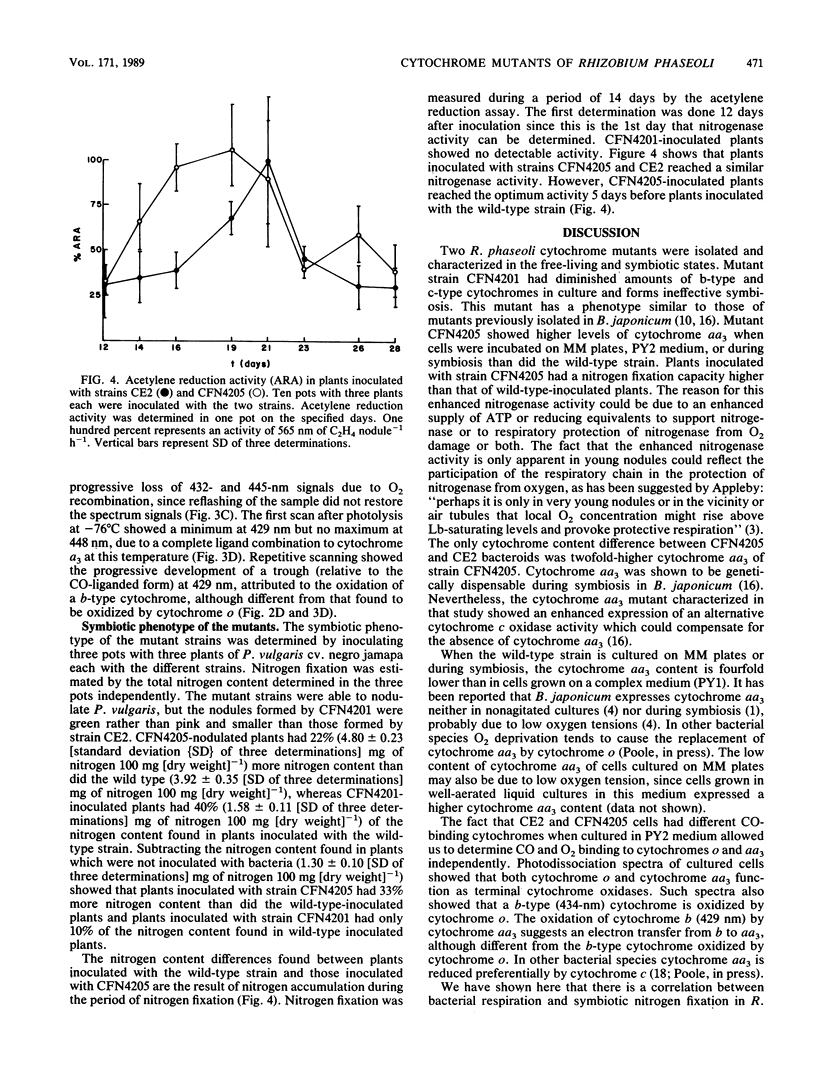
Abstract
Cultured cells of a Rhizobium phaseoli wild-type strain (CE2) possess b-type and c-type cytochromes and two terminal oxidases: cytochromes o and aa3. Cytochrome aa3 was partially expressed when CE2 cells were grown on minimal medium, during symbiosis, and in well-aerated liquid cultures in a complex medium (PY2). Two cytochrome mutants of R. phaseoli were obtained and characterized. A Tn5-mob-induced mutant, CFN4201, expressed diminished amounts of b-type and c-type cytochromes, showed an enhanced expression of cytochrome oxidases, and had reduced levels of N,N,N',N'-tetramethyl-p-phenylenediamine, succinate, and NADH oxidase activities. Nodules formed by this strain had no N2 fixation activity. The other mutant, CFN4205, which was isolated by nitrosoguanidine mutagenesis, had reduced levels of cytochrome o and higher succinate oxidase activity but similar NADH and N,N,N',N'-tetramethyl-p-phenylenediamine oxidase activities when compared with the wild-type strain. Strain CFN4205 expressed a fourfold-higher cytochrome aa3 content when cultured on minimal and complex media and had twofold-higher cytochrome aa3 levels during symbiosis when compared with the wild-type strain. Nodules formed by strain CFN4205 fixed 33% more N2 than did nodules formed by the wild-type strain, as judged by the total nitrogen content found in plants nodulated by these strains. Finally, low-temperature photodissociation spectra of whole cells from strains CE2 and CFN4205 reveal cytochromes o and aa3. Both cytochromes react with O2 at -180 degrees C to give a light-insensitive compound. These experiments identify cytochromes o and aa3 as functional terminal oxidases in R. phaseoli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. II. Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim Biophys Acta. 1969 Jan 14;172(1):88–105. doi: 10.1016/0005-2728(69)90094-2. [DOI] [PubMed] [Google Scholar]
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Escamilla J. E., Ramírez R., Del-Arenal P., Aranda A. Respiratory systems of the Bacillus cereus mother cell and forespore. J Bacteriol. 1986 Aug;167(2):544–550. doi: 10.1128/jb.167.2.544-550.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig B., Gibson Q. H. Reaction of oxygen with cytochrome c oxidase from Paracoccus denitrificans. J Biol Chem. 1981 Oct 10;256(19):10092–10098. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Kirshbom P. M., Maier R. J. Tn5-induced cytochrome mutants of Bradyrhizobium japonicum: effects of the mutations on cells grown symbiotically and in culture. J Bacteriol. 1987 Mar;169(3):1089–1094. doi: 10.1128/jb.169.3.1089-1094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Electron transport components involved in hydrogen oxidation in free-living Rhizobium japonicum. J Bacteriol. 1982 Oct;152(1):422–430. doi: 10.1128/jb.152.1.422-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Waring A. J., Chance B. The reaction of cytochrome omicron in Escherichia coli with oxygen. Low-temperature kinetic and spectral studies. Biochem J. 1979 Nov 15;184(2):379–389. doi: 10.1042/bj1840379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hollander J. A., Stouthamer A. H. The electron transport chain of Rhizobium trifolii. Eur J Biochem. 1980 Oct;111(2):473–478. doi: 10.1111/j.1432-1033.1980.tb04962.x. [DOI] [PubMed] [Google Scholar]


