Abstract
The majority of the phosphotyrosine recovered from partial acid hydrolysates of 32P-labeled Escherichia coli is derived from a single prominent protein. We show here by biochemical, genetic, and immunological criteria that this protein is actually glutamine synthetase adenylylated (not phosphorylated) at tyrosine. Furthermore, all of the phosphotyrosine detectable in partial acid hydrolysates of 32P-labeled Salmonella typhimurium was eliminated in a strain deficient in both glutamine synthetase and uridylyltransferase, an enzyme which uridylylates the regulatory protein PII at a tyrosine residue. These results suggest that protein-tyrosine phosphorylation represents a rare modification in eubacterial cells.
Full text
PDF
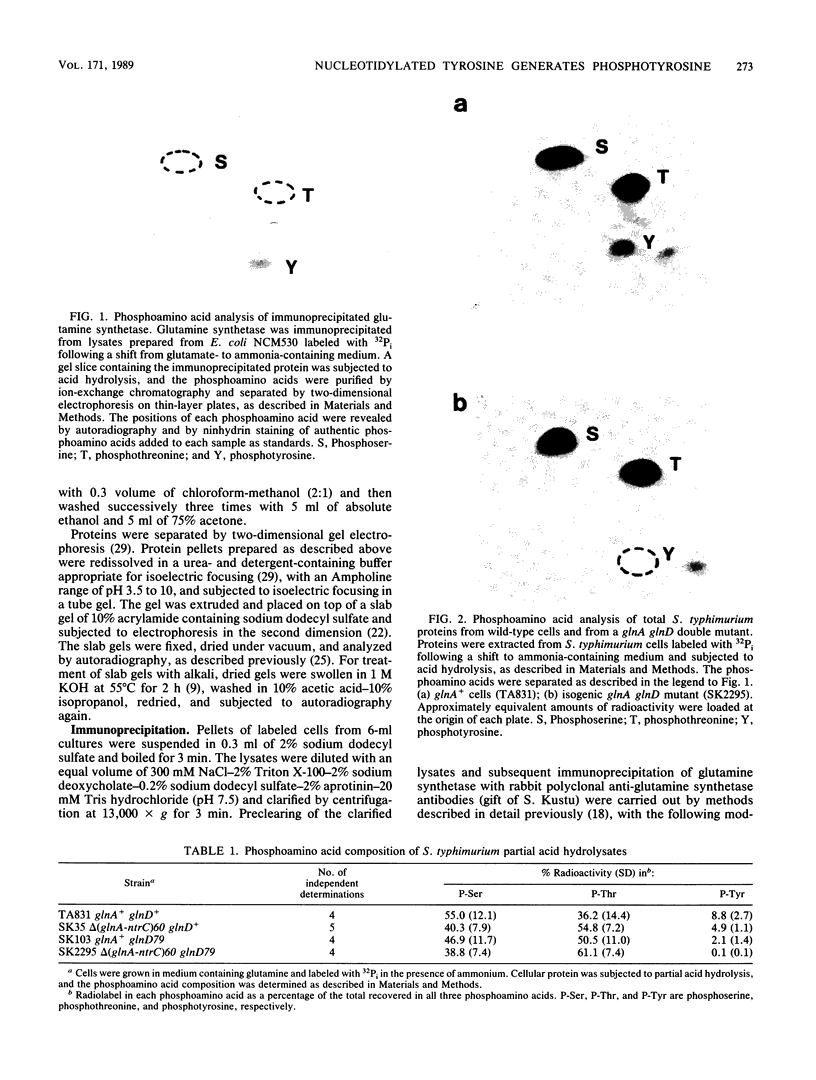
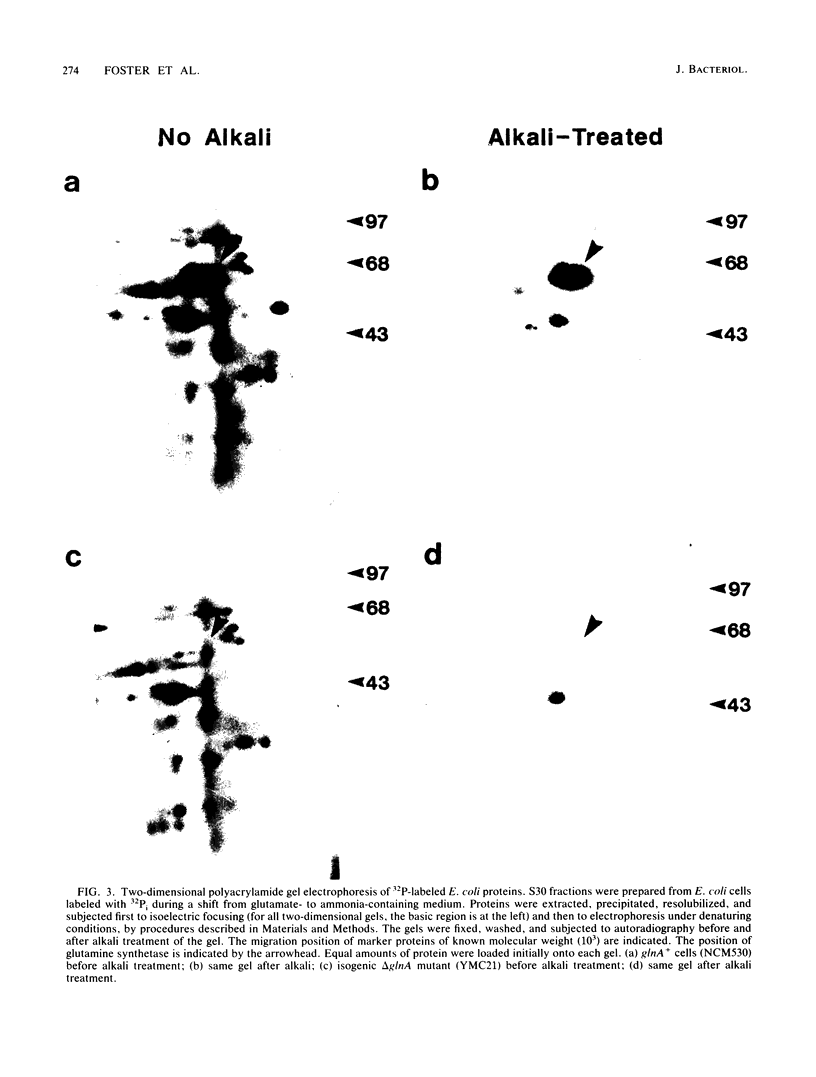


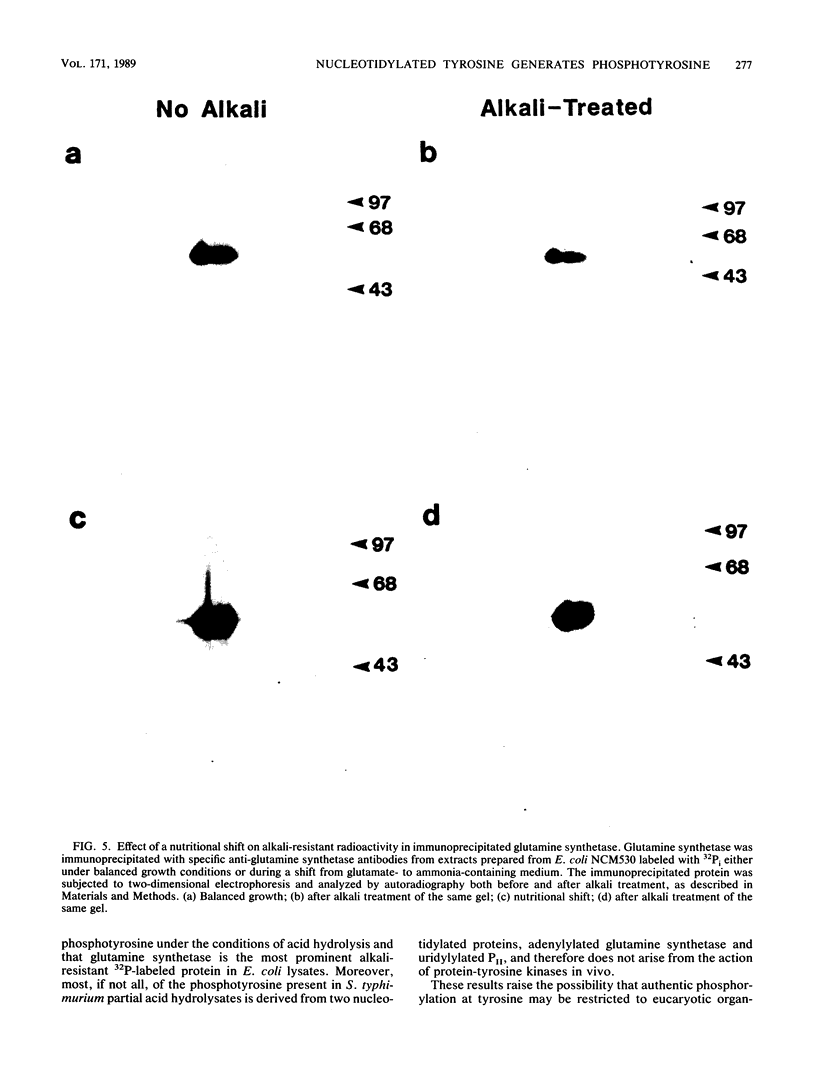


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Purich D., Stadtman E. R. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975 Aug 25;250(16):6264–6272. [PubMed] [Google Scholar]
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft S., Rhee S. G., Neumann C., Kustu S. Mutations that alter the covalent modification of glutamine synthetase in Salmonella typhimurium. J Bacteriol. 1978 Jun;134(3):1046–1055. doi: 10.1128/jb.134.3.1046-1055.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F. R., Levin M. S., Foor F., Tyler B. Regulation of glutamine synthetase formation in Escherichia coli: characterization of mutants lacking the uridylyltransferase. J Bacteriol. 1978 May;134(2):569–577. doi: 10.1128/jb.134.2.569-577.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Segal A., Stadtman E. R. Modulation of glutamine synthetase adenylylation and deadenylylation is mediated by metabolic transformation of the P II -regulatory protein. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2949–2953. doi: 10.1073/pnas.68.