Abstract
The neuroprotective actions of cannabidiol and other cannabinoids were examined in rat cortical neuron cultures exposed to toxic levels of the excitatory neurotransmitter glutamate. Glutamate toxicity was reduced by both cannabidiol, a nonpsychoactive constituent of marijuana, and the psychotropic cannabinoid (−)Δ9-tetrahydrocannabinol (THC). Cannabinoids protected equally well against neurotoxicity mediated by N-methyl-d-aspartate receptors, 2-amino-3-(4-butyl-3-hydroxyisoxazol-5-yl)propionic acid receptors, or kainate receptors. N-methyl-d-aspartate receptor-induced toxicity has been shown to be calcium dependent; this study demonstrates that 2-amino-3-(4-butyl-3-hydroxyisoxazol-5-yl)propionic acid/kainate receptor-type neurotoxicity is also calcium-dependent, partly mediated by voltage sensitive calcium channels. The neuroprotection observed with cannabidiol and THC was unaffected by cannabinoid receptor antagonist, indicating it to be cannabinoid receptor independent. Previous studies have shown that glutamate toxicity may be prevented by antioxidants. Cannabidiol, THC and several synthetic cannabinoids all were demonstrated to be antioxidants by cyclic voltametry. Cannabidiol and THC also were shown to prevent hydroperoxide-induced oxidative damage as well as or better than other antioxidants in a chemical (Fenton reaction) system and neuronal cultures. Cannabidiol was more protective against glutamate neurotoxicity than either ascorbate or α-tocopherol, indicating it to be a potent antioxidant. These data also suggest that the naturally occurring, nonpsychotropic cannabinoid, cannabidiol, may be a potentially useful therapeutic agent for the treatment of oxidative neurological disorders such as cerebral ischemia.
Cannabinoid components of marijuana are known to exert behavioral and psychotropic effects but also to possess therapeutic properties including analgesia (1), ocular hypotension (2), and antiemesis (3). This report examines another potential therapeutic role for cannabinoids as neuroprotectants and describes their mechanism of action in rat cortical neuronal cultures.
During an ischemic episode, large quantities of the excitatory neurotransmitter glutamate are released. This event causes neuronal death by over-stimulating N-methyl-d-aspartate receptors (NMDAr) and 2-amino-3-(4-butyl-3-hydroxyisoxazol-5-yl)propionic acid (AMPA) and kainate-type receptors and results in metabolic stress and accumulation of toxic levels of intracellular calcium (4). In vitro and in vivo studies (4, 5, 6) have demonstrated that such neurotoxicity can be reduced by antioxidants or antagonists to NMDAr and AMPA/kainate receptors. Antioxidants such as α-tocopherol (5, 6) are effective neuroprotectants because of their ability to reduce the toxic reactive oxygen species (ROS) formed during ischemic metabolism. Cannabinoids like (−)Δ9-tetrahydrocannabinol (THC) and its psychoactive analogues also have been reported to be neuroprotective against glutamate toxicity in vitro (7). Cannabinoids have been suggested to prevent glutamate neurotoxicity by activating cannabinoid receptors (7, 8), which can reduce calcium influx through voltage sensitive calcium channels (8, 9). A synthetic cannabinoid (HU-211) also has been demonstrated to be neuroprotective even though it does not activate cannabinoid receptors. This compound is an atypical cannabinoid, however, in that it, unlike other cannabinoids, directly antagonizes NMDAr (10) and possesses some antioxidant properties (11). The present study examines classical cannabinoids as neuroprotectants in vitro but focuses on the nonpsychoactive cannabinoid cannabidiol. Like THC, cannabidiol is a natural component of the marijuana plant, Cannabis sativa, although unlike THC, cannabidiol does not activate cannabinoid receptors and so is devoid of psychoactive effects (12). This study reports that cannabidiol and other cannabinoids such as THC are potent antioxidants that protect neurons from glutamate-induced death without cannabinoid receptor activation.
MATERIALS AND METHODS
Materials.
Cannabidiol, THC, and reagents other than those specifically listed below were purchased from Sigma. Cyclothiazide, glutamatergic ligands, and MK-801 were obtained from Tocris Cookson (Bristol, U.K.). Dihydrorhodamine was supplied by Molecular Probes. Tert-butyl hydroperoxide, tetraethylammonium chloride, ferric citrate, and sodium dithionite were all purchased from Aldrich. Agatoxin and conotoxin were obtained through Biomol (Plymouth Meeting, PA). All culture media were GIBCO/BRL products.
