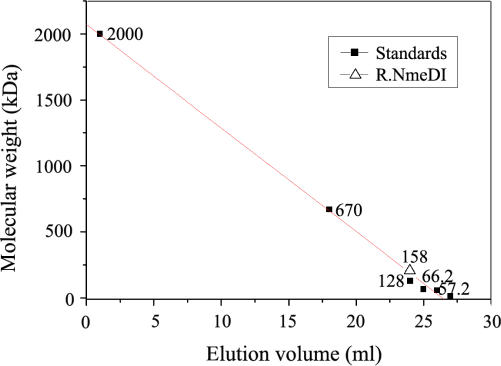
The quaternary structure of the R.NmeDI enzyme was determined by gel filtration chromatography. The active R.NmeDI enzyme was eluted from a Sepharose G200 column at a volume corresponding to a size of ∼160 kDa (Figure 4). This is consistent with the mass of R.NmeDI in the tetramer form (calculated tetramer molecular mass 158 kDa).
Figure 4.
Determination of the quaternary structure of R.NmeDI. Estimation of the subunit structure of the native R.NmeDI by gel filtration on a Sepharose G200 column. The quaternary structure of R.NmeDI was calculated by interpolation from the standard curve obtained using dextran blue (2 MDa) and proteins with known molecular masses [thyroglobulin (670 kDa), restriction endonuclease R.Cfr10I (calculate tetramer molecular weight 128 kDa), bovine serum albumin (molecular weight 66.2 kDa), restriction endonuclease EcoRV (calculate dimer molecular weight 57.2 kDa) and lysozyme (molecular weight 14.4 kDa)]. The calibration line was calculated by linear regression using Origin Lab software.
R.NmeDI endonuclease requires two recognition sequences for effective cleavage
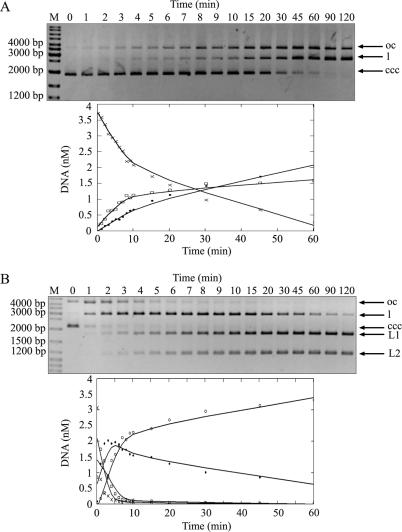
Several non-orthodox Type IIP REases are able to cleave substrate DNA effectively when more than one recognition sequence is present in the substrate DNA molecules (11–13). To test the dependence of the R.NmeDI cleavage rate on the number of recognition sites we compared the rate of cleavage of pUC18 and pBluescript KS II (+) containing one and two recognition sites, respectively. The data presented in Figure 5A show that pUC18 DNA is cleaved first in one strand converting the supercoiled form of DNA into an open circle form and that cleavage of the second strand resulting in the full-length form is never completed.
Figure 5.
R.NmeDI activity on plasmids with one or two recognition sequences. Reactions were carried out with 0.15 μM of R.NmeDI and 14.2 nM of supercoiled DNAs at 37°C. Aliquots were withdrawn at various times and the reactions were stopped by the addition of 1 μl of phenol and analyzed on 0.8% TBE agarose gel. pUC18 (A) containing one R.NmeDI site and pBluescript KS II (+) (B) containing two R.NmeDI sites were used as a substrate in the reactions. The top panels show agarose gels of time-course series of enzymatic digestion products taken from the reaction. All forms of plasmid DNA: supercoiled (ccc) (×-×), open circle (oc) (□-□), linear (l) (•-•) are marked by arrows. Additionally two linear products of digestion of pBluescript KS II (+) with the enzyme are marked as L1 and L2 (◦-◦). The graphs show the analysis of the data produced by fitting to a first-order rate equation using MathCAD software. The DNA in the substrate and product bands was determined using Gel/ChemiDoc (BioRad) analyzer and quantified with the accompanying software package (Quantity One). The amounts present in individual bands were expressed as a fraction of the total intensity of DNA as visualized by ethidium bromide staining.
