Abstract
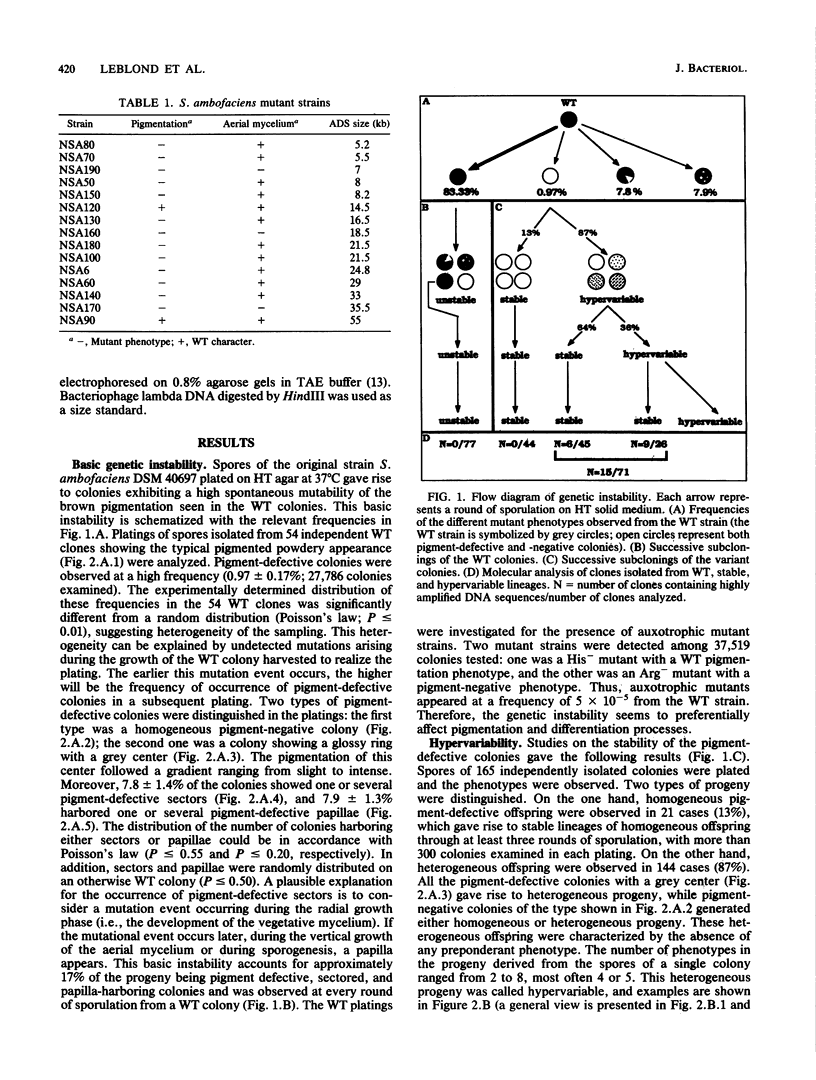
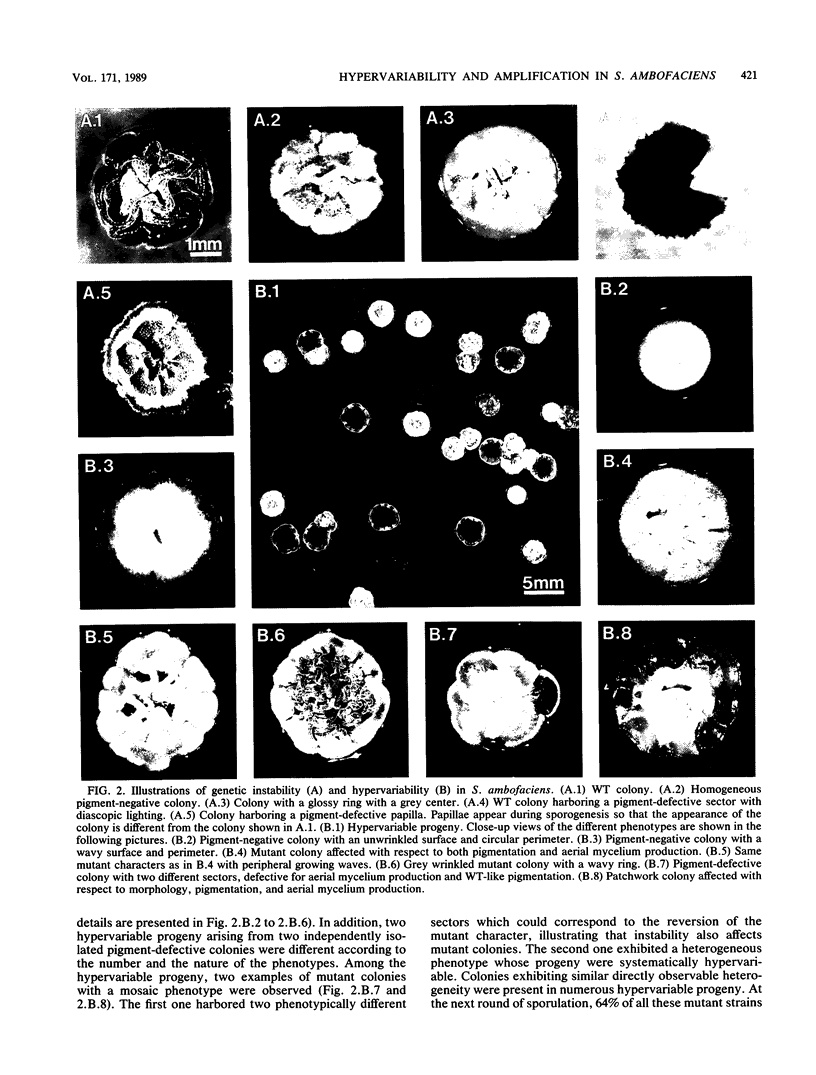
The wild-type strain Streptomyces ambofaciens DSM 40697 exhibits a high degree of genetic instability. Pigment-defective colonies were observed in the progeny of wild-type colonies at a frequency of about 0.01. While only 13% of these pigment-defective colonies gave rise to homogeneous progeny exhibiting the mutant parental phenotype, 87% of the mutant colonies gave rise to hetergeneous progeny without a preponderant phenotype. This new phenomenon of instability was called hypervariability. In addition, 21% of the mutant strains arising in hypervariable progeny contained highly reiterated DNA sequences, while amplified DNA sequences could be detected in neither stable pigment-defective mutant clones nor in wild-type clones. These results indicate a frequent association between genetic instability and hypervariability and a frequent association between hypervariability and amplification of DNA sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbuchner J., Cullum J. DNA amplification and an unstable arginine gene in Streptomyces lividans 66. Mol Gen Genet. 1984;195(1-2):134–138. doi: 10.1007/BF00332735. [DOI] [PubMed] [Google Scholar]
- Altenbuchner J., Cullum J. Structure of an amplifiable DNA sequence in Streptomyces lividans 66. Mol Gen Genet. 1985;201(2):192–197. doi: 10.1007/BF00425659. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Stonesifer J. Phenotypic changes associated with loss of expression of tylosin biosynthesis and resistance genes in Streptomyces fradiae. J Antibiot (Tokyo) 1985 Sep;38(9):1226–1236. doi: 10.7164/antibiotics.38.1226. [DOI] [PubMed] [Google Scholar]
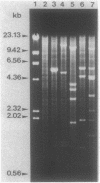
- Demuyter P., Leblond P., Decaris B., Simonet J. M. Characterization of two families of spontaneously amplifiable units of DNA in Streptomyces ambofaciens. J Gen Microbiol. 1988 Jul;134(7):2001–2007. doi: 10.1099/00221287-134-7-2001. [DOI] [PubMed] [Google Scholar]