Solution Preparation.
Solutions of cannabinoids, cyclothiazide, and other lipophiles were prepared by evaporating a 10 mM ethanolic solution (under a stream of nitrogen) in a siliconized microcentrifuge tube. Dimethyl sulfoxide (<0.05% of final volume) was added to ethanol to prevent the lipophile from completely drying onto the tube wall. After evaporation, 1 ml of culture media was added, and the drug was dispersed by using a high power sonic probe. Special attention was used to ensure the solution did not overheat or generate foam. After dispersal, all solutions were made to their final volume in siliconized glass tubes by mixing with an appropriate quantity of culture media.
Neuronal Cultures.
Primary cortical neuron cultures were prepared according to the method of Ventra et al (13). In brief, fetuses were extracted by C-section from a 17-day pregnant Wistar rat, and the fetal brains were placed into phosphate buffered saline. The cortices then were dissected out, cut into small pieces, and incubated with papain for 9 min at 37°. After this time, the tissue was dissociated by passage through a fire-polished Pasteur pipette, and the resultant cell suspension was separated by centrifugation over a gradient consisting of 10 mg/ml BSA and 10 mg/ml ovomucoid (a trypsin inhibitor) in Earle’s balanced salt solution. The pellet then was resuspended in high glucose, phenol red-free DMEM containing 10% fetal bovine serum, 2 mM glutamine, 100 units of penicillin, and 100 μg/ml streptomycin (DMEM). Cells were counted, were tested for vitality by using the trypan blue exclusion test, and were seeded onto poly-d-lysine coated 24 multiwell plates. After 96 hr, 10 μM fluorodeoxyuridine and 10 μM uridine were added to block glial cell growth. This protocol results in a highly neuron-enriched culture (13).
Preparation of (Type I) Astrocytes and Conditioned Media.
Astrocyte-conditioned DMEM (phenol red-free) was used throughout the AMPA/kainate toxicity procedure and after glutamate exposure in the NMDAr-mediated toxicity protocol. Media were conditioned by 24 hr of treatment over a confluent layer of type I astrocytes prepared from 2-day-old Wistar rat pups (14). In brief, cortices were dissected, were cut into small pieces, were digested enzymatically with 0.25% trypsin, and then were dissociated mechanically by passage through a plastic pipette. The cell suspension then was plated into untreated 75-cm2 T-flasks, and, after 24 hr, the media were replaced and unattached cells were removed. Once astrocytes achieved confluency, cells were divided into four flasks. Media for experiments were conditioned by a 24-hr exposure to these astrocytes, after which time they were frozen at −20°C until use. Astrocyte cultures were used to condition DMEM for no longer than 2 months.
NMDAr-Mediated Toxicity Procedure.
NMDAr-mediated glutamate toxicity was examined by exposing neurons (cultured for 14–18 days) to 250 μM glutamate for 10 min in a phenol red-free and magnesium-free saline. The saline was composed of 125 mM NaCl, 25 mM Glucose, 10 mM Hepes (pH 7.4), 5 mM KCl, 1.8 mM calcium chloride, and 5% BSA. After exposure, cells were washed twice with saline and were incubated for 18 hr in conditioned DMEM. Toxicity was prevented completely by addition of the NMDAr antagonist MK-801 (500 nM) (data not shown).
AMPA and Kainate Receptor-Mediated Toxicity Procedures.
Unlike NMDAr, which are regulated by magnesium ions, AMPA/kainate receptors rapidly desensitize after ligand binding. To examine AMPA and kainate receptor-mediated toxicity, neurons were cultured for 7–13 days and then were exposed to 100 μM glutamate and 50 μM cyclothiazide (used to prevent AMPA receptor desensitization). Cells were incubated with glutamate in the presence of 500 nM MK-801 for 18–20 hr before analysis. Specific AMPA and kainate receptor ligands also were used to separately examine the effects of cannabinoids on AMPA and kainate receptor-mediated events. Fluorowillardiine (1.5 μM) and 4-methyl glutamate (10 μM) were used to investigate AMPA and kainate (15) receptor-mediated toxicity, respectively. When specifically examining kainate receptor activity, cyclothiazide was replaced with 0.15 mg/ml Concanavalin-A.
