Abstract
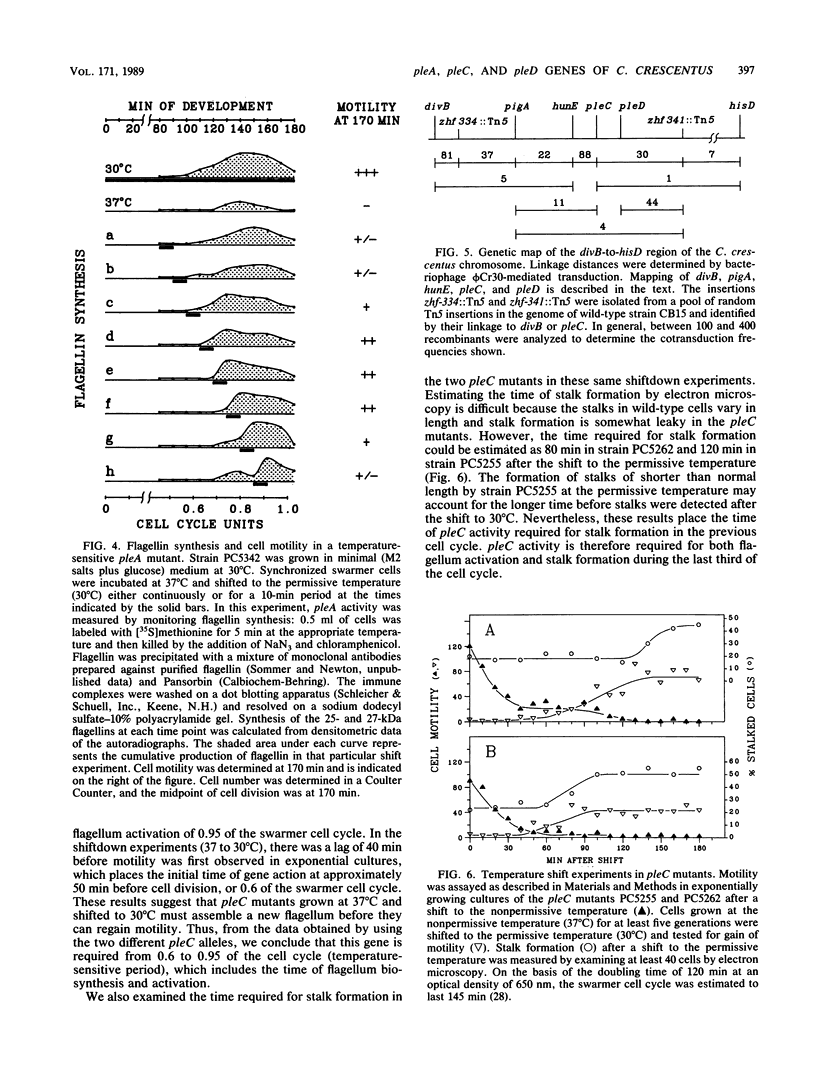
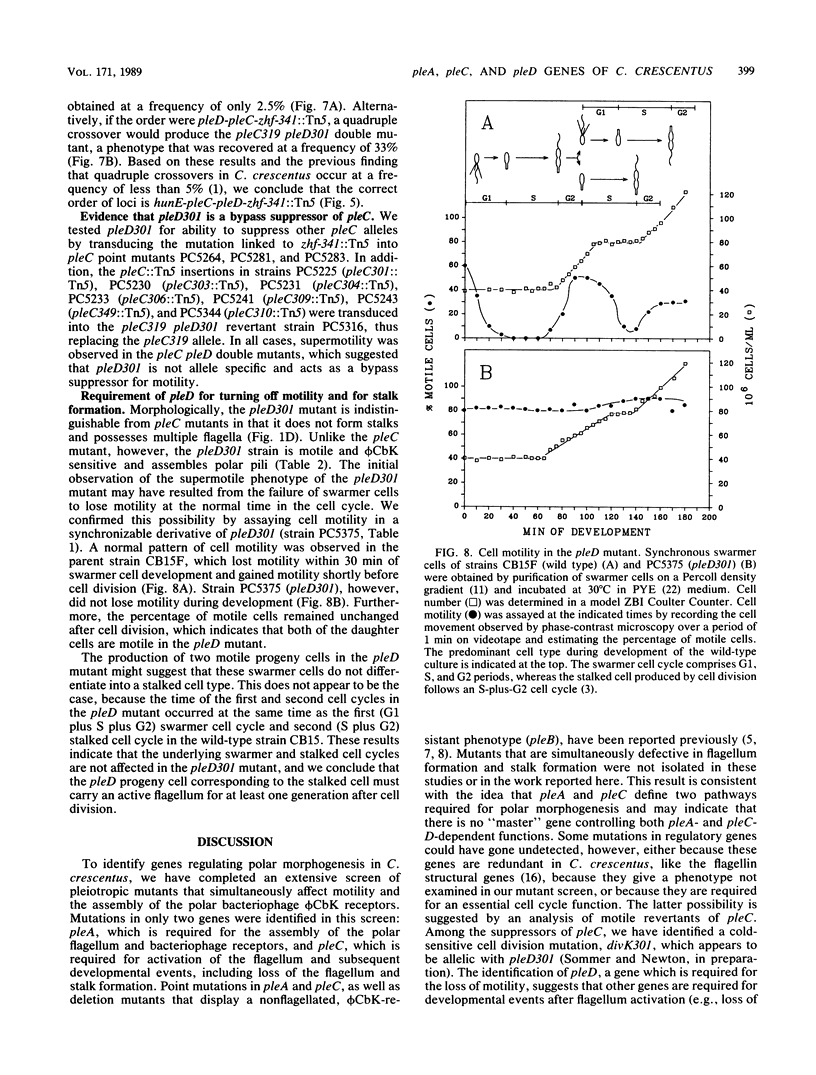
We have identified mutations in three pleiotropic genes, pleA, pleC, and pleD, that are required for differentiation in Caulobacter crescentus. pleA and pleC mutants were isolated in an extensive screen for strains defective in both motility and adsorption of polar bacteriophage phi CbK; using temperature-sensitive alleles, we determined the time at which the two genes act. pleA was required for a short period at 0.7 of the swarmer cell cycle for flagellum biosynthesis, whereas pleC was required during an overlapping period from 0.6 to 0.95 of the cell cycle to activate flagellum rotation as well as to enable loss of the flagellum and stalk formation by swarmer cells after division. The third pleiotropic gene, pleD, is described here for the first time. A pleD mutation was identified as a bypass suppressor of a temperature-sensitive pleC allele. Strains containing this mutation were highly motile, did not shed the flagellum or form stalks, and retained motility throughout the cell cycle. Since pleD was required to turn off motility and was a bypass suppressor of pleC, we conclude that it acts after the pleA and pleC gene functions in the cell cycle. No mutants defective in both flagellum biosynthesis and stalk formation were identified. Consequently, we propose that the steps required for formation of swarmer cells and subsequent development into stalked cells are organized into at least two developmental pathways: a pleA-dependent sequence of events, responsible for flagellum biosynthesis in predivisional cells, and a pleC-pleD-dependent sequence, responsible for flagellum activation in predivisional cells and loss of motility and stalk formation in progeny swarmer cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. T., Rhodes C. S., Ferber D. M., Jenkins B., Kuhl S. A., Ely B. Construction of a genetic map for Caulobacter crescentus. J Bacteriol. 