Abstract
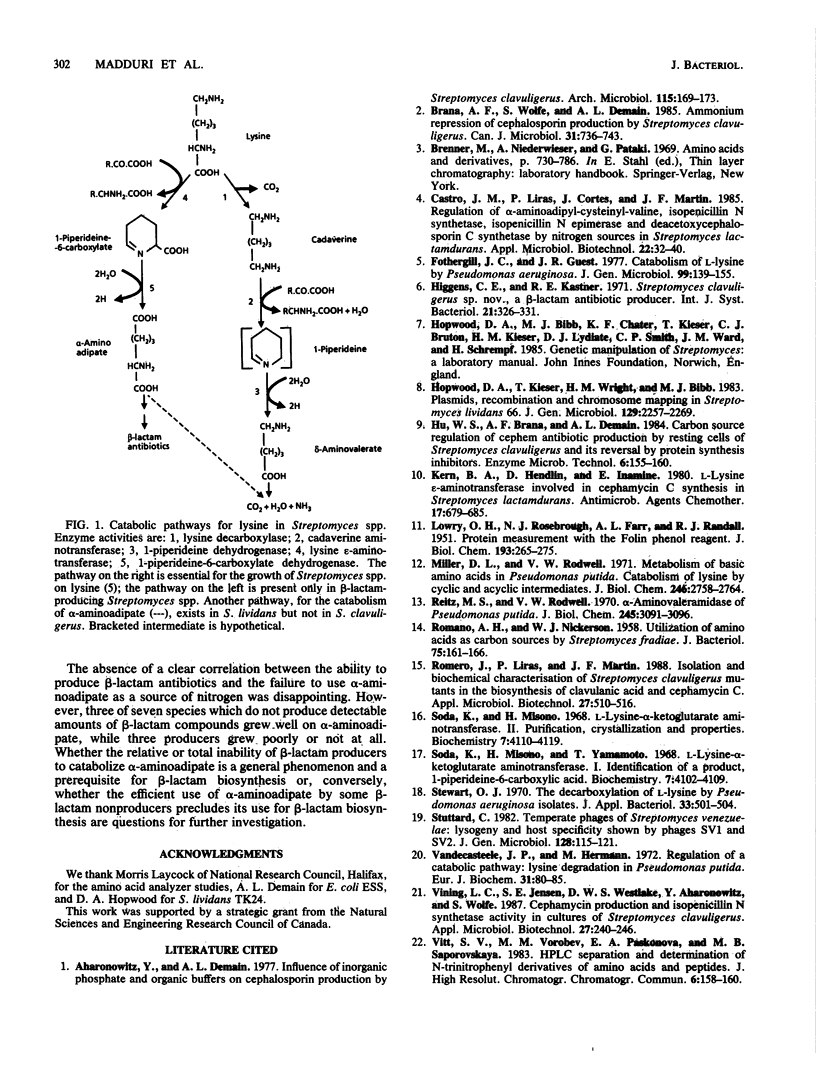
Genetic and biochemical evidence was obtained for lysine catabolism via cadaverine and delta-aminovalerate in both the beta-lactam producer Streptomyces clavuligerus and the nonproducer Streptomyces lividans. This pathway is used when lysine is supplied as the sole source of nitrogen for the organism. A second pathway for lysine catabolism is present in S. clavuligerus but not in S. lividans. It leads to alpha-aminoadipate, a precursor for beta-lactam biosynthesis. Since it does not allow S. clavuligerus to grow on lysine as the sole nitrogen source, this pathway may be used exclusively to provide a precursor for beta-lactam biosynthesis. beta-Lactam producers were unable to grow well on alpha-aminoadipate as the only nitrogen source, whereas three of seven species not known to produce beta-lactam grew well under the same conditions. Lysine epsilon-aminotransferase, the initial enzyme in the alpha-aminoadipate pathway for lysine catabolism, was detected in cell extracts only from the beta-lactam producers. These results suggest that synthesis of alpha-aminoadipate is exclusively a secondary metabolic trait, present or expressed only in beta-lactam producers, while genes governing the catabolism of alpha-aminoadipate are present or fully expressed only in beta-lactam nonproducers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Demain A. L. Influence of inorganic phosphate and organic buffers on cephalosporin production by Streptomyces clavuligerus. Arch Microbiol. 1977 Nov 18;115(2):169–173. doi: 10.1007/BF00406371. [DOI] [PubMed] [Google Scholar]
- Braña A. F., Wolfe S., Demain A. L. Ammonium repression of cephalosporin production by Streptomyces clavuligerus. Can J Microbiol. 1985 Aug;31(8):736–743. doi: 10.1139/m85-138. [DOI] [PubMed] [Google Scholar]
- Fothergill J. C., Guest J. R. Catabolism of L-lysine by Pseudomonas aeruginosa. J Gen Microbiol. 1977 Mar;99(1):139–155. doi: 10.1099/00221287-99-1-139. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Kern B. A., Hendlin D., Inamine E. L-lysine epsilon-aminotransferase involved in cephamycin C synthesis in Streptomyces lactamdurans. Antimicrob Agents Chemother. 1980 Apr;17(4):679–685. doi: 10.1128/aac.17.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Rodwell V. W. Metabolism of basic amino acids in Pseudomonas putida. Catabolism of lysine by cyclic and acyclic intermediates. J Biol Chem. 1971 May 10;246(9):2758–2764. [PubMed] [Google Scholar]
- Reitz M. S., Rodwell V. W. Delta-aminovaleramidase of Pseudomonas putida. J Biol Chem. 1970 Jun;245(12):3091–3096. [PubMed] [Google Scholar]
- Soda K., Misono H., Yamamoto T. L-Lysine:alpha-ketoglutarate aminotransferase. I. Identification of a product, delta-1-piperideine-6-carboxylic acid. Biochemistry. 1968 Nov;7(11):4102–4109. doi: 10.1021/bi00851a045. [DOI] [PubMed] [Google Scholar]
- Stewart D. J. The decarboxylation of L-lysine by Pseudomonas aeruginosa isolates. J Appl Bacteriol. 1970 Sep;33(3):501–504. doi: 10.1111/j.1365-2672.1970.tb02226.x. [DOI] [PubMed] [Google Scholar]