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H. K., Rhee S. G. Separation of glutamine synthetase species with different states of adenylylation by chromatography on monoclonal anti-AMP antibody affinity columns. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4677–4681. doi: 10.1073/pnas.81.15.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cortay J. C., Duclos B., Cozzone A. J. Phosphorylation of an Escherichia coli protein at tyrosine. J Mol Biol. 1986 Jan 20;187(2):305–308. doi: 10.1016/0022-2836(86)90236-6. [DOI] [PubMed] [Google Scholar]
- Cortay J. C., Rieul C., Duclos B., Cozzone A. J. Characterization of the phosphoproteins of Escherichia coli cells by electrophoretic analysis. Eur J Biochem. 1986 Sep 1;159(2):227–237. doi: 10.1111/j.1432-1033.1986.tb09858.x. [DOI] [PubMed] [Google Scholar]
- Garcia E., Rhee S. G. Cascade control of Escherichia coli glutamine synthetase. Purification and properties of PII uridylyltransferase and uridylyl-removing enzyme. J Biol Chem. 1983 Feb 25;258(4):2246–2253. [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Purification and properties of phosphorylated isocitrate dehydrogenase of Escherichia coli. J Biol Chem. 1979 Aug 25;254(16):7915–7920. [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Matsumura P., Simon M. I. Protein phosphorylation is involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7609–7613. doi: 10.1073/pnas.84.21.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Keener J., Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon H. S., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Kustu S. Evidence that nitrogen regulatory gene ntrC of Salmonella typhimurium is transcribed from the glnA promoter as well as from a separate ntr promoter. Mol Gen Genet. 1984;193(1):135–142. doi: 10.1007/BF00327426. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mangum J. H., Magni G., Stadtman E. R. Regulation of glutamine synthetase adenylylation and deadenylylation by the enzymatic uridylylation and deuridylylation of the PII regulatory protein. Arch Biochem Biophys. 