Abstract
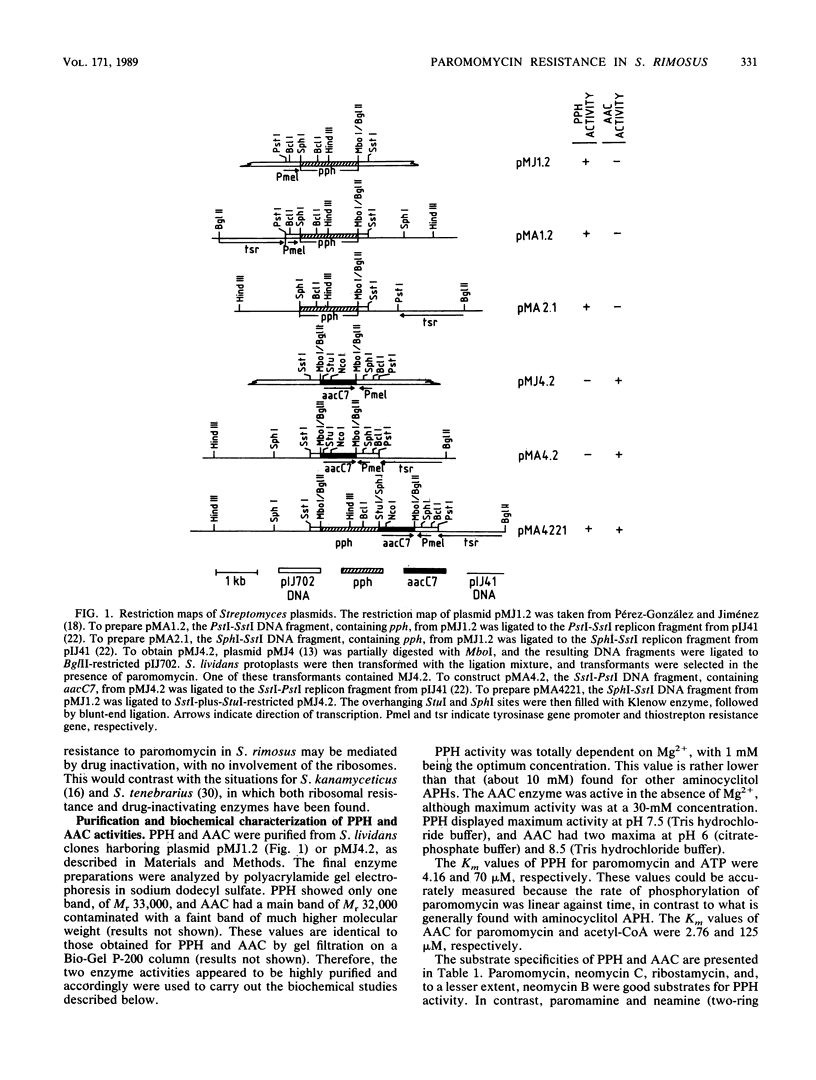
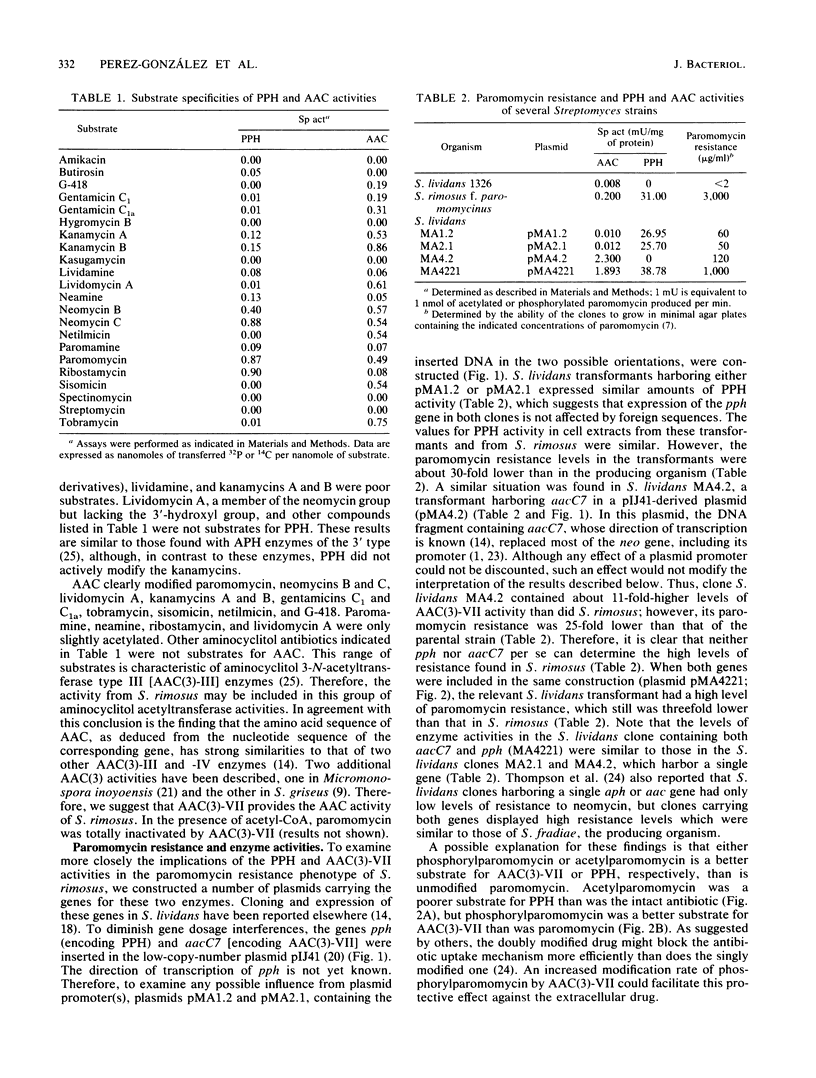
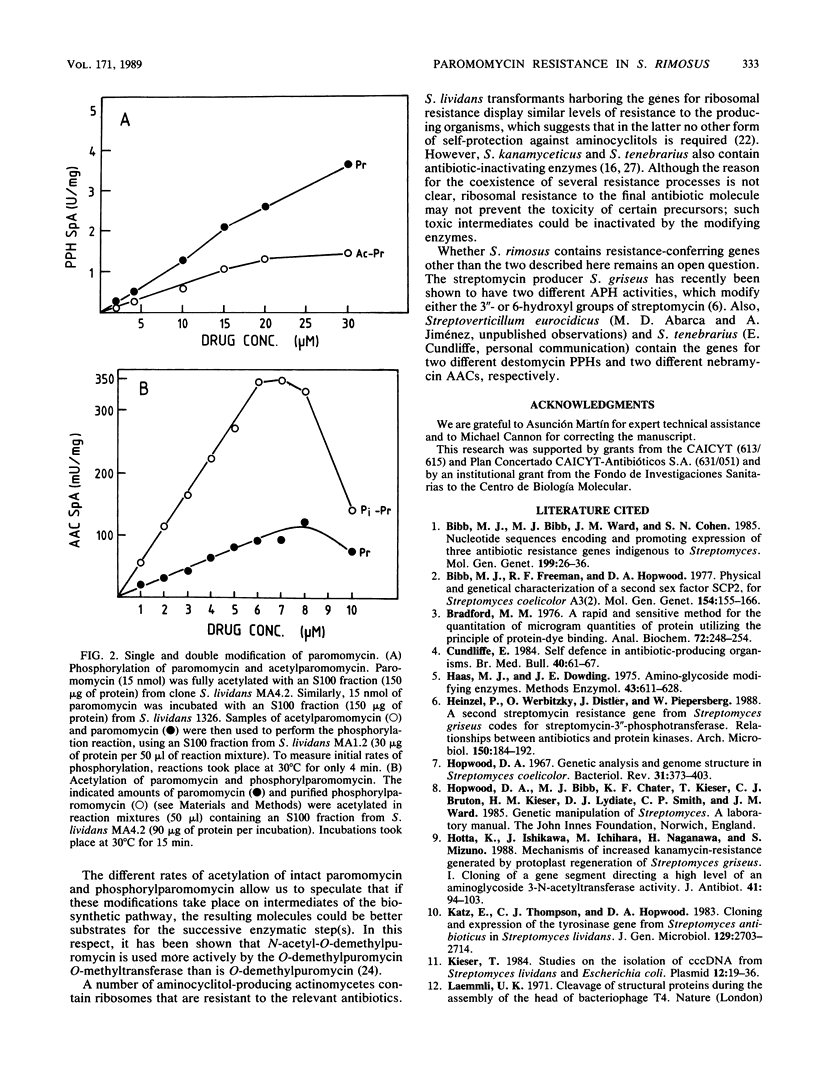
The mechanism conferring resistance to paromomycin in Streptomyces rimosus forma paromomycinus, the producing organism, was studied at the level of both protein synthesis and drug-inactivating enzymes. Ribosomes prepared from this organism grown in either production or nonproduction medium were fully sensitive to paromomycin. A paromomycin acetyltransferase and a paromomycin phosphotransferase, both characteristic of the producer, were highly purified from extracts prepared from two Streptomyces lividans transformants harboring the relevant genes inserted in pIJ702-derived plasmids. In vitro, paromomycin was inactivated by either activity. In vivo, however, S. lividans clones containing the gene for either enzyme inserted in the low-copy-number plasmid pIJ41 were resistant to only low levels of paromomycin. In contrast, an S. lividans transformant containing both genes inserted in the same pIJ41-derived plasmid displayed high levels of resistance to paromomycin. These results indicate that both genes are required to determine the high levels of resistance to this drug in the producing organism. Paromomycin is doubly modified by the enzymes. However, whereas acetylparomomycin was a poorer substrate than paromomycin for the phosphotransferase, phosphorylparomomycin was modified more actively than was the intact drug by the acetyltransferase. These findings are discussed in terms of both a permeability barrier to paromomycin and the possible role(s) of the two enzymes in the biosynthetic pathway of this antibiotic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Bibb M. J., Ward J. M., Cohen S. N. Nucleotide sequences encoding and promoting expression of three antibiotic resistance genes indigenous to Streptomyces. Mol Gen Genet. 