1982 Mar;149(3):889–896. doi: 10.1128/jb.149.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer R., Bryan R., Gomes S. L., Purucker M., Shapiro L. Temporal and spatial control of flagellar and chemotaxis gene expression during Caulobacter cell differentiation. Cold Spring Harb Symp Quant Biol. 1985;50:831–840. doi: 10.1101/sqb.1985.050.01.101. [DOI] [PubMed] [Google Scholar]
- Degnen S. T., Newton A. Chromosome replication during development in Caulobacter crescentus. J Mol Biol. 1972 Mar 14;64(3):671–680. doi: 10.1016/0022-2836(72)90090-3. [DOI] [PubMed] [Google Scholar]
- Ely B., Croft R. H., Gerardot C. J. Genetic mapping of genes required for motility in Caulobacter crescentus. Genetics. 1984 Nov;108(3):523–532. doi: 10.1093/genetics/108.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Croft R. H. Transposon mutagenesis in Caulobacter crescentus. J Bacteriol. 1982 Feb;149(2):620–625. doi: 10.1128/jb.149.2.620-625.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Asada M., Koyasu S., Yoshida H., Yaginuma K., Okada Y. Regulation of polar morphogenesis in Caulobacter crescentus. J Bacteriol. 1981 Jan;145(1):559–572. doi: 10.1128/jb.145.1.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Iba H., Okada Y. Stalkless mutants of Caulobacter crescentus. J Bacteriol. 1977 Jul;131(1):280–287. doi: 10.1128/jb.131.1.280-287.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iida H., Okada Y. Regulation of polar surface structures in Caulobacter crescentus: pleiotropic mutations affect the coordinate morphogenesis of flagella, pili and phage receptors. Mol Gen Genet. 1976 Dec 8;149(2):167–173. doi: 10.1007/BF00332885. [DOI] [PubMed] [Google Scholar]
