Abstract
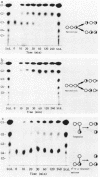
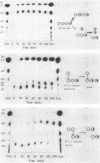
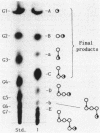
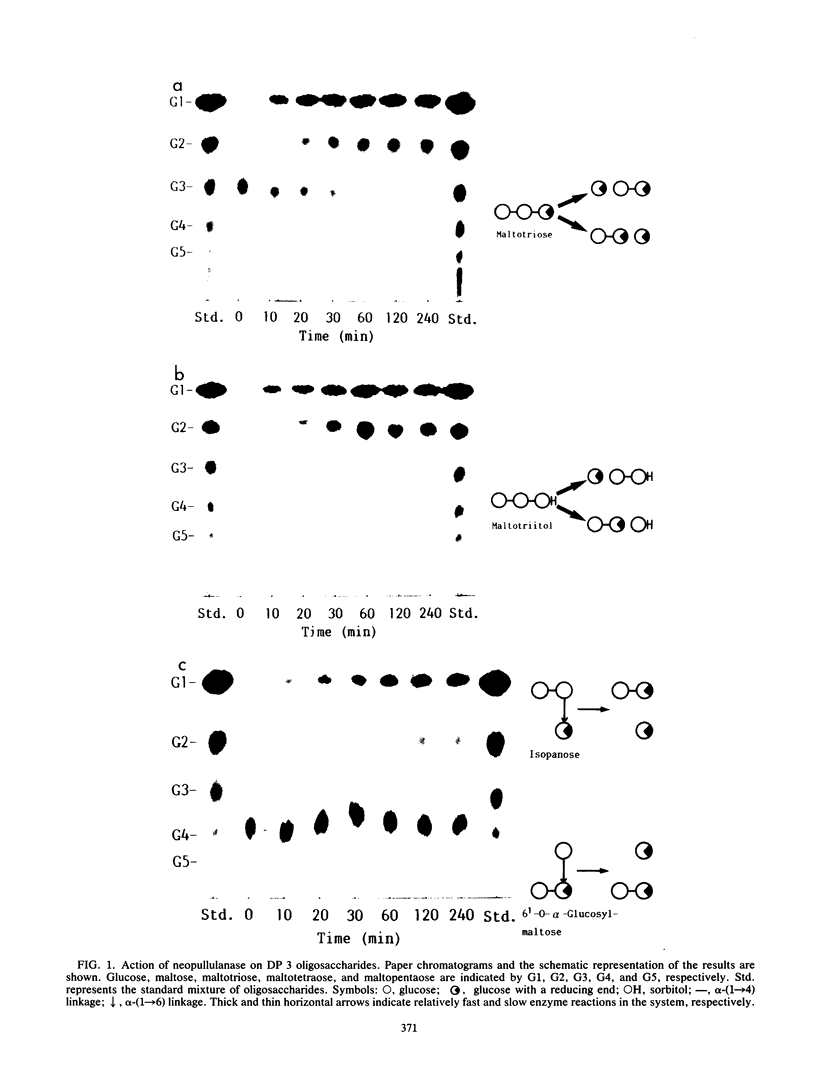
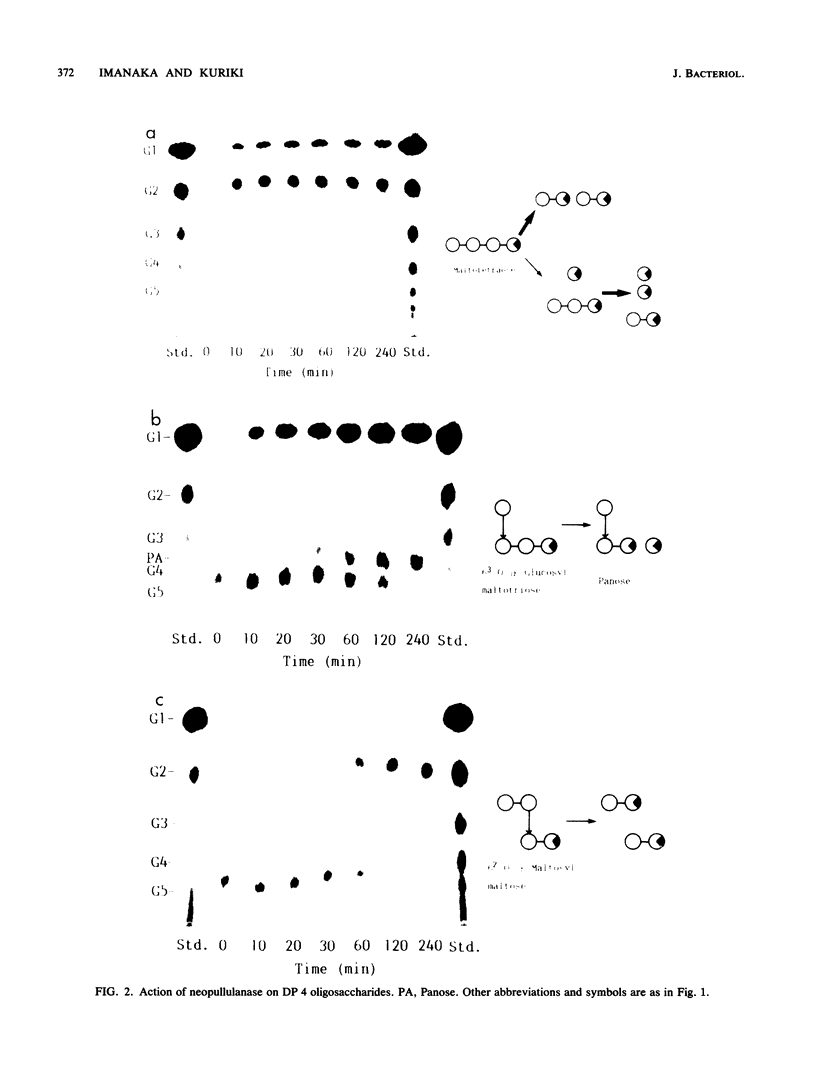
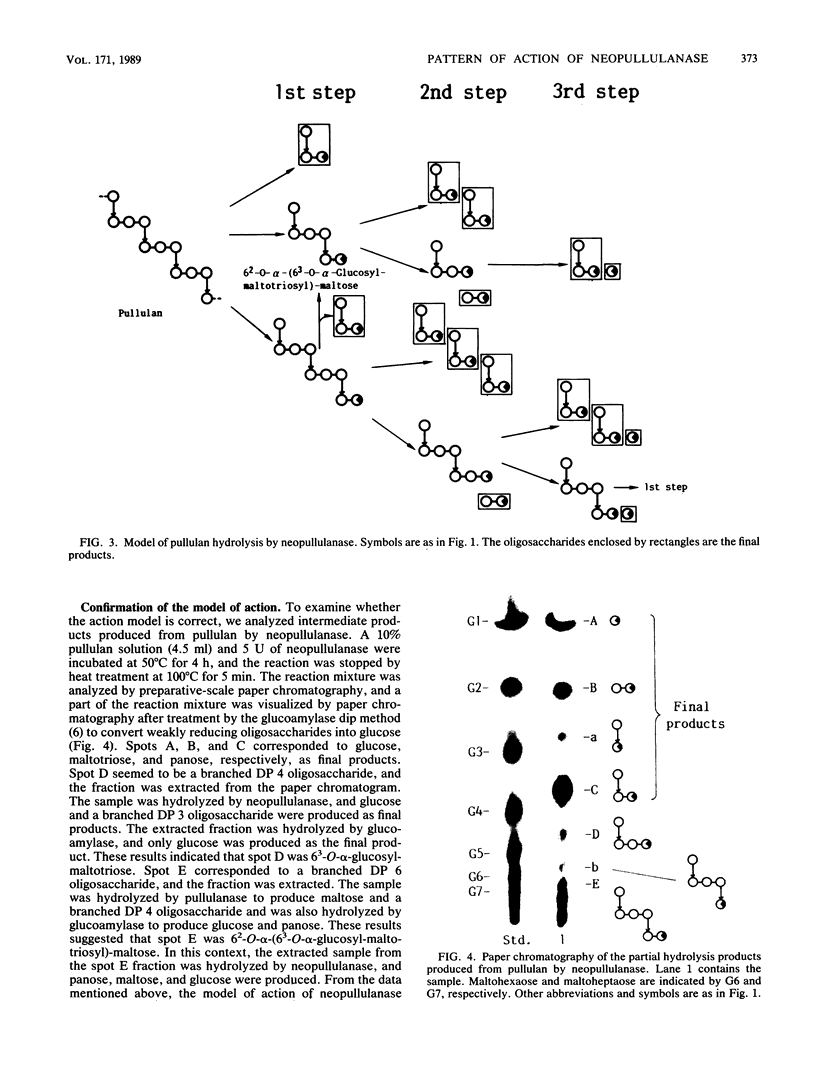
The action of neopullulanase from Bacillus stearothermophilus on many oligosaccharides was tested. The enzyme hydrolyzed not only alpha-(1----4)-glucosidic linkages but also specific alpha-(1----6)-glucosidic linkages of several branched oligosaccharides. When pullulan was used as a substrate, panose, maltose, and glucose, in that order, were produced as final products at a final molar ratio of 3:1:1. According to these results, we proposed a model for the pattern of action of neopullulanase on pullulan as follows. In the first step, the enzyme hydrolyzes only alpha-(1----4)-glucosidic linkages on the nonreducing side of alpha-(1----6) linkages of pullulan and produces panose and several intermediate products composed of some panose units. In the second step, taking 6(2)-O-alpha-(6(3)-O-alpha-glucosyl-maltotriosyl)-maltose as an example of one of the intermediate products, the enzyme hydrolyzes either alpha-(1----4) (the same position as that described above) or alpha-(1----6) linkages and produces panose or 6(3)-O-alpha-glucosyl-maltotriose plus maltose, respectively. In