DNA containing two recognition sites is converted into the open circle and full-length forms and the final reaction product more rapidly than DNA having only one recognition site (Figure 5B). The presence of a second recognition site in trans (pUC18 DNA and oligonucleotides containing one 5′ RCCGGY 3′ sequence) does not increase the rate of cleavage (data not shown).
Summarizing, R.NmeDI cleaves supercoiled DNA with two recognition sequences more quickly and effectively than DNA with only a single site. Moreover, R.NmeDI preferentially interacts with two recognition sites in cis rather than in trans.
R.NmeDI does not show site preference for cleavage
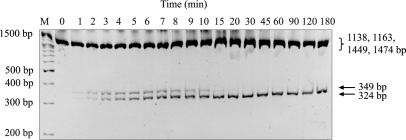
To test whether the enzymatic excision of a small DNA fragment containing the R.NmeDI recognition sequence is the consequence of one cleavage event or two independent cleavage events, we studied the cleavage intermediates. pBluescript KS II (+) DNA (2961 bp) was linearized with R.Cfr42I (site located at position 661 nt) and then digested for varying lengths of time with R.NmeDI (one recognized sequence is located at position 328 nt and another at 2126 nt). If R.NmeDI cleaves simultaneously on both sides of the recognition sequence, in addition to the 25-bp fragment, three fragments of 324, 1138 and 1449 bp should be generated. If R.NmeDI cleaves in two steps, the intermediate fragments of 349, 1163 (two such fragments) and 1474 bp should be generated first followed by the final products of 324, 1138 and 1449 bp created, in addition to the 25-bp fragment. The results presented in Figure 6 demonstrate the generation of the 349-bp intermediate cleavage product. This intermediate fragment is the result of the initial cleavage of DNA only upstream of recognition sequence at position 328 nt. The larger fragments were not resolved on this gel. Since the relative fluorescence of all the fragments obtained is similar, it has been concluded that cleavage on either side of the recognition sequence occurs randomly and at equal rates.
Figure 6.
Analysis of the preference of R.NmeDI cleavage site. A total of 14.2 nM pBluescript KS II (+) was linearized with R.Cfr42I, and then digested with 0.15 μM R.NmeDI for varying lengths of time. Samples were analyzed on 4% polyacrylamide gel and visualized by staining with ethidium bromide. M, DNA molecular weight marker: 10 000, 8000, 6000, 5000, 4000, 3500, 3000, 2500, 2000, 1500, 1200, 1000, 900, 800, 700, 600, 500, 400, 300, 200 and 100 bp.
The in vivo restriction activity of R.NmeDI REase
The NmeDI R-M system is able to restrict λvir phage in vivo. The efficiency of plaque-forming units of the virulent form of λ phage on E. coli ER1821 (pAK12) encoding the NmeDI R-M system was 1000 times lower than on control strains E. coli ER1821 containing pMPMA4Ω plasmid or E. coli ER1821 which did not contain a plasmid. The restriction level of bacteriophage λvir by NmeDI R-M is 10−3 (Table 1).
Table 1.
The efficiency of plating (eop) of λvir phage by the E. coli ER1821 strain carrying the R-M NmeDI system
| Bacterial strain | The efficiency of plating of λvir |
|---|---|
| E. coli ER1821 (pMPMA4Ω::nmeDIM + nmeDIR) | 1 × 10−3 |
| E. coli ER1821 (pMPMA4Ω) | 1 |
| E. coli ER1821 | 1 |
EOP (efficiency of plating): p.f.u. ml−1 formed on the indicator strain divided by p.f.u. ml −1 formed on the propagating strain. Data are means of at least three independent.
