Abstract
Objectives
We evaluated lung maturation responses to the mixture of betamethasone phosphate (Beta-PO4) + betamethasone acetate (Beta-Ac) in comparison to Beta-PO4 or Beta-Ac in fetal sheep.
Study Design
Ewes carrying singleton pregnancies at 122d gestation were randomized to: single doses of 0.5 mg/kg Beta-PO4 + Beta-Ac, 0.25 mg/kg Beta-PO4, 0.5 mg/kg Beta-PO4 or 0.25 mg/kg Beta-Ac given 48h before delivery. These treatments were compared to saline placebo and 2 doses of 0.5 mg/kg Beta-PO4 + Beta-Ac given 48 and 24h prior to delivery. Fetal lung maturation was evaluated.
Results
The 2 doses of the Beta-PO4 + Beta-Ac mixture gave the best lung maturation. Single doses of the Beta-PO4 + Beta-Ac mixture and Beta-Ac also induced lung maturation. There were no consistent responses to either dose of Beta-PO4.
Conclusions
Beta-PO4 alone is ineffective with the dosing schedule used, while Beta-Ac can induce lung maturation. However, the best responses result from the mixture of Beta-PO4 + Beta-Ac.
Keywords: Respiratory Distress Syndrome, preterm, corticosteroids, fetal sheep, lung development
INTRODUCTION
Antenatal corticosteroid treatments are standard of care for women at risk of early preterm labor. However, there remain questions about the optimal corticosteroid, the dose and the formulation, as reviewed in the new meta-analysis of corticosteroids (1). The betamethasone preparation used clinically is a 1 to 1 mixture of soluble and rapid acting betamethasone-sodium-phosphate (Beta-PO4) and the slow release betamethasone-acetate (Beta-Ac) originally selected by Liggins and Howie (2). The Beta-PO4 and Beta-Ac mixture is the primary corticosteroid preparation used world-wide because its use is associated with better neonatal outcomes than dexamethasone (3, 4). Because there continue to be concerns about potential toxicity of corticosteroids in pregnancy, strategies to minimize maternal and fetal exposures are desirable. We proposed that the long half-life of Beta-Ac may not contribute to the beneficial fetal responses (3). We also suggested that two 12 mg doses may not be required because infants delivered 24h after the first dose have benefit (1, 3). If true, maternal treatments with 6 mg Beta-PO4 might be equivalent to the standard 24 mg dose given as two 12 mg doses. Ultimately it would be desirable to develop an optimal agent and dosing strategy for antenatal corticosteroids. We report a comparison of fetal lung responses in sheep to the clinical betamethasone preparation and its two components - Beta-PO4 and Beta-Ac.
MATERIALS AND METHODS
Betamethasone treatments
The animal studies were approved by the animal ethics committees at Cincinnati Children’s Hospital and by the Western Australian Department of Agriculture. Singleton pregnant Merino ewes were weighed and control ewes received IM saline injections 24h apart on days 122 and 123 of gestation. Other groups received: an IM dose of 0.5 mg/kg of the 1 to 1 clinical preparation of Beta-PO4 and Beta-Ac on day 122 (0.5 Beta-Phos + Beta-Ac × 1), doses of 0.5 mg/kg with the same phosphate and acetate mixture on days 122 and 123 (0.5 Beta-Phos + Beta-Ac × 2), one dose of 0.25 mg/kg Beta-PO4 on day 122 (0.25 Beta-Phos × 1), one dose of 0.5 mg/kg Beta-PO4 on day 122 (0.5 Beta-Phos × 1), or 0.25 mg/kg Beta-Ac on day 122 (0.25 Beta-Ac × 1). The phosphate and acetate mixture was the Celestone Chronodose® (Schering Plough, Australia) routinely used clinically and given as the optimal dose to cause lung maturation in fetal sheep (5). The Beta-PO4 (Sigma Chemical, St. Louis, MO) was solubilized in saline, filtered for sterility, and given in the same volume as the clinical drug. The Beta-Ac (Sigma Chemical) was solubilized in 70% ethanol and diluted into saline to generate a fine suspension. The fine suspension was visually similar to the suspension of the Beta-Ac in Celestone. This suspension also was given by IM injection in the same volume.