Although the neuron preparation technique described above results in a largely neuronal culture, a minority of astrocytic cells remain. Astrocytes are highly resistant to glutamate toxicity (16) because of their lack of functional NMDAr (17, 18), although glutamate toxicity in astrocytes has been observed to prevent AMPA receptor desensitization if cyclothiazide is present (19). To examine whether AMPA/kainate-type toxicity affects astrocytes in our cultures, astrocytes (as prepared above) were exposed to glutamate under the same conditions used on neuron-enriched cultures. Under these conditions, astrocytes were resistant to glutamate toxicity, with 20 hr of exposure resulting in a lactate dehydrogenase release of only 5% above background, compared with 100–200% of the background observed in neuron-enriched cultures (data not shown). It was concluded, therefore, that astrocyte contamination does not contribute substantially to the effects of glutamate in our neuronal cultures.
ROS Toxicity Assay.
To examine the effects of cannabinoids on ROS toxicity, 7- to 13-day-old cultured neurons were incubated with 300 μM tert-butyl hydroperoxide (an oxidant) in conditioned DMEM. Tert-butyl hydroperoxide was used because its miscibility with both water and lipids allows oxidation to occur in both cytosolic and membrane-delimited cellular compartments.
Toxicity Assay.
Cell toxicity was assessed 18–20 hrs after insult by measuring lactate dehydrogenase release into the (phenol red-free) culture media. Experiments were conducted with triple or quadruple values at each point, and all plates contained positive (glutamate alone) and baseline controls. The assay was validated by comparison with a tetrazolium-based viability assay (XTT) (20). Results were similar with either system, although lactate dehydrogenase release was used in this study because it provided a greater signal to noise ratio than the XTT assay.
Cyclic Voltametry.
Cyclic voltametry was performed with an EG & G Princeton Applied Research potentiostat/galvanostat (model 273/par 270 software). The working electrode was a glassy carbon disk with a platinum counter electrode and silver/silver chloride reference. Tetraethylammonium chloride in acetonitrile (0.1 M) was used as an electrolyte. Cyclic voltametry scans were done from 0 to +1.8 V at scan rate of 100 mV per second.
Iron-Catalysed Dihydrorhodamine Oxidation (Fenton Reaction).
The antioxidant activities of each of the compounds were evaluated by their ability to prevent oxidation of dihydrorhodamine to the fluorescent compound rhodamine. Oxidant was generated by ferrous catalysis (diothionite-reduced ferric citrate) of tert-butyl hydroperoxide in a 50:50 water-to-acetonitrile (vol/vol) solution. Dihydrorhodamine (50 μM) was incubated with 300 μM tert-butyl hydroperoxide and 0.5 μM iron for 5 min. After this time, oxidation was assessed by spectrofluorimetry (Excitation = 500 nm, Emission = 570 nm). Various concentrations of cannabinoids and butylhydroxytoluene (BHT) were included to examine their ability to prevent dihydrorhodamine oxidation.
Data Analysis.
Data are reported as mean values plus and minus standard error. Significance was examined by using a Student’s t test, (P ≤ 0.05). Kinetic data was analyzed by using GraphPad’s prism software package (GraphPad, San Diego) for PC.
RESULTS
Cannabidiol Blocks NMDAr and AMPA and Kainate Receptor-Mediated Neurotoxicity.