- Dyson P., Schrempf H. Genetic instability and DNA amplification in Streptomyces lividans 66. J Bacteriol. 1987 Oct;169(10):4796–4803. doi: 10.1128/jb.169.10.4796-4803.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett F., Cullum J. DNA deletions in spontaneous chloramphenicol-sensitive mutants of Streptomyces coelicolor A 3(2) and Streptomyces lividans 66. Mol Gen Genet. 1987 May;207(2-3):499–502. doi: 10.1007/BF00331621. [DOI] [PubMed] [Google Scholar]
- Freeman R. F., Bibb M. J., Hopwood D. A. Chloramphenicol acetylransferase-independent chloramphenicol resistance in Streptomyces coelicolor A3(2). J Gen Microbiol. 1977 Feb;98(2):453–465. doi: 10.1099/00221287-98-2-453. [DOI] [PubMed] [Google Scholar]
- Freeman R. F., Hopwood D. A. Unstable naturally occurring resistance to antibiotics in streptomyces. J Gen Microbiol. 1978 Jun;106(2):377–381. doi: 10.1099/00221287-106-2-377. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Hintermann G., Simonet J. M., Crameri R., Piret J., Hütter R. Certain chromosomal regions in Streptomyces glaucescens tend to carry amplifications and deletions. Mol Gen Genet. 1985;200(3):375–384. doi: 10.1007/BF00425720. [DOI] [PubMed] [Google Scholar]
- Hintermann G., Crameri R., Vögtli M., Hütter R. Streptomycin-sensitivity in Streptomyces glaucescens is due to deletions comprising the structural gene coding for a specific phosphotransferase. Mol Gen Genet. 1984;196(3):513–520. doi: 10.1007/BF00436201. [DOI] [PubMed] [Google Scholar]
- Hintermann G., Zatchej M., Hütter R. Cloning and expression of the genetically unstable tyrosinase structural gene from Streptomyces glaucescens. Mol Gen Genet. 1985;200(3):422–432. doi: 10.1007/BF00425726. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Kumada Y., Beppu T. Unstable genetic determinant of A-factor biosynthesis in streptomycin-producing organisms: cloning and characterization. J Bacteriol. 1984 May;158(2):481–487. doi: 10.1128/jb.158.2.481-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornemann U., Otto C. J., Hoffman G. G., Bertinuson A. C. Spectinomycin resistance and associated DNA amplification in Streptomyces achromogenes subsp. rubradiris. J Bacteriol. 