Assessments of lung function
The ewes were anesthetized with ketamine and zylazine and killed with a penetrating captive bolt just prior to delivery of the fetus. The fetal head was delivered through an abdominal incision and, following local injection of Lidocaine, a 4 mm endotracheal tube was secured by rapid tracheotomy (6). The fetus then was delivered, weighed, and mechanical ventilation was initiated using 100% heated and humidified oxygen. The initial ventilator settings were a rate of 40 breaths/min, a peak inspiratory pressure of 35 cmH20, a positive end expiratory pressure of 5 cmH20 and an inspiratory time of 0.7 sec (7). An umbilical artery catheter was placed to permit blood sampling. The lambs were ventilated for 30 min with blood gas measurements at 10, 20, and 30 min. Tidal volume was measured continuously with a tidal volume monitor (Florian Infant Monitor, Acutronic Medical Systems, Switzerland) (8). Peak inspiratory pressure only was changed to target a Pco2 of about 60 mmHg using a tidal volume of less than 10 ml/kg. Peak inspiratory pressures were limited to 40 cmH20 pressure to avoid pneumothorax. Compliance/kg was calculated as tidal volume divided by peak minus end expiratory pressure and by body weight. Ventilation efficiency index is an integrated measure of ventilation that is calculated as:
Ventilation efficiency index = 3800/(P●F●PaCO2), where 3800 is a CO2 production constant, P is ventilatory pressure and F is ventilator rate for these lambs without spontaneous breathing (9). The investigators delivering the lambs, ventilating the lambs, and assessing the lungs were not aware of the Beta treatment that the ewe had received.
Assessments of lungs
After the collection of a final arterial blood sample at 30 min of age, the lambs were deeply anesthetized with pentobarbital and the tracheal tube was clamped for 3 min to achieve atelectasis by oxygen absorption. The lamb was then exsanguinated, the chest was opened, and the lungs were evaluated visually for pulmonary interstitial emphysema (10). A deflation pressure-volume curve then was measured after air inflation of the lungs to a pressure of 40 cmH20 (11). After the removal of the lungs from the chest, each lung was weighed, and the left lung was lavaged 3 times by infusion and withdrawal of a sufficient volume of 4°C normal saline solution to fully fill the lung (7). The bronchoalveolar lavages (BAL) were pooled; the total volume was measured, and the aliquots were saved for later analyses.
Surfactant measurements
Saturated phosphatidylcholine (Sat PC) was isolated from chloroform-methanol (2:1) extracts of BAL fluid by neutral alumina column chromatography after exposure of lipid extracts to osmium tetroxide (12). Sat PC was quantified by phosphorus assay (13). The mRNAs for Surfactant Proteins-A, B, and C were measured using S1 nuclease protection assays, as described previously (14).
Statistical analysis
Data are presented as mean ± SEM, and statistical comparisons were performed with Instat software. A differences between groups was considered significant for a probability value of <.05. Data were compared between controls and each of the 5 corticosteroid treatment groups with one-way analysis of variance with the Tukey post hoc test for multiple comparisons. The pressure-volume curves were compared by a repeated measures ANOVA. Selected group comparisons to controls by two tailed t-tests are indicated in the text.
RESULTS
Description of animals
The numbers, birth weights, and sex of the lambs in each group are given in Table I. The birth weight of the 0.5 Beta-PO4 + Beta-Ac × 2 group was lower than the control group by t-test (p<0.05), but there were no other differences in birth weights in other groups. The animals had similar cord blood gas values at delivery (data not shown).
Table I.
Description of Animals
| Treatment Group and Dose | N | Weight (kg) | Sex (M/F) |
|---|---|---|---|
| Saline Control | 12 | 2.48?.09 | 7/5 |
| 0.5 mg/kg Beta-PO4 +Beta-Ac × 1 | 13 | 2.44?0.10 | 7/6 |
| 0.5 mg/kg Beta-PO4 +Beta-Ac × 2 | 14 | 2.22? ?.08* | 6/8 |
| 0.25 mg/kg Beta-PO4 × 1 | 15 | 2.33?0.06 | 6/9 |
| 0.5 mg/kg Beta-PO4 × 1 | 12 | 2.48?0.10 | 6/6 |
| 0.25 mg/kg Beta Ac × 1 | 9 | 2.34?0.13 | 4/5 |
p<0.05 vs. control by t-test
Ventilation outcomes
The newborn lambs were ventilated for 30 min in a standardized fashion to evaluate lung mechanisms and gas exchange (Table II). The 30 min blood gas pH values were higher for the two 0.5 Beta-PO4 + Beta-Ac treated groups than controls and qualitatively better than for the other treatment groups. The Pco2 values were lower for the 0.25 Beta-PO4, 0.5 Beta-PO4 and 0.25 Beta-Ac groups relative to control. Only the two dose 0.5 Beta-PO4 + Beta-Ac group had a significant increase in Po2, required less peak inspiratory pressure and achieved reasonable Pco2 values. At autopsy this same group had a striking increase in pulmonary interstitial emphysema relative to control and the other groups.