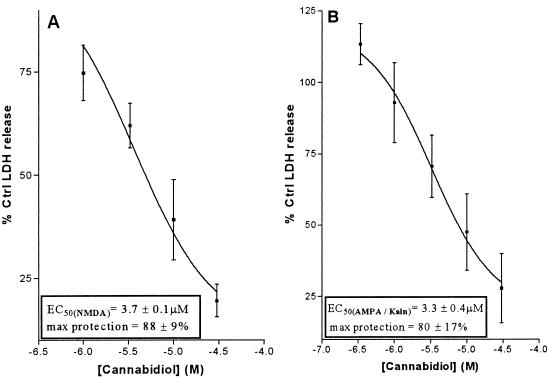
Glutamate neurotoxicity can be mediated by NMDAr, AMPA receptors, or kainate receptors. To examine NMDAr-mediated toxicity, rat cortical neurons were exposed to glutamate for 10 min in a magnesium-free medium, and the level of lactate dehydrogenase released was used as an index of cell injury. To examine AMPA/kainate receptor-mediated toxicity, neurons were incubated for 20 hr with glutamate or a specific AMPA or kainate receptor ligand (fluorowillardiine or 4-methyl-glutamate, respectively). An NMDAr antagonist (MK-801) and an agent to prevent receptor desensitization also were included. Cannabidiol prevented cell death equally well (EC50 of 2–4 μM) in both NMDAr and AMPA/kainate toxicity models (Fig. 1 A and B). Similar data also was observed when glutamate was replaced with either AMPA-specific or kainate receptor-specific ligands (data not shown). These results suggest that cannabidiol protects similarly, regardless of whether toxicity is mediated by NMDA, AMPA, or kainate receptors.
Figure 1.
Effect of cannabidiol on NMDAr- (A) and AMPA/kainate receptor- (B) mediated neurotoxicity. Data shown represents mean values ± SEM from a single experiment with four replicates. Each experiment was repeated on at least four occasions with essentially the same results. Cannabinoids were present during (and, in the case of NMDAr mediated toxicity, after) the glutamate exposure periods. See Materials and Methods for further experimental details.
AMPA/Kainate Toxicity Is Calcium Dependent.
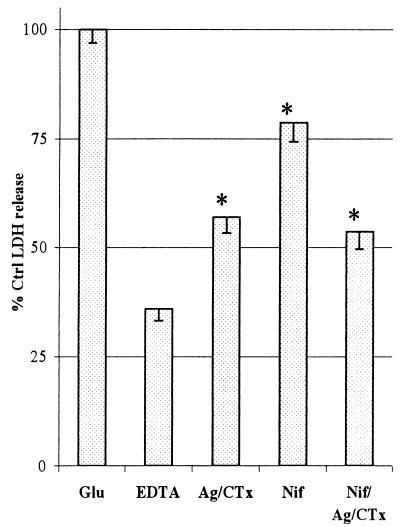
Increased calcium influx is known to be a key factor in NMDAr-induced cell death (4), but its role in AMPA and kainate toxicity is less clear. It has been suggested that AMPA/kainate receptors may not directly allow entry of sufficient calcium to kill cells. However, AMPA/kainate receptors flux large amounts of sodium, which can depolarize cell membranes. Such depolarization may activate both voltage-sensitive calcium channels (21) and facilitate NMDAr activation (22, 23). In this way, AMPA/kainate receptor stimulation may lead indirectly to accumulation of toxic intracellular calcium levels. Addition of the calcium chelator EDTA reduced toxicity in a concentration-dependent manner, demonstrating the involvement of calcium in AMPA/kainate-type toxicity (data not shown). EDTA (2 mM) eliminated ≈70% of glutamate toxicity (Fig. 2) even though the presence of MK-801 prevented NMDAr activation. Toxicity also was reduced by inhibitors to L-, N-, and P/Q- type calcium channels (nifedipine, agatoxin IVa, and conotoxin GVIa, respectively; Fig. 2), indicating that voltage-sensitive calcium channels also are involved in AMPA/kainate-type toxicity. However, a combination of these calcium channel inhibitors did not completely block EDTA-preventable (calcium-dependant) cell death.
Figure 2.
The involvement of calcium and calcium channels in AMPA/kainate-mediated toxicity. The effects of 2 mM EDTA and various combinations of the voltage-sensitive calcium channel inhibitors ω-Agatoxin IVa (Ag) (250 nM), ω-Conotoxin GVIa (CTx) (500 nM), and Nifedipine (Nif) (1 μM) were used to probe the role and source of calcium in AMPA/kainate receptor-mediated toxicity. Data represents mean values ± SEM from four experiments, each with four replicates. Cannabinoids were present throughout the glutamate exposure period. See Materials and Methods for further experimental details. Significant difference between EDTA and other treatments is indicated with an asterisk.
Neuroprotection by Tetrahydrocannabinol.