the third step, the alpha-(1----4) linkage of 6(3)-O-alpha-glucosyl-maltotriose is hydrolyzed by the enzyme, and glucose and another panose are produced. To confirm the model of the pattern of action, we extracted intermediate products produced from pullulan by neopullulanase and analyzed the structures by glucoamylase, pullulanase, and neopullulanase analyses. The experimental results supported the above-mentioned model of the pattern of action of neopullulanase on pullulan.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdullah M., French D. Substrate specificity of pullulanase. Arch Biochem Biophys. 1970 Apr;137(2):483–493. doi: 10.1016/0003-9861(70)90466-2. [DOI] [PubMed] [Google Scholar]
- Coleman R. D., Yang S. S., McAlister M. P. Cloning of the debranching-enzyme gene from Thermoanaerobium brockii into Escherichia coli and Bacillus subtilis. J Bacteriol. 1987 Sep;169(9):4302–4307. doi: 10.1128/jb.169.9.4302-4307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainuma K., French D. Action of pancreatic amylase on starch oligosaccharides containing single glucose side chains. FEBS Lett. 1969 Nov 29;5(4):257–261. doi: 10.1016/0014-5793(69)80363-7. [DOI] [PubMed] [Google Scholar]
- Kainuma K., Kobayashi S., Harada T. Action of Pseudomonas isoamylase on various branched oligo and poly-saccharides. Carbohydr Res. 1978 Mar;61:345–357. doi: 10.1016/s0008-6215(00)84494-8. [DOI] [PubMed] [Google Scholar]