DISCUSSION
The new restriction endonuclease R.NmeDI encoded by N. meningitidis 2120 has been characterized in this article. R.NmeDI is a novel restriction endonuclease that recognizes the palindromic sequence (12/7) RCCGGY (7/12) (R = A/G, Y = C/T), and cleaves both DNA strands upstream and downstream of its recognition sequence excising small 25-bp fragments. The enzyme in its active form is a tetramer that cleaves supercoiled DNA with two recognition sequences more quickly and effectively than plasmid DNA with only a single site. The reaction rate for plasmid DNA with a single site is not improved even in the presence of oligonucleotides containing the 5′ RCCGGY 3′ sequence. This observation indicates that the enzyme interacts with two recognition sites in cis, probably through the formation of the DNA loop (14). The R.NmeDI enzyme converts a two-site plasmid into the final restriction fragments via the formation of an intermediate product. This intermediate product is formed because the DNA is cleaved in two steps. In the first step, DNA is digested on one side of the recognition sequence, and is followed by a second step in which cleavage takes place on the other side.
Cleavage on both sides of the recognition sequence is characteristic of Type IIB systems but all IIB enzymes studied so far have been found to recognize discontinuous (usually asymmetric) sites and a distinctive subunit/domain organization that is not present in the R.NmeDI enzyme. On the other hand, excision of small 25-bp fragments by R.NmeDI bears some similarities to the action of BcgI (15) and HaeIV (16) belonging to Type IIB system. However, conversely to these enzymes, R.NmeDI recognizes non-interrupted sequences and ATP and AdoMet do not have any influence on its activity.
Type IIB enzymes excise DNA fragments by cutting either side of their sites and some of them (R.BplI and R.AloI) (1) recognize palindromic sequences. Some of the Type IIB systems need no cofactor other than Mg2+ to cut DNA, like R.NmeDI (only some IIB enzymes need AdoMet) and most of the IIB enzymes need two sites to cut DNA, like R.NmeDI. R.NmeDI may be the prototype of a new subtype of Type II REases combining the features of several subtypes of Type II REases. On the one hand, R.NmeDI is similar to Type IIE or IIF enzymes that require two copies of the target site for effective cleavage (2,12). However, in contrast to Type IIF, R.NmeDI does not cleave both targets coordinately but carries out the process in two steps. Furthermore, in contrast to Type IIE, R.NmeDI cleaves dsDNA outside its recognition sequence and its activity is not stimulated by oligonucleotide duplexes containing the specific recognition site. In the case of Type IIE enzymes, one of the target sequences serves as an allosteric effector for the effective cleavage of the other recognition site.
Another intriguing possibility is that R.NmeDI is a Type IIS enzyme but with a palindromic recognition site. All other Type IIS enzymes recognize asymmetric sites and cleave at fixed distances from the recognition site on one side only, the upstream side. However, a Type IIS enzyme with a palindromic recognition site may bind in one orientation and excise the upstream DNA and may then return to the same site, re-bind in the opposite orientation and excise downstream DNA, thus eventually releasing the recognition sites on two-site DNA at equal rates. This is supported by the fact that with the exception of Type IIS enzymes most restriction enzymes, which use two sites, destroy the site as they cleave DNA. By cleavage at positions remote from their sites, the two-site DNA remaining after IIS action at one site still possesses two copies of the recognition sequence that can still interact with each other. Many Type IIS enzymes are monomers in solution but dimerize during cleavage of DNA, however, some are known to be tetramers (17). Type IIS systems generally have two MTases, one for each strand, but a Type IIS with a palindromic would need only one, as for example the R-M NmeDI system.
R.NmeDI is an example of a restriction endonuclease, which like R.Cfr10I and R.BsrFI recognizes sequence 5′ RCCGGY 3′, but unlike these enzymes has different reaction mechanisms. Examples of similar observations regarding R-M systems have been reported by Gowers et al. (18). These authors reported about seven different restriction enzymes with five different reaction mechanisms which all act on the same sequence.
To summarize, R.NmeDI represents a new subtype of Type II REases because of its unique properties.
ACKNOWLEDGEMENTS
This work was supported by KBN grant nos. 3 P04A 040 23 and 2 P04A 053 26. We would like to thank Kamil Olejnik for technical assistance with cloning. Funding to pay the Open Access publication charges for this article was provided by Faculty of Biology University of Warshaw.
Conflict of interest statement. None declared.