Table II.
Values after 30 min. of Ventilation
| Groups | pH | Pco 2 (mmHg) | Po2 (mmHg) | Peak Inspiratory Pressure (cmH20) | Tidal Volume (ml/kg) | Pulmonary Interstitial Emphysema (N/Total) | |
|---|---|---|---|---|---|---|---|
| A | Saline Control | 6.96?0.03 | 116? 8 | 86?22 | 39.3?0.5 | 7.4?0.5 | 1/12 |
| B | 0.5 mg/kg Beta-PO4 + Beta-Ac × 1 | 7.11?0.02* | 76?3* | 86?13 | 37.3?0.9 | 8.9?0.5 | 3/13 |
| C | 0.5 mg/kg Beta-PO4 + Beta-Ac × 2 | 7.23?0.02*‡ | 61?3*,† | 188?36§ | 33.2?1.1*‡ | 9.3?0.2* | 9/14 |
| D | 0.25 mg/kg Beta-PO4 × 1 | 7.03?0.02 | 98±? * | 72? 6 | 38.1?0.9 | 8.5? 0.4 | 3/15 |
| E | 0.5 mg/kg Beta-PO4 × 1 | 7.00±?.02 | 92±6* | 139±14 | 39.1±0.6 | 7.1±0.2 | 1/12 |
| F | 0.25 mg/kg Beta-Ac × 1 | 7.03?0.03 | 85±4* | 100?34 | 37.0?1.1 | 7.8?0.3 | 2/9 |
Different from control by ANOVA
Different from all groups except B by ANOVA
Different from all other groups by ANOVA
Different from A, B, and D by ANOVA
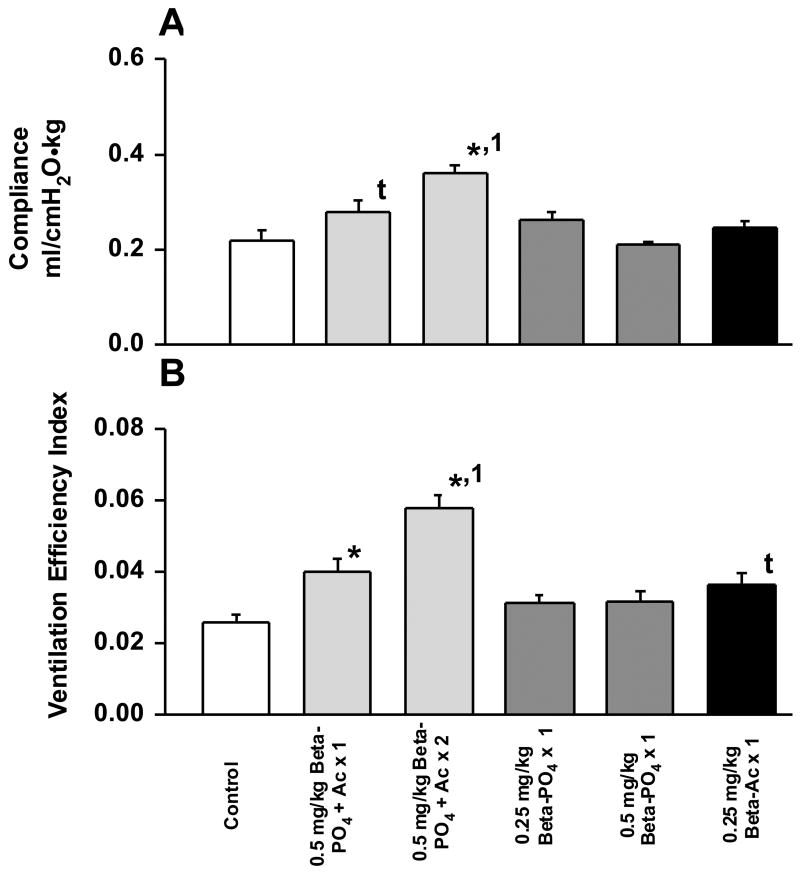
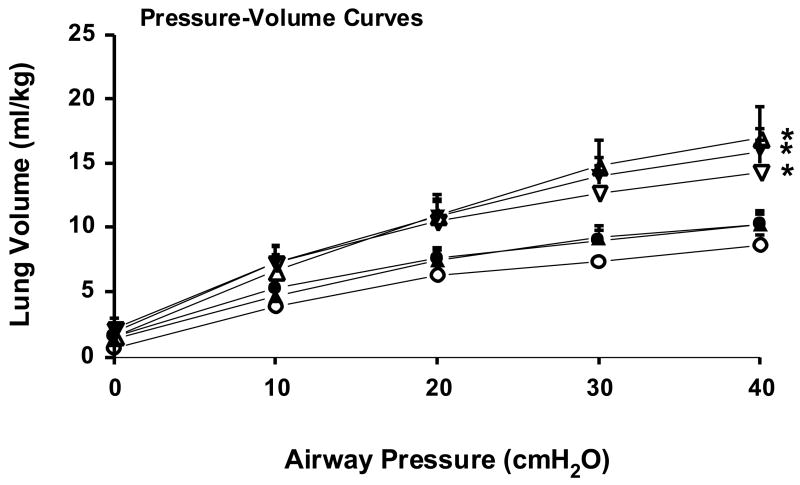
Lung function was evaluated at 30 min by measuring total thoracic compliance, ventilation efficiency index as a measure of gas exchange, and the lung gas volumes on the deflation limbs of the pressure-volume curves. Thoracic compliance was increased by the 2 dose 0.5 Beta-PO4 + Beta-Ac treatment relative to all groups and the single dose of 0.5 Beta-PO4 + Beta-Ac increased compliance relative to the control by t-test (p=0.04) (Fig. 1). The two 0.5 Beta-PO4 + Beta-Ac groups increased ventilation efficiency relative to control and the two Beta-PO4 groups. The 0.5 Beta-PO4 + Beta-Ac × 2 group had a higher ventilation efficiency index than all other groups. The 0.25 Beta-Ac group had increased ventilation efficiency