Abstract
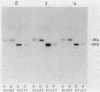
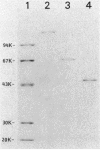
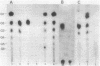
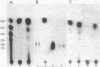
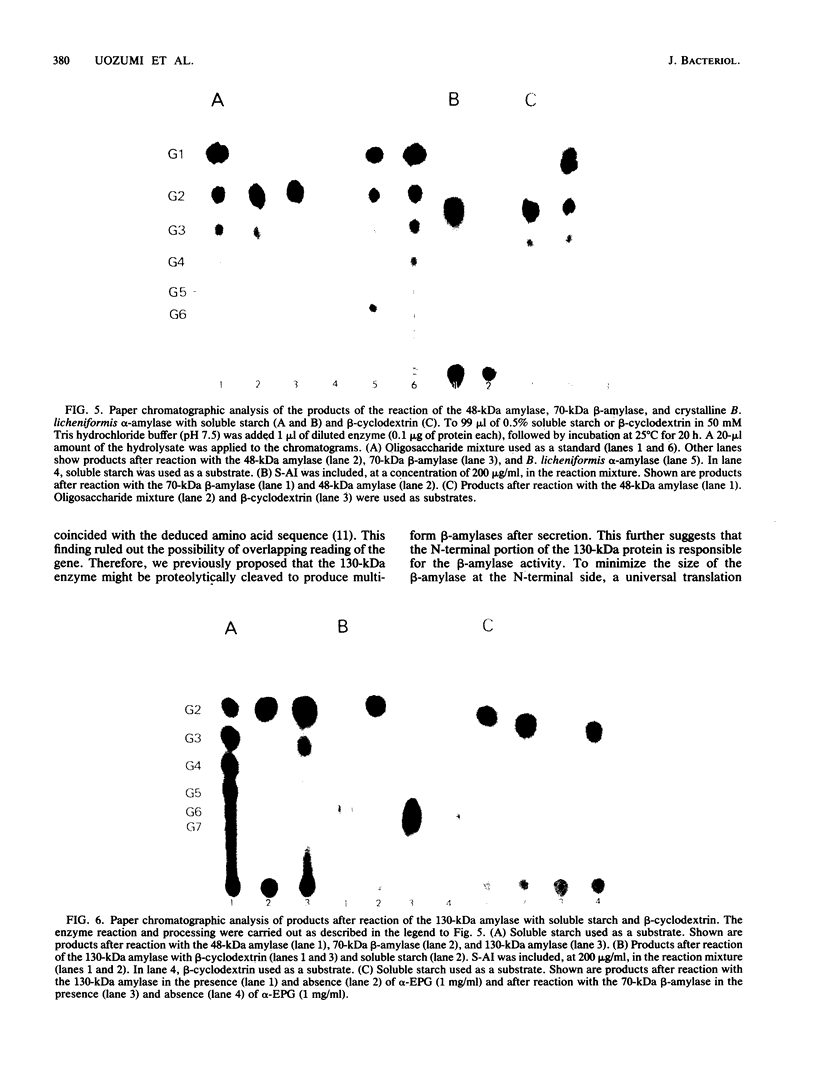
The Bacillus polymyxa amylase gene comprises 3,588 nucleotides. The mature amylase comprises 1,161 amino acids with a molecular weight of 127,314. The gene appeared to be divided into two portions by the direct-repeat sequence located at almost the middle of the gene. The 5' region upstream of the direct-repeat sequence was shown to be responsible for the synthesis of beta-amylase. The 3' region downstream of the direct-repeat sequence contained four sequences homologous with those in other alpha-amylases, such as Taka-amylase A. The 48-kilodalton (kDa) amylase isolated from B. polymyxa was proven to have alpha-amylase activity. The amino acid sequences of the peptides generated from the 48-kDa amylase showed complete agreement with the predicted amino acid sequence of the C-terminal portion. The B. polymyxa amylase gene was therefore concluded to contain in-phase beta- and alpha-amylase-coding sequences in the 5' and 3' regions, respectively. A precursor protein, a 130-kDa amylase, directed by a plasmid, pYN520, carrying the entire amylase gene, had both beta- and alpha-amylase activities. This represents the first report of a single protein precursor in procaryotes that gives rise to two enzymes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Hickey D. A. The alpha-amylase gene in Drosophila melanogaster: nucleotide sequence, gene structure and expression motifs. Nucleic Acids Res. 1986 Nov 11;14(21):8399–8411. doi: 10.1093/nar/14.21.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P., Cornet P., Aubert J. P. Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum. J Bacteriol. 1985 Apr;162(1):102–105. doi: 10.1128/jb.162.1.102-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg F., Rhodes C. Cloning and characterization of the beta-amylase gene from Bacillus polymyxa. J Bacteriol. 1986 Mar;165(3):819–824. doi: 10.1128/jb.165.3.819-824.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori F., Sashihara N., Kudo T., Horikoshi K. Nucleotide sequences of two cellulase genes from alkalophilic Bacillus sp. strain N-4 and their strong homology. J Bacteriol. 1986 Nov;168(2):479–485. doi: 10.1128/jb.168.2.479-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara H., Sasaki T., Tsuboi A., Yamagata H., Tsukagoshi N., Udaka S. Complete nucleotide sequence of a thermophilic alpha-amylase gene: homology between prokaryotic and eukaryotic alpha-amylases at the active sites. J Biochem. 1985 Jul;98(1):95–103. doi: 10.1093/oxfordjournals.jbchem.a135279. [DOI] [PubMed] [Google Scholar]
- Kawazu T., Nakanishi Y., Uozumi N., Sasaki T., Yamagata H., Tsukagoshi N., Udaka S. Cloning and nucleotide sequence of the gene coding for enzymatically active fragments of the Bacillus polymyxa beta-amylase. J Bacteriol. 1987 Apr;169(4):1564–1570. doi: 10.1128/jb.169.4.1564-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto N., Yamagata H., Kato T., Tsukagoshi N., Udaka S. Cloning and sequencing of the gene encoding thermophilic beta-amylase of Clostridium thermosulfurogenes. J Bacteriol. 1988 Dec;170(12):5848–5854. doi: 10.1128/jb.170.12.5848-5854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis M., Williamson M., Buxton B., Pywell J., Hejgaard J., Svendsen I. Primary structure and differential expression of beta-amylase in normal and mutant barleys. Eur J Biochem. 1987 Dec 15;169(3):517–525. doi: 10.1111/j.1432-1033.1987.tb13640.x. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacks S. A., Springhorn S. S. Renaturation of enzymes after polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. J Biol Chem. 1980 Aug 10;255(15):7467–7473. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. M., Virolle M. J., Chang S. Y., Chang S., Bibb M. J. alpha-Amylase gene of Streptomyces limosus: nucleotide sequence, expression motifs, and amino acid sequence homology to mammalian and invertebrate alpha-amylases. J Bacteriol. 1987 Dec;169(12):5745–5754. doi: 10.1128/jb.169.12.5745-5754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Kusunoki M., Harada W., Kakudo M. Structure and possible catalytic residues of Taka-amylase A. J Biochem. 1984 Mar;95(3):697–702. doi: 10.1093/oxfordjournals.jbchem.a134659. [DOI] [PubMed] [Google Scholar]