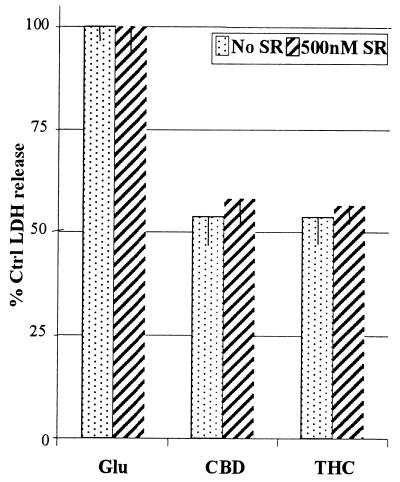
Unlike cannabidiol, THC is a ligand for the brain cannabinoid receptor (24), and this action has been proposed to explain the ability of THC to protect neurons from NMDAr toxicity in vitro (7). However, in AMPA/kainate receptor toxicity assays, THC and cannabidiol were similarly protective, suggesting that cannabinoid neuroprotection may be independent of cannabinoid receptor activation. This was confirmed by inclusion of a cannabinoid receptor antagonist, SR-141716A (Fig. 3). Neither THC or cannabidiol neuroprotection was affected by cannabinoid receptor antagonist.
Figure 3.
Effect of THC, cannabidiol, and cannabinoid receptor antagonist on glutamate induced neurotoxicity. Neurons exposed to glutamate in an AMPA/kainate receptor toxicity model were incubated with 10 μM cannabidiol or THC in the presence or absence of SR141716A (500nM). See Materials and Methods for experimental details. Data represents mean values ± SEM from four experiments, each with three replicates.
Cannabinoids as Antioxidants.
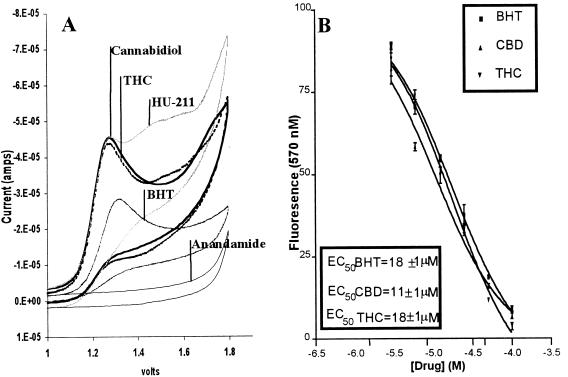
Cells use easily oxidizable compounds such as glutathione, ascorbate, and α-tocopherol as antioxidants that protect important cellular structures (e.g., DNA, proteins, and membranes) from ROS damage. Studies have suggested that ROS damage may be involved in glutamate neurotoxicity (5, 6). To investigate whether cannabinoids could protect neurons against glutamate by reacting with ROS, the antioxidant properties of cannabidiol and other cannabinoids were assessed. Cyclic voltametry, a procedure that measures the ability of a compound to accept or donate electrons under a variable voltage potential, was used to measure the oxidation potentials of several natural and synthetic cannabinoids. Cannabidiol, THC, and the synthetic cannabinoid HU-211 all donated electrons at a similar potential as the antioxidant BHT. Anandamide (arachidonyl-ethanolamide), which is not a cannabinoid in structure but is an endogenous ligand for the cannabinoid receptor, did not undergo oxidation in this assay (Fig. 4A). Three other cannabinoids, cannabinol, nabilone, and levanantrodol, also were tested, and they, too, exhibited oxidation profiles similar to cannabidiol and THC (data not shown).
Figure 4.
(A) A comparison of the oxidation potentials of cannabinoids and the antioxidant BHT. The oxidation profiles of (750 μM) BHT, cannabinoids, and anandamide were compared by cyclic voltametry. Anandamide, a cannabinoid receptor ligand with a noncannabinoid structure, was used as a nonresponsive control. Experiments were repeated three times with essentially the same results. See Materials and Methods for experimental details. (B) Effect of cannabidiol and THC on dihydrorhodamine oxidation. Cannabinoids were compared with BHT for their ability to prevent tert-butyl hydroperoxide-induced oxidation of dihydrorhodamine. See Materials and Methods for experimental details. Data represent mean values ± SEM from a single experiment with three replicates. This experiment was repeated four times with essentially the same results.