- Kuriki T., Okada S., Imanaka T. New type of pullulanase from Bacillus stearothermophilus and molecular cloning and expression of the gene in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1554–1559. doi: 10.1128/jb.170.4.1554-1559.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZUR J. H., ANDO T. The hydrolysis of glucosyl oligosaccharides with alpha-D-(1-4) and alpha-D-(1-6) bonds by fungal amyloglucosidase. J Biol Chem. 1960 Feb;235:297–302. [PubMed] [Google Scholar]
- Plant A. R., Clemens R. M., Morgan H. W., Daniel R. M. Active-site- and substrate-specificity of Thermoanaerobium Tok6-B1 pullulanase. Biochem J. 1987 Sep 1;246(2):537–541. doi: 10.1042/bj2460537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- UEDA S., FUJITA K., KOMATSU K., NAKASHIMA Z. I. Polysaccharide produced by the genus Pullularia. I. Production of polysaccharide by growing cells. Appl Microbiol. 1963 May;11:211–215. doi: 10.1128/am.11.3.211-215.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeki K., Yamamoto T. Enzymatic determination of structure of singly branched hexaose dextrins formed by liquefying -amylase of Bacillus subtilis. J Biochem. 1972 Jul;72(1):101–109. doi: 10.1093/oxfordjournals.jbchem.a129874. [DOI] [PubMed] [Google Scholar]