- Nishide T., Emi M., Nakamura Y., Matsubara K. Corrected sequences of cDNAs for human salivary and pancreatic alpha-amylases [corrected]. Gene. 1984 May;28(2):263–270. doi: 10.1016/0378-1119(84)90265-8. [DOI] [PubMed] [Google Scholar]
- Normark S., Bergström S., Edlund T., Grundström T., Jaurin B., Lindberg F. P., Olsson O. Overlapping genes. Annu Rev Genet. 1983;17:499–525. doi: 10.1146/annurev.ge.17.120183.002435. [DOI] [PubMed] [Google Scholar]
- Palva I. Molecular cloning of alpha-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene. 1982 Jul-Aug;19(1):81–87. doi: 10.1016/0378-1119(82)90191-3. [DOI] [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- Rhodes C., Strasser J., Friedberg F. Sequence of an active fragment of B. polymyxa beta amylase. Nucleic Acids Res. 1987 May 11;15(9):3934–3934. doi: 10.1093/nar/15.9.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyt J. F., French D. Multiple attach hypothesis of alpha-amylase action: action of porcine pancreatic, human salivary, and Aspergillus oryzae alpha-amylases. Arch Biochem Biophys. 1967 Oct;122(1):8–16. doi: 10.1016/0003-9861(67)90118-x. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Saito N. A thermophilic extracellular -amylase from Bacillus licheniformis. Arch Biochem Biophys. 1973 Apr;155(2):290–298. doi: 10.1016/0003-9861(73)90117-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Pittet A. C., Young R. A., Hagenbüchle O., Tosi M., Gellman S., Wellauer P. K. The mouse alpha-amylase multigene family. Sequence organization of members expressed in the pancreas, salivary gland and liver. J Mol Biol. 1982 Mar 5;155(3):247–266. doi: 10.1016/0022-2836(82)90004-3. [DOI] [PubMed] [Google Scholar]
- Sharrock W. J. Cleavage of two yolk proteins from a precursor in Caenorhabditis elegans. J Mol Biol. 1984 Apr 15;174(3):419–431. doi: 10.1016/0022-2836(84)90329-2. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Takahashi W., Yamagata H., Yamaguchi K., Tsukagoshi N., Udaka S. Genetic transformation of Bacillus brevis 47, a protein-secreting bacterium, by plasmid DNA. J Bacteriol. 1983 Dec;156(3):1130–1134. doi: 10.1128/jb.156.3.1130-1134.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Ihara H., Yamagata H., Udaka S. Cloning and expression of a thermophilic alpha-amylase gene from Bacillus stearothermophilus in Escherichia coli. Mol Gen Genet. 1984;193(1):58–63. doi: 10.1007/BF00327414. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Iritani S., Sasaki T., Takemura T., Ihara H., Idota Y., Yamagata H., Udaka S. Efficient synthesis and secretion of a thermophilic alpha-amylase by protein-producing Bacillus brevis 47 carrying the Bacillus stearothermophilus amylase gene. J Bacteriol. 1985 Dec;164(3):1182–1187. doi: 10.1128/jb.164.3.1182-1187.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A. U., Weiss R. L. A single precursor protein for two separable mitochondrial enzymes in Neurospora crassa. J Biol Chem. 1987 Apr 25;262(12):5823–5830. [PubMed] [Google Scholar]
- Yamada H., Tsukagoshi N., Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1981 Oct;148(1):322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuuki T., Nomura T., Tezuka H., Tsuboi A., Yamagata H., Tsukagoshi N., Udaka S. Complete nucleotide sequence of a gene coding for heat- and pH-stable alpha-amylase of Bacillus licheniformis: comparison of the amino acid sequences of three bacterial liquefying alpha-amylases deduced from the DNA sequences. J Biochem. 1985 Nov;98(5):1147–1156. doi: 10.1093/oxfordjournals.jbchem.a135381. [DOI] [PubMed] [Google Scholar]