1985;199(1):26–36. doi: 10.1007/BF00327505. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. Self defence in antibiotic-producing organisms. Br Med Bull. 1984 Jan;40(1):61–67. doi: 10.1093/oxfordjournals.bmb.a071949. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Heinzel P., Werbitzky O., Distler J., Piepersberg W. A second streptomycin resistance gene from Streptomyces griseus codes for streptomycin-3"-phosphotransferase. Relationships between antibiotic and protein kinases. Arch Microbiol. 1988;150(2):184–192. doi: 10.1007/BF00425160. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K., Ishikawa J., Ichihara M., Naganawa H., Mizuno S. Mechanism of increased kanamycin-resistance generated by protoplast regeneration of Streptomyces griseus. I. Cloning of a gene segment directing a high level of an aminoglycoside 3-N-acetyltransferase activity. J Antibiot (Tokyo) 1988 Jan;41(1):94–103. doi: 10.7164/antibiotics.41.94. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Leboul J., Davies J. Enzymatic modification of hygromycin B in Streptomyces hygroscopicus. J Antibiot (Tokyo) 1982 Apr;35(4):527–528. doi: 10.7164/antibiotics.35.527. [DOI] [PubMed] [Google Scholar]
- López-Cabrera M., Pérez-González J. A., Heinzel P., Piepersberg W., Jiménez A. Isolation and nucleotide sequencing of an aminocyclitol acetyltransferase gene from Streptomyces rimosus forma paromomycinus. J Bacteriol. 1989 Jan;171(1):321–328. doi: 10.1128/jb.171.1.321-328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpartida F., Zalacaín M., Jiménez A., Davies J. Molecular cloning and expression in streptomyces lividans of a hygromycin B phosphotransferase gene from Streptomyces hygroscopicus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):6–12. doi: 10.1016/0006-291x(83)91533-4. