Abstract
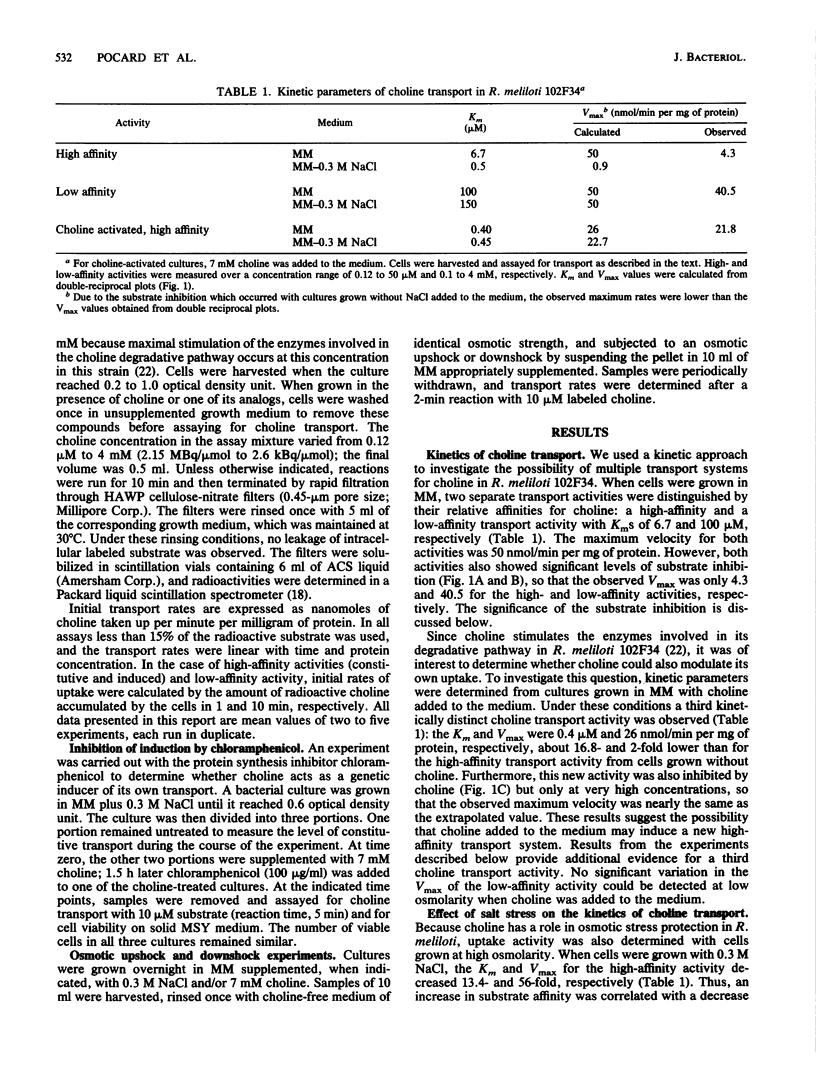
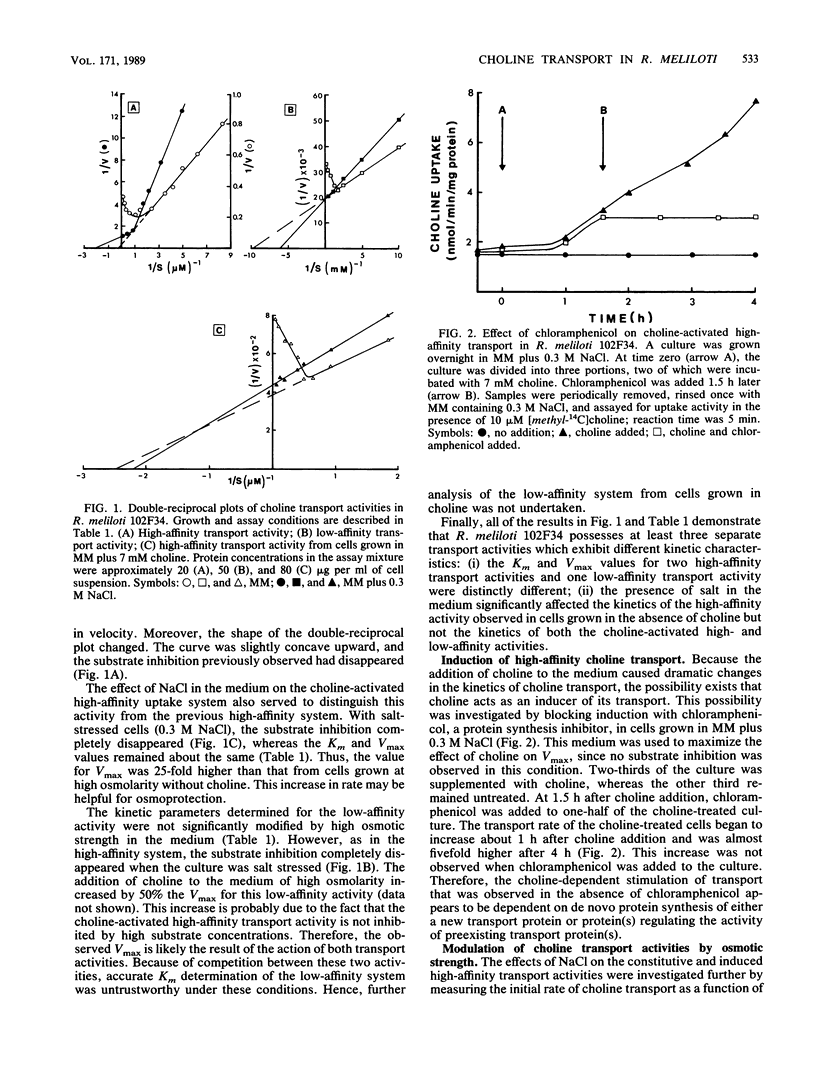
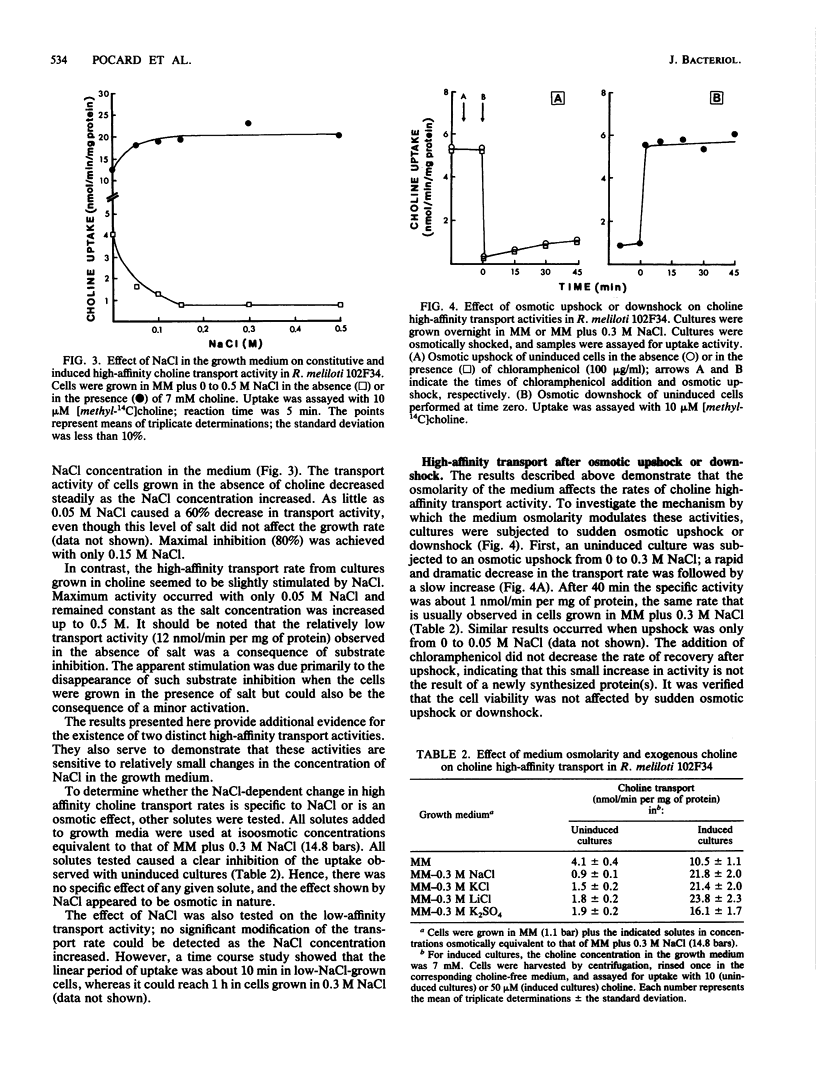
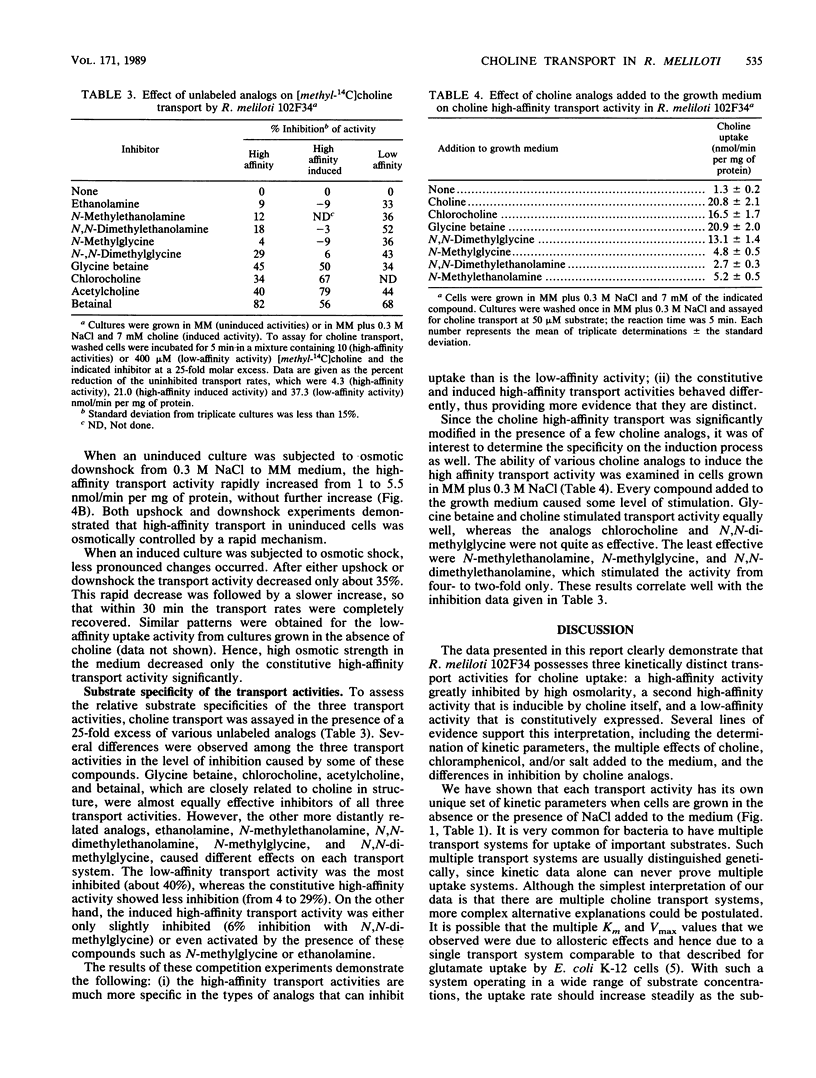
Choline has both a nutritional and osmoregulatory role in Rhizobium meliloti (T. Bernard, J. A. Pocard, B. Perroud, and D. Le Rudulier, Arch. Microbiol. 143:359-364, 1986). In view of this fact, choline transport was studied in R. meliloti 102F34 to determine how the rate of choline uptake is modulated. The effects of the cultural conditions on the kinetics of transport are presented. A high-affinity activity and a low-affinity activity were found in cells grown in minimal medium. The addition of 0.3 M NaCl or other osmolytes to the medium resulted in a marked decrease in the high-affinity activity, whereas the low-affinity activity remained fairly constant. Furthermore, results from osmotic upshock and downshock experiments indicate that the response of the cell to high osmolarity is rapid; hence, the mechanism of regulation by salt likely does not involve gene induction. A second high-affinity transport activity was induced by choline itself. Like the