relative to control by t-test (p=0.012). The maximal lung volumes measured at 40 cmH20 airway pressure were increased similarly and significantly by the treatments with the Beta mixture and the Beta-Ac alone relative to control and Beta-PO4. The only treatments that improved the deflation limbs of the pressure-volume curves were the two 0.5 Beta-PO4 + Beta-Ac groups and the 0.25 Beta-Ac group (Fig. 2). The lack of much increase in volumes at 0 and 10 cmH20 pressure suggest little effect of surfactant to stabilize deflation for any of the treatment groups. The patterns of physiologic responses for blood gas values and lung mechanics indicate that the single dose of 0.25 Beta-Ac improved lung function but not as much as either a single or two doses of the mixture of 0.5 Beta-PO4 + Beta-Ac. There was no consistent improvement in lung function in response to either dose of Beta-PO4 for the dosing schedule used for this experiment.
Fig. 1.
Compliance and ventilation efficiency index at 30 min of age. A. The 2 dose 0.5 mg/kg Beta-PO4 + Beta-Ac treatment increased compliance relative to control and all other groups. The single dose 0.5 mg/kg Beta-PO4 + Beta-Ac increased compliance relative to control by t-test. B. The ventilation efficiency index was increased by the single and two dose 0.5 mg/kg Beta-PO4 + Beta-Ac mixture relative to control, and the two dose treatment was different from all other treatments. The Beta-PO4 treatments had no effect on compliance or ventilation efficiency index, while the 0.25 mg/kg Beta-Ac increased ventilation efficiency index relative to control by t-test. * - different from control by ANOVA, 1 - different from all other groups by ANOVA, t - different from control by t-test.
Fig. 2.