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Mashiko H., Ogawara H. Cloning of the kanamycin resistance gene from a kanamycin-producing Streptomyces species. J Bacteriol. 1984 Jan;157(1):79–83. doi: 10.1128/jb.157.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J. M., Malpartida F., Rico M., Jiménez A. Biochemical basis of resistance to hygromycin B in Streptomyces hygroscopicus--the producing organism. J Gen Microbiol. 1985 Jun;131(6):1289–1298. doi: 10.1099/00221287-131-6-1289. [DOI] [PubMed] [Google Scholar]
- Piendl W., Böck A. Ribosomal resistance in the gentamicin producer organism Micromonospora purpurea. Antimicrob Agents Chemother. 1982 Aug;22(2):231–236. doi: 10.1128/aac.22.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González J. A., Vara J., Jiménez A. The mechanism of resistance to puromycin and to the puromycin-precursor O-demethyl-puromycin in Streptomyces alboniger. J Gen Microbiol. 1985 Nov;131(11):2877–2883. doi: 10.1099/00221287-131-11-2877. [DOI] [PubMed] [Google Scholar]
- Salas J. A., Cundliffe E. Characterization in Micromonospora inyoensis of aminoglycoside acetyltransferase activity not previously encountered among actinomycetes. J Gen Microbiol. 1985 Mar;131(3):451–457. doi: 10.1099/00221287-131-3-451. [DOI] [PubMed] [Google Scholar]
- Skeggs P. A., Thompson J., Cundliffe E. Methylation of 16S ribosomal RNA and resistance to aminoglycoside antibiotics in clones of Streptomyces lividans carrying DNA from Streptomyces tenjimariensis. Mol Gen Genet. 1985;200(3):415–421. doi: 10.1007/BF00425725. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Kieser T., Ward J. M., Hopwood D. A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982 Nov;20(1):51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Skinner R. H., Thompson J., Ward J. M., Hopwood D. A., Cundliffe E. Biochemical characterization of resistance determinants cloned from antibiotic-producing streptomycetes. J Bacteriol. 