1973 Oct;158(2):514–525. doi: 10.1016/0003-9861(73)90543-2. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Radke K., Hughes S., Quintrell N., Bishop J. M., Varmus H. E. Mutants of Rous sarcoma virus with extensive deletions of the viral genome. Virology. 1979 Jul 30;96(2):530–546. doi: 10.1016/0042-6822(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G. Regulation of bacterial metabolism by protein phosphorylation. Essays Biochem. 1987;23:1–27. [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieven G., Thorner J., Martin G. S. Protein-tyrosine kinase activity in Saccharomyces cerevisiae. Science. 1986 Jan 24;231(4736):390–393. doi: 10.1126/science.2417318. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase: a new form of the enzyme with altered regulatory and kinetic properties. Proc Natl Acad Sci U S A. 1967 Aug;58(2):642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Vallejos R. H., Holuigue L., Lucero H. A., Torruella M. Evidence of tyrosine kinase activity in the photosynthetic bacterium Rhodospirillum rubrum. Biochem Biophys Res Commun. 1985 Jan 31;126(2):685–691. doi: 10.1016/0006-291x(85)90239-6. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr Evidence for protein kinase activities in the prokaryote Salmonella typhimurium. J Biol Chem. 1978 Nov 10;253(21):7605–7608. [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr The identification of distinct protein kinases and phosphatases in the prokaryote Salmonella typhimurium. J Biol Chem. 1981 May 10;256(9):4640–4648. [PubMed] [Google Scholar]
- Wang J. Y., Queen C., Baltimore D. Expression of an Abelson murine leukemia virus-encoded protein in Escherichia coli causes extensive phosphorylation of tyrosine residues. J Biol Chem. 1982 Nov 25;257(22):13181–13184. [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]