Deflation limbs of pressure-volume curves measured after 30 min of ventilation. The curves for the 2 doses of 0.5 mg/kg Beta-PO4 + Beta-Ac (△), 1 dose of 0.5 mg/kg Beta-PO4 + Beta-Ac (▼), and 1 dose of 0.25 mg/kg Beta-Ac (▽) were different from the control curve (○), but not from each other. The 0.25 mg/kg Beta-PO4 (●) and 0.5 mg/kg Beta-PO4 (▲) curves were not different from control. Pressure-volume curves were measured for all control and Beta-PO4 treated animals, for 8 Beta-Ac treated animals, for 10 0.5 mg/kg Beta-PO4 + Beta-Ac × 1 treated animals, and for 7 0.5 mg/kg Beta-PO4 + Beta-Ac × 2 treated animals because some animals had air leaks caused by pulmonary interstitial emphysema. * - different from control by repeated measures ANOVA for the curves.
Surfactant components
The amounts of Sat PC recovered by BAL were similar across all groups (Table III), as has been reported previously 48h after antenatal corticosteroid treatment (7) The mixture of 0.5 Beta-PO4 + Beta-Ac increased the mRNA levels for SP-A, SP-B, and SP-C by approximately 2-fold relative to control values. The 0.5 Beta-PO4 and the 0.25 Beta-Ac also significantly increased the mRNA levels for all three surfactant proteins. The 0.25 Beta-PO4 did not increase these mRNA levels.
Table III.
Surfactant Sat PC and Surfactant Protein mRNA
| Group | Sat PC In BALF (μmol/kg) | SP-A mRNA | SP-B mRNA | SP-C mRNA | |
|---|---|---|---|---|---|
| A | Saline Control | 0.10±0.01 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 |
| B | 0.5 mg/kg Beta-PO4+Ac × 1 | 0.14±0.03 | 1.9±0.1*,† | 1.9±0.1*,† | 2.1±0.1* |
| C | 0.5 mg/kg Beta-PO4+Ac × 2 | 0.11±0.01 | 1.8±0.1*,† | 2.3±0.2*,† | 1.9±0.2* |
| D | 0.25 mg/kg Beta-PO4 × 1 | 0.18±0.03 | 1.3±0.2 | 1.2±0.2 | 1.3±0.2 |
| E | 0.5 mg/kg Beta-PO4 × 1 | 0.12±0.02 | 1.4±0.1‡ | 1.3±0.1‡ | 1.6±0.2‡ |
| F | 0.25 mg/kg Beta-Ac × 1 | 0.11±0.03 | 1.6±0.2‡ | 1.4±0.1‡ | 1.6±0.1‡ |
Different from control by ANOVA
Different from D,E, F by ANOVA
Different from control by t-test
COMMENT
Although corticosteroids for antenatal treatments of women at risk of preterm delivery are standard of care (1), there is minimal clinical information to guide the choice of corticosteroid or dose. Also, the formulation and dose of the corticosteroid have not been developed and tested for this unique indication in pregnant women. Our hypothesis about the betamethasone mixture used clinically was that Beta-Ac did not contribute to the clinical response because clinical effects occur within 24h and the half life of Beta-Ac is reported to be 7d (3, 16). Our second hypothesis was that two doses separated by 24h is unnecessary because 24h is enough to detect benefit in women and lung responses in fetal sheep are apparent by 15h (1, 17). Both hypotheses were not supported by these results in preterm sheep. The two doses of Beta-PO4 + Beta-Ac separated by 24h improved lung maturation more than did the single dose 48h prior to delivery. Furthermore, the 0.25 mg/kg Beta-PO4 given at an equivalent Beta-PO4 content as the 0.5 mg Beta-PO4 + Beta-Ac mixture had essentially no effects on lung maturation and twice the dose had minimal effects. Therefore, Beta-PO4 by itself does not effectively induce lung maturation as dosed in this study. The surprise was that Beta-Ac seemed to be effective.
These results need to be considered within the context of several aspects of antenatal corticosteroid treatments that remain to be explained. In sheep fetal injections with the Beta-PO4 + Beta-Ac mixture using ultrasound guidance causes some lung maturation but no fetal growth restriction (11). In contrast, maternal treatments result in a better lung maturation response, but cause fetal growth restriction despite exposure of the fetus to much lower peak levels of betamethasone (11, 18). A large dose of cortisol given IV to the fetus does not cause lung maturation (5) but, repetitive doses of cortisol given over 6h to the fetal sheep do cause lung maturation (6). When taken together, these observations suggest that the lung maturational effects of betamethasone depend on a continuous exposure over hours, but that maternal or fetal plasma levels of betamethasone may not need to be very high. The differences in fetal growth restriction responses may result from unidentified effects of the corticosteroid on the mother and/or the placenta.