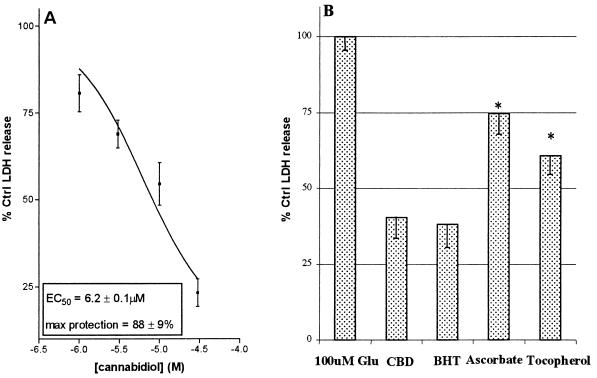
The ability of cannabinoids to be oxidized readily suggests that they may possess antioxidant properties comparable to BHT. These properties were examined further in a Fenton reaction (iron-catalyzed ROS generation). Tert-butyl hydroperoxide was used to generate ROS and oxidize dihydrorhodamine into the fluorescent compound rhodamine. Cannabidiol, THC, and BHT all prevented dihydrorhodamine oxidation in a similar, concentration-dependent manner (Fig. 4B), indicating cannabinoids to be comparable to BHT in antioxidant potency. To confirm that cannabinoids act as antioxidants in the intact cell, neurons were incubated with tert-butyl hydroperoxide and varying concentrations of cannabidiol (Fig. 5A). The oxidant was chosen for its solubility in both aqueous and organic solvents, thereby facilitating oxidation in both cytosolic and membrane cell compartments. As observed in studies with glutamate, cannabidiol protected neurons against ROS toxicity in a concentration-related manner. Cannabidiol also was compared with antioxidants in an AMPA/kainate toxicity protocol. Neurons were exposed to glutamate and equimolar cannabidiol, α-tocopherol, BHT, or ascorbate (Fig. 5B). Although all of the antioxidants attenuated glutamate toxicity, cannabidiol was significantly more protective than either α-tocopherol or ascorbate.
Figure 5.
(A) The effect of cannabidiol on oxidative toxicity in neuronal cultures. Tert-butyl hydroperoxide-induced toxicity was examined in the presence or absence of cannabidiol. (B) Comparison of antioxidants and cannabidiol for their ability to prevent glutamate toxicity in neurons. The effects of cannabidiol, BHT, ascorbate, and α-tocopherol (10 μM) were examined in a model of AMPA/kainate receptor-dependent toxicity. All drugs were present throughout the glutamate exposure period. Each experiment represents the mean of four replicates repeated on three occasions. See Materials and Methods for further experimental details. Significant differences between cannabidiol and other antioxidants are indicated with an asterisk.
DISCUSSION
The nonpsychoactive marijuana constituent cannabidiol was found to prevent both glutamate neurotoxicity and ROS-induced cell death. The psychoactive principle of Cannabis, THC, also blocked glutamate neurotoxicity with a similar potency to cannabidiol. In both cases, neuroprotection was unaffected by cannabinoid receptor antagonist. This suggests that cannabinoids may have potentially useful therapeutic effects that are independent of psychoactivity-inducing cannabinoid receptors (12) and so are not necessarily accompanied by psychotropic side effects.
Cannabidiol blocked glutamate toxicity in cortical neurons with equal potency regardless of whether the insult was mediated by NMDAr, AMPA receptors, or kainate receptors. This suggests that either cannabinoids antagonize all three glutamate receptors with the same affinity, or, more likely, their site of action is downstream of initial receptor activation events. Neurotoxic concentrations of glutamate induce massive calcium influx through NMDAr (4) that ultimately kills the cell. This study has demonstrated that the toxic effects of glutamate are also calcium-dependent when mediated by AMPA/kainate receptors. Both EDTA (a calcium chelator) and voltage-sensitive calcium channel inhibitors reduced AMPA-/kainate-type neurotoxicity, indicating that a portion of calcium influx-associated AMPA-/kainate-receptor activation is mediated by secondary activation of calcium channels. However, the mixture of calcium channel inhibitors and NMDAr antagonist did not eliminate completely glutamate toxicity or reduce cell death as efficiently as EDTA. This suggests that although toxicity resulting from AMPA/kainate receptor stimulation may be caused by calcium entering the cell by several routes, it is not caused exclusively by calcium channel activity. These studies also demonstrate that NMDAr activation is not required for AMPA-/kainate-type toxicity [as suggested (22)].