- Hodgson D., Shaw P., Letts V., Henry S., Shapiro L. Genetic analysis and characterization of a Caulobacter crescentus mutant defective in membrane biogenesis. J Bacteriol. 1984 May;158(2):430–440. doi: 10.1128/jb.158.2.430-440.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenel E. D., Newton A. Localization of surface structures during procaryotic differentiation: role of cell division in Caulobacter crescentus. Differentiation. 1982;21(2):71–78. doi: 10.1111/j.1432-0436.1982.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Huguenel E., Newton A. Isolation of flagellated membrane vesicles from Caulobacter crescentus cells: evidence for functional differentiation of polar membrane domains. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3409–3413. doi: 10.1073/pnas.81.11.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977 May;86(1):25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Farmer S., Agabian N. Adsorption properties of stage-specific Caulobacter phage phiCbK. Virology. 1977 Mar;77(1):401–407. doi: 10.1016/0042-6822(77)90436-6. [DOI] [PubMed] [Google Scholar]
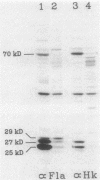
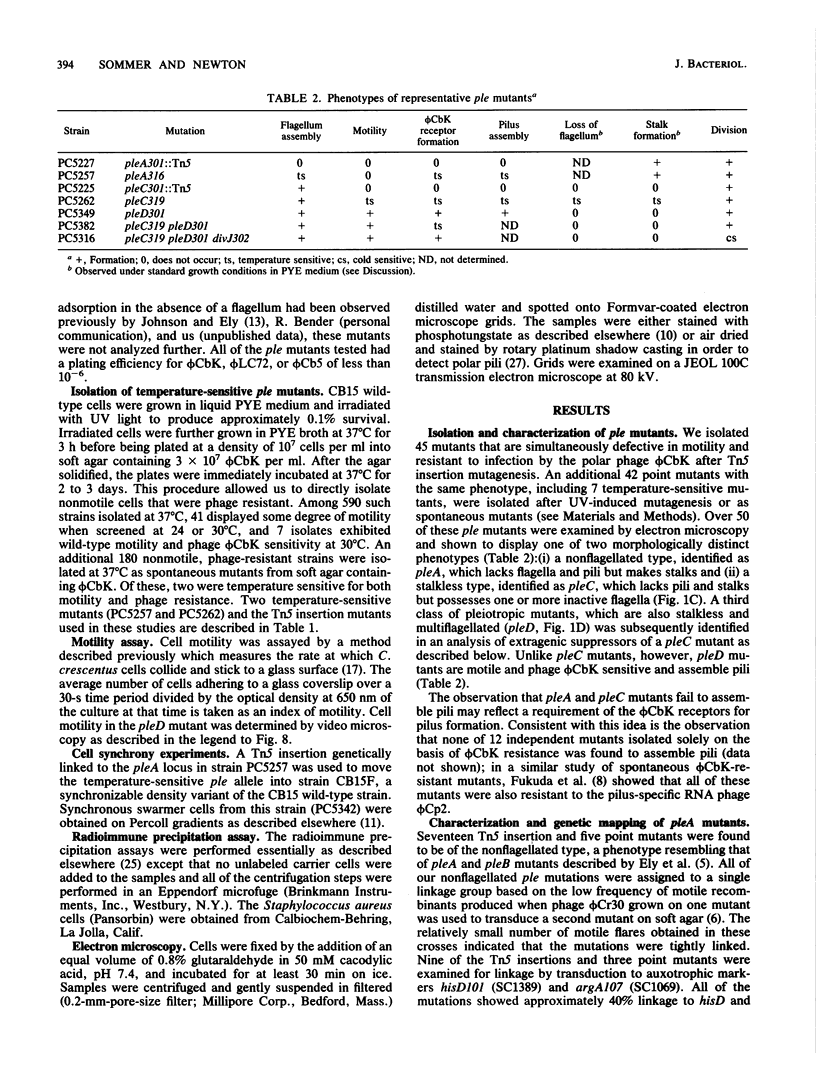
- Minnich S. A., Ohta N., Taylor N., Newton A. Role of the 25-, 27-, and 29-kilodalton flagellins in Caulobacter crescentus cell motility: method for construction of deletion and Tn5 insertion mutants by gene replacement. J Bacteriol. 1988 Sep;170(9):3953–3960. doi: 10.1128/jb.170.9.3953-3960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. Role of transcription in the temporal control of development in Caulobacter crescentus (stalk-rifampin-RNA synthesis-DNA synthesis-motility). Proc Natl Acad Sci U S A. 1972 Feb;69(2):447–451. doi: 10.1073/pnas.69.