The kinetics of Beta appearance and clearance in the maternal and fetal compartments are best modeled as polyexperimental curves with terminal half-lives used to describe clearance (19). Betamethasone given to the fetus is cleared from the fetus 4 times faster than it lost from the fetus when the ewe is treated (19). The terminal half-life of Beta-PO4 in the ewe is about 4h while the value recently reported for the Beta-PO4 - Beta-Ac mixture is 14h, long relative to Beta-PO4 but much shorter than reported in standard texts (20). While the different preparations of Beta will have effects while the drug is in the circulation, this corticosteroid binds tightly to receptors and can have prolonged effects that extend well beyond plasma availability. A further complication for the interpretation of plasma levels of Beta is the presence of pro-drug (Beta-PO4 or Beta-Ac) in plasma that may be hydrolysed prior to assay, which will artificially elevate plasma Beta levels (21). The link between plasma corticosteroid levels and lung maturational responses in the fetus remains poorly characterized. Based on fetal cardiovascular responses, Schwab suggested that the clinical dose may be excessive (21).
Although the 2 dose Beta-PO4 + Beta-Ac treatment caused the largest improvement in lung function, the lungs also had more ventilation mediated injury as detected by the pulmonary interstitial emphysema. Fetal sheep at 124d gestation are profoundly surfactant deficient and corticosteroids require 4–7 days to increase surfactant pool sizes (7). The primary effect of the corticosteroid on the fetal lung is to decrease the mesenchyme and increase potential volume for gas (22). The physiologic result is a lung that contains more gas at high pressures on the pressure-volume curve but does not have increased stability at low transpulmonary pressures. This more distensible lung with immature support structures is prone to rupture when ventilated (10).
A challenge for the field is to develop a corticosteroid preparation that is better suited to the indication of corticosteroid treatment for women at risk of preterm delivery. The Beta-PO4 in the currently preferred mixture of Beta-PO4 and Beta-Ac may not be helpful to the fetus. The high maternal plasma levels of Beta also may add risk. The Beta-Ac dose and formulation also did not achieve good lung maturation. There are other chemical formulations of betamethasone that might be better suited to this clinical indication. However, as shown by this experiment and as indicated by the recent review of the available clinical information (1), the mixture of Beta-PO4 and Beta-Ac is the corticosteroid that is presently indicated for women at risk of preterm delivery.
Acknowledgments
Funded by The National Institutes of Health - USA (HL-65397) The National Health and Medical Research Council of Australia and Women and Infant’s Research Foundation of Western Australia
Footnotes
Society for Gynecologic Investigation - 2005 (Newnham, J.P., Moss, T.J.M., Ikegami, M., Jobe, A.H. Determining the optimum dose and pharmacologic preparation of betamethasone for enhancement of maturation in sheep. J. Soc. Gynecol. Invest. 12 (supplement), 300A, 2005
CONDENSATION: The combined preparation of betamethasone phosphate and betamethasone acetate used clinically is more effective than either betamethasone component to induce lung maturation in fetal sheep.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Liggins GC, Howie RN. A controlled trial of antepartium glucocorticoid treatment for prevention of RDS in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 3.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol. 2004;190:878–881. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Baud O, Foix-L’Helias L, Kaminski M, Audibert F, Jarreau PH, Papiernik E, Huon C, Lepercq J, Dehan M, Lacaze-Masmonteil T. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341:1190–1196. doi: 10.1056/NEJM199910143411604. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH, Polk D, Ikegami M, Newnham J, Sly P, Kohen R, Kelly R. Lung responses to ultrasound-guided fetal treatments with corticosteroids in preterm lambs. J Appl Physiol. 1993;75:2099–2105. doi: 10.1152/jappl.1993.75.5.2099. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AH, Newnham J, Moss TJ, Ikegami M. Differential effects of maternal betamethasone and cortisol on lung maturation and growth in fetal sheep. Am J Obstet Gynecol. 2003;188:22–28. doi: 10.1067/mob.2003.61. [DOI] [PubMed] [Google Scholar]