Accumulation of ROS has been shown to be involved in NMDAr-mediated cell death (4). The current study has similarly demonstrated that AMPA/kainate receptor-induced toxicity also involves ROS formation and may be prevented with antioxidant treatment. Cannabidiol and THC were found to be comparable with BHT (antioxidant) in both their ability to prevent dihydrorhodamine oxidation (Fenton reaction) and their cyclic voltametric profiles. Synthetic cannabinoids such as HU-211, nabilone, and levanantradol also exhibited similar profiles. Anandamide, which is a natural cannabinoid receptor ligand but is not structurally related to cannabinoids, did not give an antioxidant-like profile by cyclic voltametry, which indicates that cannabinoids can act as reducing agents (in a chemical system). To confirm that cannabinoids also can function as antioxidants in living cells, a lipid hydroperoxide was used to generate ROS toxicity in neuronal cultures. As observed in the Fenton reaction system, cannabidiol attenuated this ROS-induced neurotoxicity. These observations indicate that many cannabinoids exert a considerable protective antioxidant effect in neuronal cultures. The similarity of the voltamagrams observed with cannabidiol, HU-211, and several other cannabinoids also suggests that the reported antioxidant effect of HU-211 is not a feature unique to this atypical cannabinoid, (as previously implied; e.g., ref. 11) but, rather, a common property of classical cannabinoid structures. The potency of cannabidiol as an antioxidant was examined by comparing it on an equimolar basis with other commonly used antioxidants. Cannabidiol protected neurons to a greater degree than either of the dietary antioxidants, α-tocopherol or ascorbate. As in the Fenton reaction system, cannabidiol protected neurons with comparable efficacy to the potent antioxidant BHT. The similar antioxidant abilities of cannabidiol and BHT in this chemical system and their comparable protection in neuronal cultures implies that cannabidiol neuroprotection is caused by an antioxidant effect.
The antioxidative properties of cannabinoids suggest a therapeutic use as neuroprotective agents, and the particular properties of cannabidiol make it a good candidate for such development. Although cannabidiol was similar in neuroprotective capacity to BHT, cannabidiol has no known tumor-promoting effects [unlike BHT (25, 26)]. The lack of psychoactivity associated with cannabidiol allows it to be administered in higher doses than would be possible with psychotropic cannabinoids such as THC. Furthermore, the ability of cannabidiol to protect against neuronal injury without inhibiting NMDAr may reduce the occurrence of toxicity or side effects associated with NMDAr antagonists (27). Previous studies have indicated that cannabidiol is not toxic, even when chronically administered to humans (28) or given in large acute doses [700 mg/day (29)]. In vivo studies to examine the efficacy of cannabidiol as a treatment for experimentally induced ischemic stroke are currently in progress.
ABBREVIATIONS
- AMPA
2-amino-3-(4-butyl-3-hydroxyisoxazol-5-yl)propionic acid
- BHT
butylhydroxytoluene
- NMDAr
N-methyl-d-aspartate receptors
- ROS
reactive oxygen species
- THC
(−)Δ9-tetrahydrocannabinol
References
- 1.Welch S P, Stevens D L. J Pharmacol ExpTher. 1992;262:8–10. [PubMed] [Google Scholar]
- 2.Merritt J C, Crawford W J, Alexander P C, Anduze A L, Gelbart S S. Ophthalmology. 1980;87:222–228. doi: 10.1016/s0161-6420(80)35258-5. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamov A, Mechoulam R. Life Sci. 1995;56:2097–2102. doi: 10.1016/0024-3205(95)00194-b. [DOI] [PubMed] [Google Scholar]
