Abstract
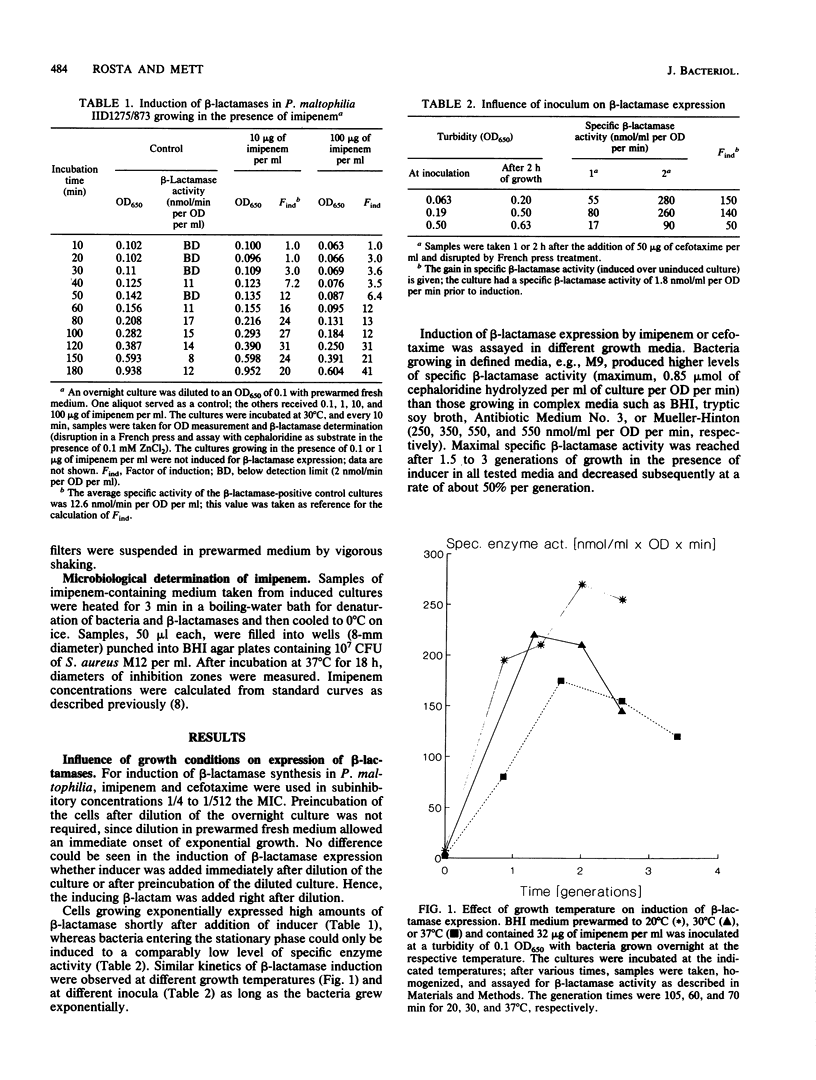
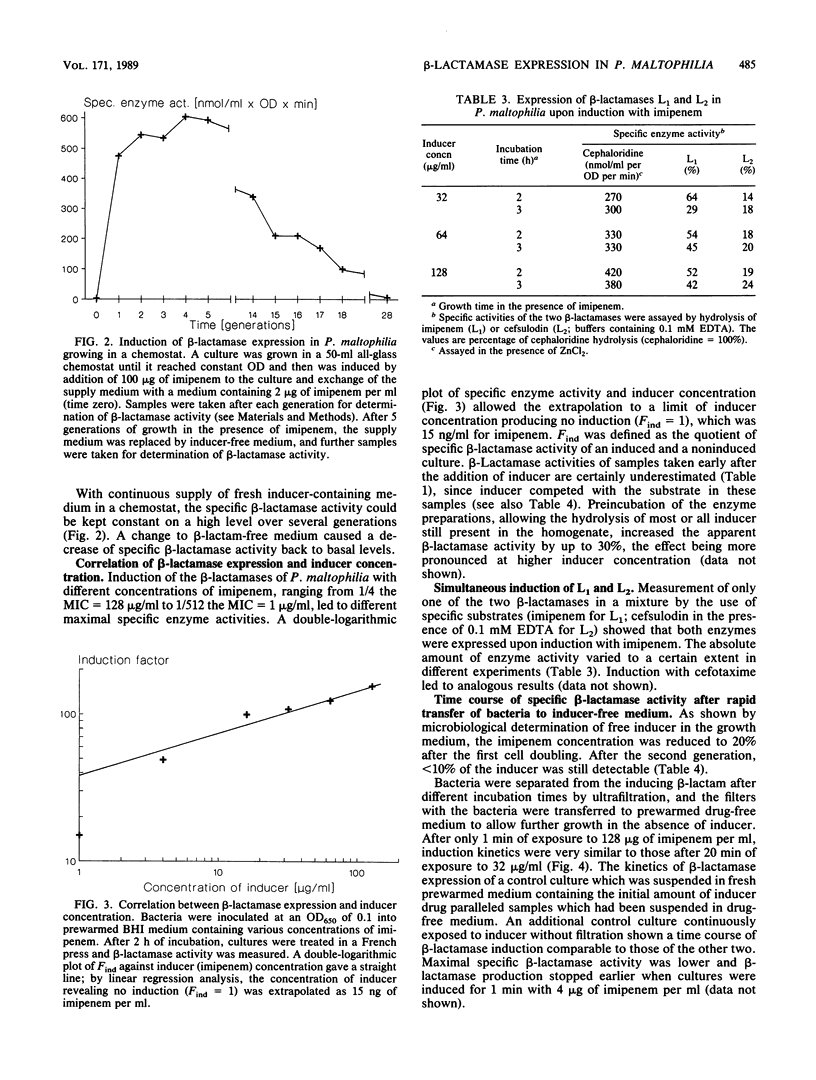
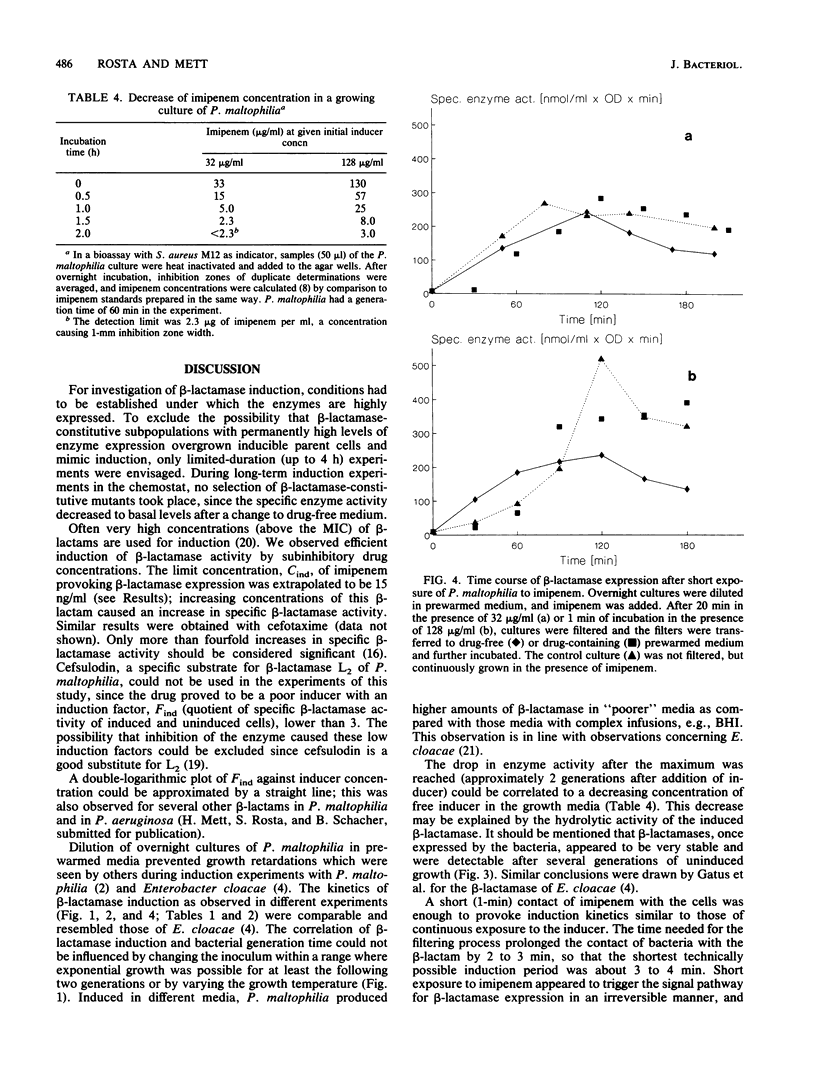
The kinetics of beta-lactamase induction in Pseudomonas maltophilia IID1275/873 were investigated. Upon induction with beta-lactam antibiotics, a correlation was seen between the increase in specific beta-lactamase activity and the generation time, as well as the concentration of inducer in the medium. The specific beta-lactamase activity increased slowly within the first 0.5 generation and then more rapidly; it decreased regularly after about 2 generations of growth in the presence of inducer. This decrease could presumably be attributed to the continuous breakdown of inducer by beta-lactamases in the culture medium. In a chemostat culture with continuous supply of fresh inducer-containing medium, the specific beta-lactamase activity could be stabilized at a high level over several generations. Removal of the beta-lactam after a certain induction time showed that a short exposure of the bacteria to inducer caused induction kinetics comparable to those resulting from continuous exposure of the cells to inducer. The two beta-lactamases of P. maltophilia, L1 and L2, were induced simultaneously under various experimental conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bicknell R., Emanuel E. L., Gagnon J., Waley S. G. The production and molecular properties of the zinc beta-lactamase of Pseudomonas maltophilia IID 1275. Biochem J. 1985 Aug 1;229(3):791–797. doi: 10.1042/bj2290791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett F., Curtis N. A., Richmond M. H. Mutant of Pseudomonas aeruginosa 18S that synthesizes type Id beta-lactamase constitutively. J Bacteriol. 