constitutive low-affinity transport activity, this activity was not greatly altered when the cells were grown in media of elevated osmotic strength. We conclude that although all three kinetically distinct transport systems are efficient at low osmolarity, only the induced high- and low-affinity activities are important for osmoregulation. The characteristics of the three transport activities from R. meliloti are compared with those from other bacterial species that use choline for growth and/or osmoregulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. D. Microbial water stress. Bacteriol Rev. 1976 Dec;40(4):803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985 Dec;164(3):1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985 Dec;164(3):1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYWARD H. R., STADTMAN T. C. Anaerobic degradation of choline. I. Fermentation of choline by an anaerobic, cytochrome-producing bacterium, Vibrio cholinicus n. sp. J Bacteriol. 1959 Oct;78:557–561. doi: 10.1128/jb.78.4.557-561.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Choline transport in Saccharomyces cerevisiae. J Bacteriol. 1980 Jul;143(1):176–181. doi: 10.1128/jb.143.1.176-181.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff J. F., Rodriguez-Valera F. Betaine is the main compatible solute of halophilic eubacteria. J Bacteriol. 1984 Oct;160(1):478–479. doi: 10.1128/jb.160.1.478-479.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortstee G. J. The aerobic decomposition of choline by microorganisms. I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as the sole carbon and nitrogen source. Arch Mikrobiol. 1970;71(3):235–244. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. I., Sydnes L. K., Landfald B., Strøm A. R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987 Feb;147(1):1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983 Jul;46(1):152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Yang S. S., Csonka L. N. Nitrogen fixation in Klebsiella pneumoniae during osmotic stress. Effect of exogenous proline or a proline overproducing plasmid. Biochim Biophys Acta. 1982 Nov 24;719(2):273–283. doi: 10.1016/0304-4165(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Munro G. F., Bell C. A., Lederman M. Multiple transport components for putrescine in Escherichia coli. J Bacteriol. 1974 Jun;118(3):952–963. doi: 10.1128/jb.118.3.952-963.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIEH H. S. AEROBIC DEGRADATION OF CHOLINE. I. FERMENTATION OF CHOLINE BY A MARINE BACTERIUM, ACHROMOBACTER CHOLINOPHAGUM N. SP. Can J Microbiol. 1964 Dec;10:837–842. doi: 10.1139/m64-109. [DOI] [PubMed] [Google Scholar]
- Sherr S. I., Law J. H. Phosphatidylcholine synthesis in Agrobacterium tumefaciens. II. Uptake and utilization of choline. J Biol Chem. 1965 Oct;240(10):3760–3765. [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes W., Keith A., Wanda P. Active transport of choline by a marine pseudomonad. J Bacteriol. 1974 Oct;120(1):197–202. doi: 10.1128/jb.120.1.197-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrvold O. B., Falkenberg P., Landfald B., Eshoo M. W., Bjørnsen T., Strøm A. R. Selection, mapping, and characterization of osmoregulatory mutants of Escherichia coli blocked in the choline-glycine betaine pathway. J Bacteriol. 1986 Mar;165(3):856–863. doi: 10.1128/jb.165.3.856-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]


