Abstract
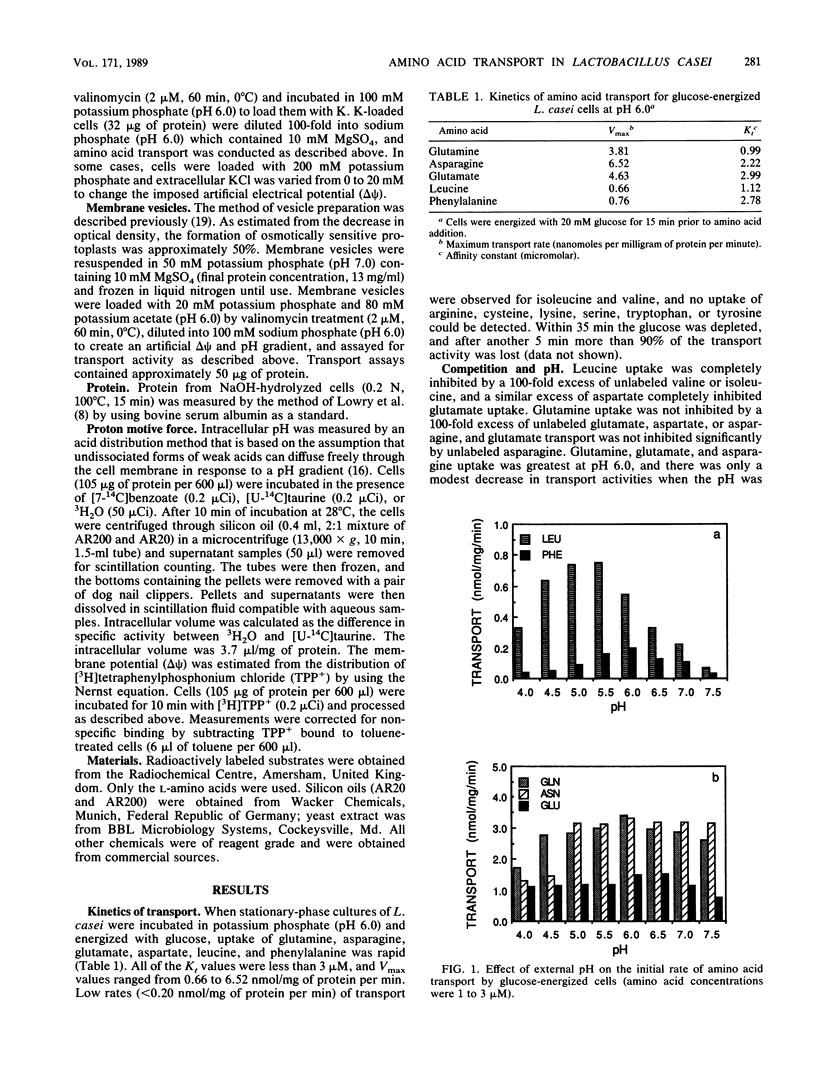
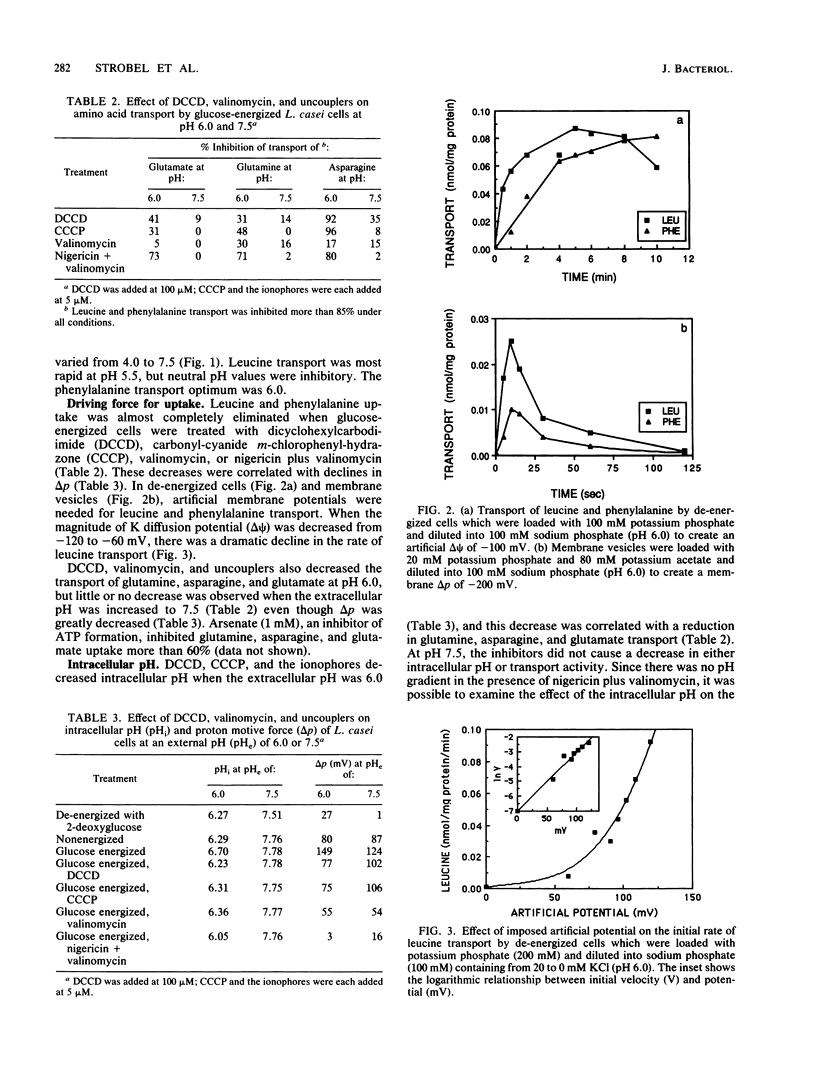
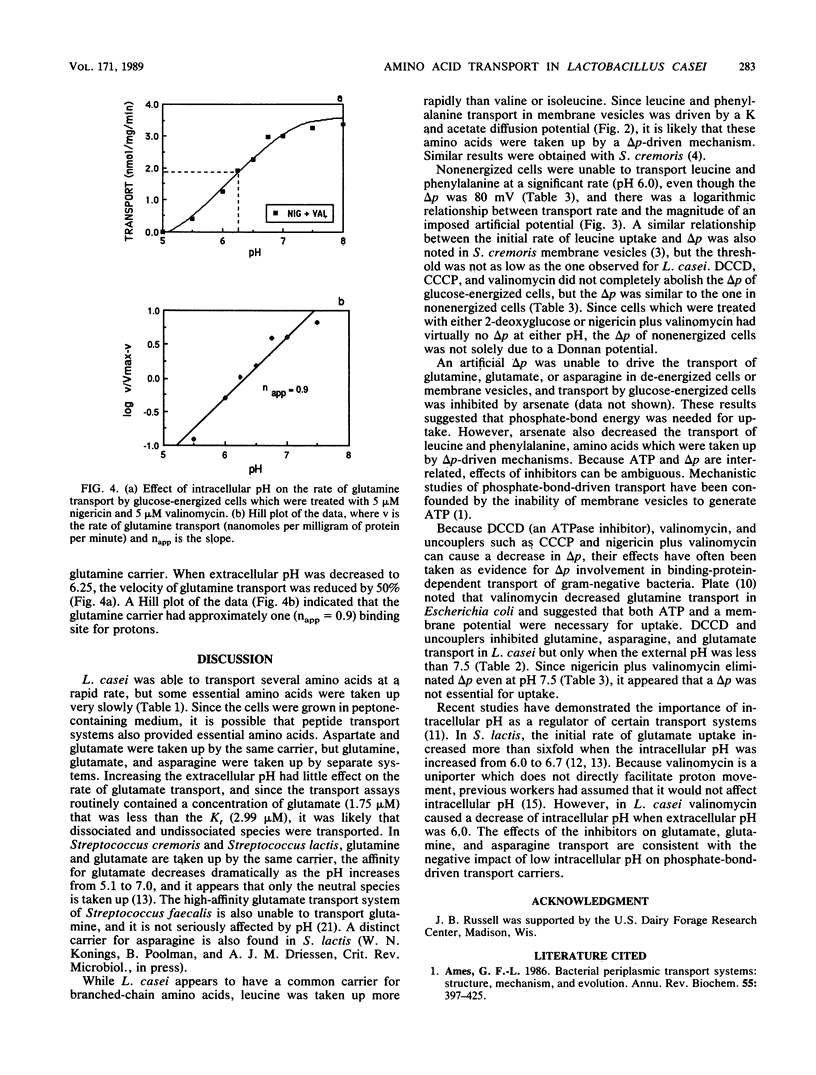
Lactobacillus casei 393 cells which were energized with glucose (pH 6.0) took up glutamine, asparagine, glutamate, aspartate, leucine, and phenylalanine. Little or no uptake of several essential amino acids (valine, isoleucine, arginine, cysteine, tyrosine, and tryptophan) was observed. Inhibition studies indicated that there were at least five amino acid carriers, for glutamine, asparagine, glutamate/aspartate, phenylalanine, or branched-chain amino acids. Transport activities had pH optima between 5.5 and 6.0, but all amino acid carriers showed significant activity even at pH 4.0. Leucine and phenylalanine transport decreased markedly when the pH was increased to 7.5. Inhibitors which decreased proton motive force (delta p) nearly eliminated leucine and phenylalanine uptake, and studies with de-energized cells and membrane vesicles showed that an artificial electrical potential (delta psi) of at least -100 mV was needed for rapid uptake. An artificial delta p was unable to drive glutamine, asparagine, or glutamate uptake, and transport of these amino acids was sensitive to a decline in intracellular pH. When intracellular pH was greater than 7.7, glutamine, asparagine, or