1976 Sep;127(3):1585–1586. doi: 10.1128/jb.127.3.1585-1586.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatus B. J., Bell S. M., Jimenez A. S. Comparison of glycine enhancement with cefoxitin induction of class 1 beta-lactamase production in Enterobacter cloacae ATCC 13047. J Antimicrob Chemother. 1988 Feb;21(2):163–170. doi: 10.1093/jac/21.2.163. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Shahrabadi M. S., Bryan L. E. Distribution of porin and lipopolysaccharide antigens on a Pseudomonas aeruginosa permeability mutant. Antimicrob Agents Chemother. 1986 Nov;30(5):802–805. doi: 10.1128/aac.30.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Intrinsic antibiotic resistance of Pseudomonas aeruginosa. J Antimicrob Chemother. 1986 Dec;18(6):653–656. doi: 10.1093/jac/18.6.653. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L. Activity of beta-lactam antibiotics against Pseudomonas aeruginosa carrying R plasmids determining different beta-lactamases. Antimicrob Agents Chemother. 1979 Aug;16(2):243–245. doi: 10.1128/aac.16.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lindquist S., Normark S. Inactivation of the ampD gene causes semiconstitutive overproduction of the inducible Citrobacter freundii beta-lactamase. J Bacteriol. 1987 May;169(5):1923–1928. doi: 10.1128/jb.169.5.1923-1928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D. M. Penicillin-binding proteins, porins and outer-membrane permeability of carbenicillin-resistant and -susceptible strains of Pseudomonas aeruginosa. J Med Microbiol. 1984 Oct;18(2):261–270. doi: 10.1099/00222615-18-2-261. [DOI] [PubMed] [Google Scholar]