- 7.Ikegami M, Polk DH, Jobe AH, Newnham J, Sly P, Kohan R, Kelly R. Effect of interval from fetal corticosteroid treatment to delivery on postnatal lung function of preterm lambs. J Appl Physiol. 1996;80:591–597. doi: 10.1152/jappl.1996.80.2.591. [DOI] [PubMed] [Google Scholar]
- 8.Mulrooney N, Champion Z, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Surfactant and Physiological Responses of Preterm Lambs to Continuous Positive Airway Pressure. Am J Respir Crit Care Med. 2005;171:1–6. doi: 10.1164/rccm.200406-774OC. [DOI] [PubMed] [Google Scholar]
- 9.Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL. Lung surfactant replacement in premature lambs with extracted lipids from bovine lung lavage: effects of dose, dispersion technique, and gestational age. Pediatr Res. 1985;19:569–577. doi: 10.1203/00006450-198506000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Willet KE, Jobe AH, Ikegami M, Newnham J, Sly PD. Pulmonary interstitial emphysema following intrauterine exposure to glucocorticoids in preterm sheep. Am J Respir Crit Care Med. 2000;162:1087–1094. doi: 10.1164/ajrccm.162.3.9906103. [DOI] [PubMed] [Google Scholar]
- 11.Jobe AH, Newnham J, Willet K, Sly P, Ikegami M. Fetal Versus Maternal and Gestational Age Effects of Repetitive Antenatal Glucocorticoids. Pediatrics. 1998;102:1116–1125. doi: 10.1542/peds.102.5.1116. [DOI] [PubMed] [Google Scholar]
- 12.Mason RJ, Nellenbogen J, Clements JA. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976;17:281–284. [PubMed] [Google Scholar]
- 13.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 14.Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant components and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280:L279–L285. doi: 10.1152/ajplung.2001.280.2.L279. [DOI] [PubMed] [Google Scholar]
- 15.Moss TJ, Doherty DA, Nitsos I, Harding R, Newnham JP. Pharmacokinetics of betamethasone after maternal or fetal intramuscular administration. Am J Obstet Gynecol. 2003;189:1751–1757. doi: 10.1016/s0002-9378(03)00825-1. [DOI] [PubMed] [Google Scholar]
- 16.Gillman AG, Hardman JG. Goodman and Gillman’s: The Pharmacologic Basis of Therapeutics. McGraw Hill Companies, Inc; 1996. [Google Scholar]
- 17.Ikegami M, Polk D, Jobe A. Minimum interval from fetal betamethasone treatment to postnatal lung responses in preterm lambs. Am J Obstet Gynecol. 1996;174:1408–1413. doi: 10.1016/s0002-9378(96)70581-1. [DOI] [PubMed] [Google Scholar]
- 18.Berry LM, Polk DH, Ikegami M, Jobe AH, Padbury JF, Ervin MG. Preterm newborn lamb renal and cardiovascular responses after fetal or maternal antenatal betamethasone. Am J Physiol. 1997;272:R1972–R1979. doi: 10.1152/ajpregu.1997.272.6.R1972. [DOI] [PubMed] [Google Scholar]
- 19.Samtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Area/moment and compartmental modeling of pharmacokinetics during pregnancy: applications to maternal/fetal exposures to corticosteroids in sheep and rats. Pharm Res. 2004;21:2279–2292. doi: 10.1007/s11095-004-7681-7. [DOI] [PubMed] [Google Scholar]
- 20.Samtani MN, Lohle M, Grant A, Nathanielsz PW, Jusko WJ. Betamethasone pharmacokinetics after two prodrug formulations in sheep: implications for antenatal corticosteroid use. Drug Metab Dispos. 2005;33:1124–1130. doi: 10.1124/dmd.105.004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Stabilization and HPLC analysis of betamethasone sodium phosphate in plasma. J Pharm Sci. 2004;93:726–732. doi: 10.1002/jps.10577. [DOI] [PubMed] [Google Scholar]
- 22.Willet KE, McMenamin P, Pinkerton KE, Ikegami M, Jobe AH, Gurrin L, Sly PD. Lung morphometry and collagen and elastin content: changes during normal development and after prenatal hormone exposure in sheep. Pediatr Res. 2000;45:615–625. doi: 10.1203/00006450-199905010-00002. [DOI] [PubMed] [Google Scholar]