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta N., Chen L. S., Swanson E., Newton A. Transcriptional regulation of a periodically controlled flagellar gene operon in Caulobacter crescentus. J Mol Biol. 1985 Nov 5;186(1):107–115. doi: 10.1016/0022-2836(85)90261-x. [DOI] [PubMed] [Google Scholar]
- Osley M. A., Newton A. Mutational analysis of developmental control in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):124–128. doi: 10.1073/pnas.74.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Newton A. Temporal control of the cell cycle in Caulobacter crescentus: roles of DNA chain elongation and completion. J Mol Biol. 1980 Mar 25;138(1):109–128. doi: 10.1016/s0022-2836(80)80007-6. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M., Stanier R. Y. The development of cellular stalks in bacteria. J Cell Biol. 1966 Mar;28(3):423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. Generation of polarity during Caulobacter cell differentiation. Annu Rev Cell Biol. 1985;1:173–207. doi: 10.1146/annurev.cb.01.110185.001133. [DOI] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Purification and characterization of a polyhook protein from Caulobacter crescentus. J Bacteriol. 1979 May;138(2):575–583. doi: 10.1128/jb.138.2.575-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Regulation of periodic protein synthesis in the cell cycle: control of initiation and termination of flagellar gene expression. Cell. 1981 Apr;24(1):49–57. doi: 10.1016/0092-8674(81)90500-6. [DOI] [PubMed] [Google Scholar]
- Sommer J. M., Newton A. Sequential regulation of developmental events during polar morphogenesis in Caulobacter crescentus: assembly of pili on swarmer cells requires cell separation. J Bacteriol. 1988 Jan;170(1):409–415. doi: 10.1128/jb.170.1.409-415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]