Abstract
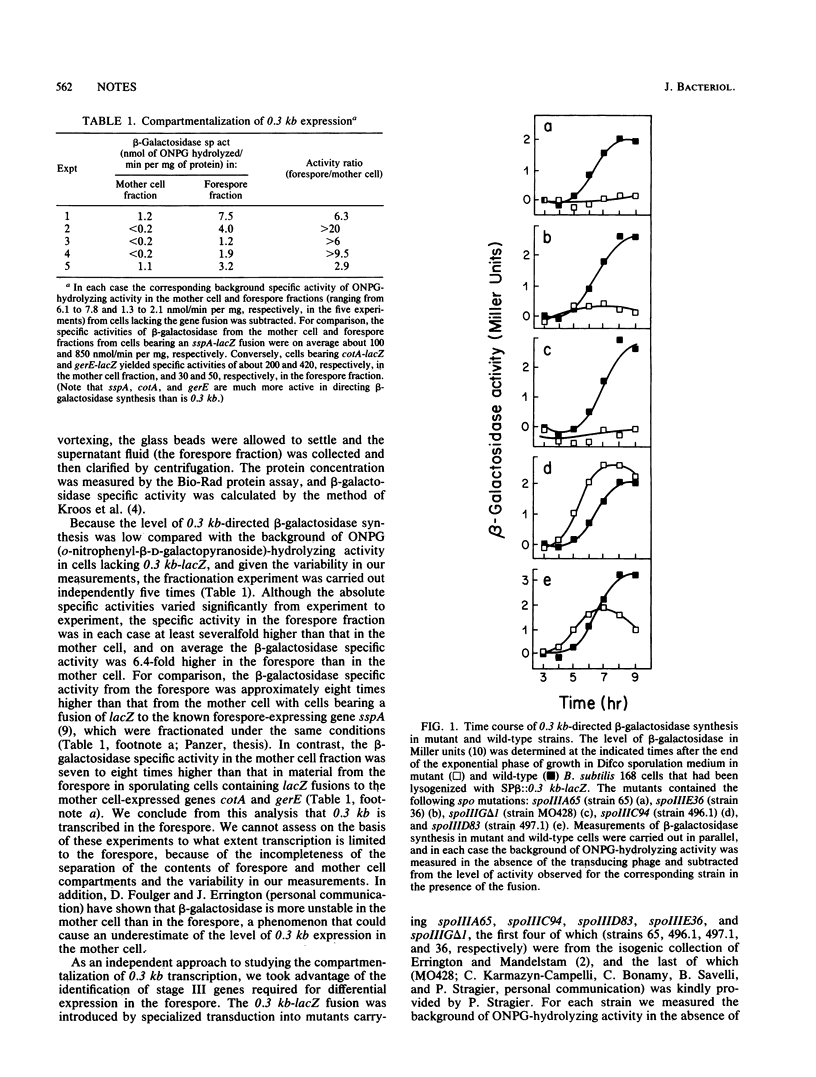
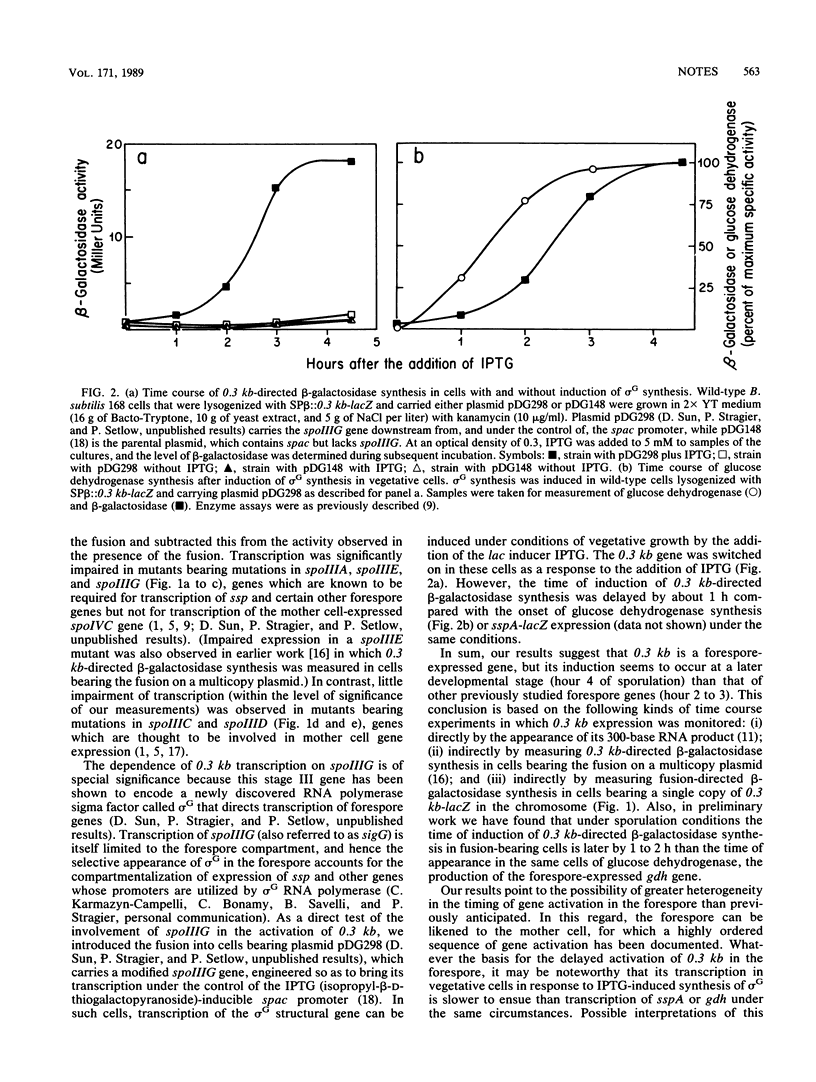
We present evidence indicating that the previously studied, sporulation-induced gene 0.3 kb, which encodes a stable RNA present at late developmental stages, is transcribed in the forespore chamber of sporulating cells of Bacillus subtilis. Compartmentalized gene expression was demonstrated on the basis of subcellular fractionation experiments in which severalfold-higher levels of 0.3 kb-directed beta-galactosidase specific activity were observed in forespore extracts than in extracts from the mother cell and dependence studies in which 0.3 kb transcription was found to be blocked in mutants bearing mutations in spoIIIA, spoIIIE, and spoIIIG, genes which are known to govern forespore gene expression. Also, 0.3 kb transcription could be switched on during growth in cells in which transcription of the forespore regulatory gene spoIIIG was engineered to be activated in response to the lac inducer IPTG (isopropyl-beta-D-thiogalactopyranoside). Although it is transcribed in the forespore, 0.3 kb is switched on at a later developmental stage than other previously studied forespore-expressed genes, and hence it appears to be representative of an additional temporal class of compartmentalized gene expression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke S., Lopez-Diaz I., Mandelstam J. Use of lacZ gene fusions to determine the dependence pattern of the sporulation gene spoIID in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2987–2994. doi: 10.1099/00221287-132-11-2987. [DOI] [PubMed] [Google Scholar]
- Errington J., Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern and the spore compartment expression of sporulation operon spoVA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2977–2985. doi: 10.1099/00221287-132-11-2977. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kuspa A., Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986 Sep;117(1):252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- Kunkel B., Sandman K., Panzer S., Youngman P., Losick R. The promoter for a sporulation gene in the spoIVC locus of Bacillus subtilis and its use in studies of temporal and spatial control of gene expression. J Bacteriol. 