1987 Jun;169(6):2360–2366. doi: 10.1128/jb.169.6.2360-2366.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa J., Koyama Y., Mizuno S., Hotta K. Mechanism of increased kanamycin-resistance generated by protoplast regeneration of Streptomyces griseus. II. Mutational gene alteration and gene amplification. J Antibiot (Tokyo) 1988 Jan;41(1):104–112. doi: 10.7164/antibiotics.41.104. [DOI] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984 Nov 16;226(4676):792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Ono H., Hintermann G., Crameri R., Wallis G., Hütter R. Reiterated DNA sequences in a mutant strain of Streptomyces glaucescens and cloning of the sequence in Escherichia coli. Mol Gen Genet. 1982;186(1):106–110. doi: 10.1007/BF00422920. [DOI] [PubMed] [Google Scholar]
- PRIDHAM T. G., ANDERSON P., FOLEY C., LINDENFELSER L. A., HESSELTINE C. W., BENEDICT R. G. A selection of media for maintenance and taxonomic study of Streptomyces. Antibiot Annu. 1956:947–953. [PubMed] [Google Scholar]
- Potekhin Ia A., Danilenko V. N. Determinant ustoichivosti k kanamitsinu Streptomyces rimosus: amplifikatsiia v sostave khromosomy i obratimaia geneticheskaia nestabil'nost'. Mol Biol (Mosk) 1985 May-Jun;19(3):805–817. [PubMed] [Google Scholar]
- Robinson M., Lewis E., Napier E. Occurrence of reiterated DNA sequences in strains of Streptomyces produced by an interspecific protoplast fusion. Mol Gen Genet. 1981;182(2):336–340. doi: 10.1007/BF00269680. [DOI] [PubMed] [Google Scholar]
- Schrempf H. Deletion and amplification of DNA sequences in melanin-negative variants of Streptomyces reticuli. Mol Gen Genet. 1983;189(3):501–505. doi: 10.1007/BF00325917. [DOI] [PubMed] [Google Scholar]
- Schrempf H. Plasmid loss and changes within the chromosomal DNA of Streptomyces reticuli. J Bacteriol. 1982 Aug;151(2):701–707. doi: 10.1128/jb.151.2.701-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Young M., Cullum J. A plausible mechanism for large-scale chromosomal DNA amplification in streptomycetes. FEBS Lett. 1987 Feb 9;212(1):10–14. doi: 10.1016/0014-5793(87)81547-8. [DOI] [PubMed] [Google Scholar]