1982 Aug;151(2):678–685. doi: 10.1128/jb.151.2.678-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara J., Malpartida F., Hopwood D. A., Jiménez A. Cloning and expression of a puromycin N-acetyl transferase gene from Streptomyces alboniger in Streptomyces lividans and Escherichia coli. Gene. 1985;33(2):197–206. doi: 10.1016/0378-1119(85)90094-0. [DOI] [PubMed] [Google Scholar]
- Vara J., Perez-Gonzalez J. A., Jimenez A. Biosynthesis of puromycin by Streptomyces alboniger: characterization of puromycin N-acetyltransferase. Biochemistry. 1985 Dec 31;24(27):8074–8081. doi: 10.1021/bi00348a036. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hotta K., Okami Y., Umezawa H. Mechanism of resistance to aminoglycoside antibiotics in nebramycin-producing Streptomyces tenebrarius. J Antibiot (Tokyo) 1982 Aug;35(8):1020–1025. doi: 10.7164/antibiotics.35.1020. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hotta K., Okami Y., Umezawa H. Ribosomal resistance of an istamycin producer, Streptomyces tenjimariensis, to aminoglycoside antibiotics. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1396–1401. doi: 10.1016/0006-291x(81)91979-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Hotta K., Okami Y., Umezawa H. Self-resistance of a Streptomyces which produces istamycins. J Antibiot (Tokyo) 1981 Jul;34(7):824–829. doi: 10.7164/antibiotics.34.824. [DOI] [PubMed] [Google Scholar]
- Zalacain M., Pardo J. M., Jiménez A. Purification and characterization of a hygromycin B phosphotransferase from Streptomyces hygroscopicus. Eur J Biochem. 1987 Jan 15;162(2):419–422. doi: 10.1111/j.1432-1033.1987.tb10618.x. [DOI] [PubMed] [Google Scholar]