REFERENCES
- 1.Roberts RJ, Vincze T, Posfai J, Macelis D. REBASE – restriction enzymes and DNA methyltransferases. Nucleic Acids Res. 2005;33:D230–D232. doi: 10.1093/nar/gki029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, Bitinaite J, Blumenthal RM, Degtyarev SKh, Dryden DT, et al. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 2003;31:1805–1812. doi: 10.1093/nar/gkg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pingoud A, Fuxreiter M, Pingoud V, Wende W. Type II restriction endonucleases: structure and mechanism. Cell Mol. Life Sci. 2005;62:685–707. doi: 10.1007/s00018-004-4513-1. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatek A, Kobes M, Olejnik K, Piekarowicz A. Characterization of the DNA methyltransferases from Neisseria meningitidis and Neisseria gonorrhoeae FA1090 associated with mismatch nicking endonucleases. Microbiology. 2004;150:1713–1722. doi: 10.1099/mic.0.27011-0. [DOI] [PubMed] [Google Scholar]
- 5.Sambrook J, Russell D. 3rd edn. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 6.Brown NL, Smith M. A general method for defining restriction enzyme cleavage and recognition site. Methods Enzymol. 1980;65:391–404. doi: 10.1016/s0076-6879(80)65050-2. [DOI] [PubMed] [Google Scholar]
- 7.Siksnys V, Skirgaila R, Sasnauskas G, Urbanke C, Cherny D, Grazulis S, Huber R. The Cfr10I restriction enzyme is functional as a tetramer. J. Mol. Biol. 1999;291:1105–1118. doi: 10.1006/jmbi.1999.2977. [DOI] [PubMed] [Google Scholar]
- 8.Winkler FK, Prota AE. Structure and function of EcoRV endonuclease. Nucleic Acids Mol. Biol. 2004;14:179–214. [Google Scholar]
- 9.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 10.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang W, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wentzell LM, Nobbs TJ, Halford SE. The SfiI restriction endonuclease makes a four-strand DNA break at two copies of its recognition sequence. J. Mol. Biol. 1995;248:581–595. doi: 10.1006/jmbi.1995.0244. [DOI] [PubMed] [Google Scholar]
- 12.Bath AJ, Milsom SE, Gormley NA, Halford SE. Many type IIs restriction endonucleases interact with two recognition sites before cleaving DNA. J. Biol. Chem. 2002;277:4024–4033. doi: 10.1074/jbc.M108441200. [DOI] [PubMed] [Google Scholar]
- 13.Embleton ML, Siksnys V, Halford SE. DNA cleavage reactions by type II restriction enzymes that require two copies of their recognition sites. J. Mol. Biol. 2001;311:503–514. doi: 10.1006/jmbi.2001.4892. [DOI] [PubMed] [Google Scholar]
- 14.Halford SE, Welsh AJ, Szczelkun MD. Enzyme-mediated DNA looping. Ann. Rev. Biophys. Biomol. Struct. 2004;33:1–24. doi: 10.1146/annurev.biophys.33.110502.132711. [DOI] [PubMed] [Google Scholar]
- 15.Kong H, Morgan RD, Maunus RE, Schildkraut I. A unique restriction endonuclease, BcgI, from Bacillus coagulans. Nucleic Acids Res. 1993;21:987–991. doi: 10.1093/nar/21.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piekarowicz A, Golaszewska M, Sunday AO, Siwinska M, Stein DC. The HaeIV restriction modification system of Haemophilus aegyptius is encoded by a single polypeptide. J. Mol. Biol. 1999;293:1055–1065. doi: 10.1006/jmbi.1999.3198. [DOI] [PubMed] [Google Scholar]
- 17.Gormley NA, Hillberg AL, Halford SE. The Type IIs restriction endonuclease BspMI is a tetramer that acts concertedly at two copies of an asymmetric DNA sequence. J. Biol. Chem. 2002;277:4034–4041. doi: 10.1074/jbc.M108442200. [DOI] [PubMed] [Google Scholar]
- 18.Gowers DM, Bellamy SR, Halford SE. One recognition sequence, seven restriction enzymes, five reaction mechanisms. Nucleic Acids Res. 2004;32:3469–3479. doi: 10.1093/nar/gkh685. [DOI] [PMC free article] [PubMed] [Google Scholar]