- Malouin F., Bryan L. E. Modification of penicillin-binding proteins as mechanisms of beta-lactam resistance. Antimicrob Agents Chemother. 1986 Jul;30(1):1–5. doi: 10.1128/aac.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett H., Rosta S., Schacher B., Frei R. Outer membrane permeability and beta-lactamase content in Pseudomonas maltophilia clinical isolates and laboratory mutants. Rev Infect Dis. 1988 Jul-Aug;10(4):765–769. doi: 10.1093/clinids/10.4.765. [DOI] [PubMed] [Google Scholar]
- Mett H., Schacher B., Schneider P., Zak O. Interaction of the novel penem CGP 31 608 and its enantiomer with type Id beta-lactamase and penicillin-binding proteins. Eur J Clin Microbiol. 1987 Dec;6(6):674–678. doi: 10.1007/BF02013069. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol Ther. 1985;27(2):197–231. doi: 10.1016/0163-7258(85)90069-5. [DOI] [PubMed] [Google Scholar]
- Saino Y., Inoue M., Mitsuhashi S. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob Agents Chemother. 1984 Mar;25(3):362–365. doi: 10.1128/aac.25.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Then R. L. Ability of newer beta-lactam antibiotics to induce beta-lactamase production in Enterobacter cloacae. Eur J Clin Microbiol. 1987 Aug;6(4):451–455. doi: 10.1007/BF02013109. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Ashby J. P., Piddock L. J. Induction of beta-lactamases in gram-negative bacilli. J Antimicrob Chemother. 1987 Nov;20(5):767–768. doi: 10.1093/jac/20.5.767. [DOI] [PubMed] [Google Scholar]