1988 Aug;170(8):3513–3522. doi: 10.1128/jb.170.8.3513-3522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Hackett R. H., Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollington J. F., Losick R. A cloned gene that is turned on at an intermediate stage of spore formation in Bacillus subtilis. J Bacteriol. 1981 Aug;147(2):443–451. doi: 10.1128/jb.147.2.443-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N., Moran C. P., Jr Compartment-specific transcription in Bacillus subtilis: identification of the promoter for gdh. J Bacteriol. 1988 Nov;170(11):5086–5092. doi: 10.1128/jb.170.11.5086-5092.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman K., Kroos L., Cutting S., Youngman P., Losick R. Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol. 1988 Apr 5;200(3):461–473. doi: 10.1016/0022-2836(88)90536-0. [DOI] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Localization of low-molecular-weight basic proteins in Bacillus megaterium spores by cross-linking with ultraviolet light. J Bacteriol. 1979 Aug;139(2):486–494. doi: 10.1128/jb.139.2.486-494.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M. A., Lang N., Sandman K., Losick R. A promoter whose utilization is temporally regulated during sporulation in Bacillus subtilis. J Mol Biol. 1984 Jul 5;176(3):333–348. doi: 10.1016/0022-2836(84)90493-5. [DOI] [PubMed] [Google Scholar]
- Turner S. M., Errington J., Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIIC in spo mutants of Bacillus subtilis: a branched pathway of expression of sporulation operons. J Gen Microbiol. 1986 Nov;132(11):2995–3003. doi: 10.1099/00221287-132-11-2995. [DOI] [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lencastre H., Piggot P. J. Identification of different sites of expression for spo loci by transformation of Bacillus subtilis. J Gen Microbiol. 1979 Oct;114(2):377–389. doi: 10.1099/00221287-114-2-377. [DOI] [PubMed] [Google Scholar]