glutamate was transported rapidly even though the proton motive force had been abolished by inhibitors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Crielaard W., Driessen A. J., Molenaar D., Hellingwerf K. J., Konings W. N. Light-driven amino acid uptake in Streptococcus cremoris or Clostridium acetobutylicum membrane vesicles fused with liposomes containing bacterial reaction centers. J Bacteriol. 1988 Apr;170(4):1820–1824. doi: 10.1128/jb.170.4.1820-1824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Jong S., Konings W. N. Transport of branched-chain amino acids in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1987 Nov;169(11):5193–5200. doi: 10.1128/jb.169.11.5193-5200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M., Huennekens F. M. Coupling of energy to folate transport in Lactobacillus casei. J Bacteriol. 1979 Aug;139(2):552–559. doi: 10.1128/jb.139.2.552-559.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morishita T., Deguchi Y., Yajima M., Sakurai T., Yura T. Multiple nutritional requirements of lactobacilli: genetic lesions affecting amino acid biosynthetic pathways. J Bacteriol. 1981 Oct;148(1):64–71. doi: 10.1128/jb.148.1.64-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of solute transport in streptococci by external and internal pH values. Microbiol Rev. 1987 Dec;51(4):498–508. doi: 10.1128/mr.51.4.498-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., FRANKLIN J. G., PERRY K. D. Correlation of the vitamin requirements with cultural and biochemical characters of Lactobacillus spp. J Gen Microbiol. 1961 Jul;25:473–482. doi: 10.1099/00221287-25-3-473. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):848–854. doi: 10.1021/bi00624a006. [DOI] [PubMed] [Google Scholar]
- Rhee S. K., Pack M. Y. Effect of environmental pH on fermentation balance of Lactobacillus bulgaricus. J Bacteriol. 1980 Oct;144(1):217–221. doi: 10.1128/jb.144.1.217-221.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebeling V., Thauer R. K., Jungermann K. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur J Biochem. 1975 Jul 1;55(2):445–453. doi: 10.1111/j.1432-1033.1975.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Trifone J. D., Brustolon M. Distribution of the phosphoenolpyruvate:glucose phosphotransferase system in fermentative bacteria. J Bacteriol. 1979 Jul;139(1):93–97. doi: 10.1128/jb.139.1.93-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utech N. M., Reid K. G., Holden J. T. Properties of a dicarboxylic amino acid transport-deficient mutant of Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5273–5280. [PubMed] [Google Scholar]