- 4.Choi D W, Koh J Y, Peters S. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciani E, Groneng L, Voltattorni M, Rolseth V, Contestabile A, Paulsen R E. Brain Res. 1996;728:1–6. [PubMed] [Google Scholar]
- 6.MacGregor D G, Higgins M J, Jones P A, Maxwell W L, Watson M W, Graham D I, Stone T W. Brain Res. 1996;727:133–144. doi: 10.1016/0006-8993(96)00362-9. [DOI] [PubMed] [Google Scholar]
- 7.Skaper S D, Buriani A, Dal Toso R, Petrell I L, Romanello L, Facci L, Leon A. Proc Natl Acad Sci USA. 1996;93:3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampson A J, Bornheim L M, Scanziani M, Yost C S, Gray A T, Hansen B M, Leonoudakis D J, Bickler P E. J Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- 9.Twitchell W, Brown S, Mackie K. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Biegon A. Ann NY Acad Sci. 1995;765:314. doi: 10.1111/j.1749-6632.1995.tb16597.x. [DOI] [PubMed] [Google Scholar]
- 11.Eshhar N, Striem S, Kohen R, Tirosh O, Biegon A. Eur J Pharmacol. 1995;283(1–3):19–29. doi: 10.1016/0014-2999(95)00271-l. [DOI] [PubMed] [Google Scholar]
- 12.Mansbach R S, Rovetti C C, Winston E N, Lowe J A., III Psychopharmacol. 1996;124:315–22. doi: 10.1007/BF02247436. [DOI] [PubMed] [Google Scholar]
- 13.Ventra C, Porcellini A, Feliciello A R, Gallo A, Paolillo M, Mele E, Avedimento V E, Schettini G. J Neurochem. 1996;66:1752–1761. doi: 10.1046/j.1471-4159.1996.66041752.x. [DOI] [PubMed] [Google Scholar]
- 14.Grimaldi M, Pozzoli G, Navarra P, Preziosi P, Schettini G. J Neurochem. 1994;63:344–350. doi: 10.1046/j.1471-4159.1994.63010344.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L M, Gu Z Q, Costa A M, Yamada K A, Mansson P E, Giordano T, Skolnick P, Jones K A. J Pharmacol Exp Ther. 1997;280:422–427. [PubMed] [Google Scholar]
- 16.Amin N, Pearce B. Neurochem Int. 1997;30:271–276. doi: 10.1016/s0197-0186(96)00092-7. [DOI] [PubMed] [Google Scholar]
- 17.Holopainen I, Saransaari P, Oja S S. Neurochem Res. 1994;19:111–115. doi: 10.1007/BF00966803. [DOI] [PubMed] [Google Scholar]
- 18.Van Bockstaele E J, Colago E E J. J Comp Neurol. 1996;369:483–496. doi: 10.1002/(SICI)1096-9861(19960610)369:4<483::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.David J C, Yamada K A, Bagwe M R, Goldberg M P. J Neurosci. 1996;16:200–209. doi: 10.1523/JNEUROSCI.16-01-00200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehm N W, Rodgers G H, Hatfield S M, Glasebrook A L. J Immunol Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 21.Hack N, Balazs R. J Neurochem. 1995;65:1077–1084. doi: 10.1046/j.1471-4159.1995.65031077.x. [DOI] [PubMed] [Google Scholar]
- 22.Berman F W, Murray T F J. J Biochem Toxicol. 1996;11:111–119. doi: 10.1002/(SICI)1522-7146(1996)11:3<111::AID-JBT2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Deupree D L, Tang X W, Yarom M, Dickman E, Kirch R D, Schloss J V, Wu J Y. Neurochem Int. 1996;29:255–261. doi: 10.1016/0197-0186(96)00003-4. [DOI] [PubMed] [Google Scholar]
- 24.Devane W A, Dysarz F A, Johnson M R, Melvin L S, Howlett A C. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 25.Thompson J A, Bolton J L, Malkinson A M. Exp Lung Res. 1991;17:439–453. doi: 10.3109/01902149109064431. [DOI] [PubMed] [Google Scholar]
- 26.Lindenschmidt R C, Tryka A F, Goad M E, Witschi H P. Toxicology. 1986;38:151–160. doi: 10.1016/0300-483x(86)90116-2. [DOI] [PubMed] [Google Scholar]
- 27.Auer R N. Psychopharmacol Bull. 1994;30:585–591. [PubMed] [Google Scholar]
- 28.Cunha J M, Carlini E A, Pereira A E, Ramos O L, Pimentel C, Gagliardi R, Sanvito W L, Lander N, Mechoulam R. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 29.Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K. Pharmacol Biochem Behav. 1991;40:701–708. doi: 10.1016/0091-3057(91)90386-g. [DOI] [PubMed] [Google Scholar]