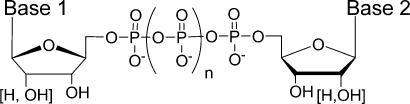Abstract
Dinucleoside polyphosphates act as agonists on purinergic P2Y receptors to mediate a variety of cellular processes. Symmetrical, naturally occurring purine dinucleotides are found in most living cells and their actions are generally known. Unsymmetrical purine dinucleotides and all pyrimidine containing dinucleotides, however, are not as common and therefore their actions are not well understood. To carry out a thorough examination of the activities and specificities of these dinucleotides, a robust method of synthesis was developed to allow manipulation of either nucleoside of the dinucleotide as well as the phosphate chain lengths. Adenosine containing dinucleotides exhibit some level of activity on P2Y1 while uridine containing dinucleotides have some level of agonist response on P2Y2 and P2Y6. The length of the linking phosphate chain determines a different specificity; diphosphates are most accurately mimicked by dinucleoside triphosphates and triphosphates most resemble dinucleoside tetraphosphates. The pharmacological activities and relative metabolic stabilities of these dinucleotides are reported with their potential therapeutic applications being discussed.
Key words: dinucleoside polyphosphates, IC50, metabolic stability, P2Y receptors, receptor selectivity, synthesis of dinucleotides
Introduction
Dinucleoside polyphosphates (NPnN′), or dinucleotides, are important extracellular signalling molecules containing a polyphosphate backbone capped at each end with a nucleoside. Several dinucleoside polyphosphates have been identified as naturally occurring compounds. They were initially isolated and characterized from Artemia [1], were later found in chromaffin cell and platelet granules, and now have been determined to be ubiquitous in prokaryotic and eukaryotic cells [2–4]. Dinucleotides are known with varying phosphate chain lengths, usually between two and eight phosphate groups. The most common dinucleotides studied both biologically and synthetically are the diadenosine polyphosphates (APnA), especially where n = 3–5. These diadenosine polyphosphates have been proposed to mediate a variety of functions including inhibition of adenosine kinase and adenylate kinase [5], stimulation of nitric oxide release from endothelial cells [6], inhibition of platelet aggregation [7], and facilitating neurotransmitter release from synaptic terminals in the central nervous system [8]. Recently, APnAs were isolated from tear fluid, underscoring their importance in physiological processes [9, 10]. Interest in the biological activity of dinucleotides rose with the discovery of the P2 nucleotide receptor family that consists of ligand-gated ion channels (P2X) and G protein coupled receptors (P2Y). Dinucleotides have varying activities at these receptors; in some cases for example, the phosphate chain length of a diadenosine polyphosphate can dictate agonist or antagonist activities at the same receptor.
Several dinucleotides have been administered in human clinical trials. AP4A for example, has been given via i.v. injection for lowering blood pressure during anesthesia [11]. Successful phase 3 trials using UP4U (diquafosol tetrasodium) as a treatment for dry eye disease [12, 32] has resulted in an approvable New Drug Application with the US FDA. A nextgeneration dinucleotide (UP4dC, INS37217, denufosol tetrasodium) is currently being tested in patients with cystic fibrosis and retinal detachment [13, 14].
Small quantities of dinucleotides have been available via enzymatic synthesis, however these methods are largely limited to purine bases and are not suited for gram scale synthesis or the synthesis of dinucleotides with unnatural nucleoside bases [1, 15–19]. Chemical procedures have also been reported for synthesis of standard and stabilized dinucleotides [20–22]. Other syntheses of dinucleotide-like structures have been developed for inosine 5′-monophosphate dehydrogenase (IMPDH) inhibitors [23–25]. Although the purine dinucleotides are more widely known and available commercially, the pyrimidine analogs where one or both bases are uridine, cytidine or thymidine, have not been systematically studied for biological activity [26–28]. The full range of purine and pyrimidine dinucleotides holds a great deal of interest to researchers in the field of purinergic receptors. The increased stability of these molecules, as well as the potential to manipulate selectivity based upon choice of nucleobases, can aid in the elucidation of cellular mechanisms. Hence, methods for the convenient synthesis of these dinucleotides were needed.
Results and discussion
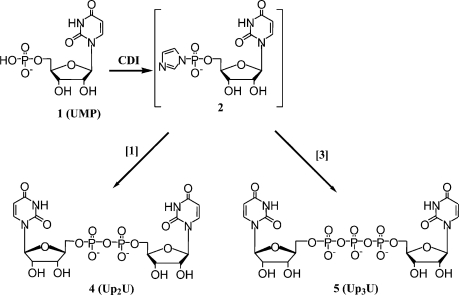
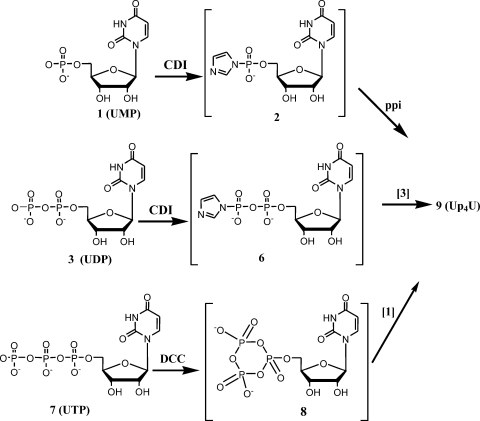
A variety of coupling protocols to prepare dinucleotides from their corresponding nucleotides were developed. In general, these procedures rely on converting the water soluble salts of nucleotides into organic soluble trialkylammonium salts, which allows the phosphate couplings to be carried out under anhydrous conditions in DMF or DMSO. The illustrated method starts with the conversion of uridine monophosphate (UMP, 1), uridine diphosphate (UDP, 3) or UTP (7) from their sodium salts into their tributylammonium salts followed by activation with either CDI or DCC in DMF. The activated intermediates were not isolated, but were directly condensed with another nucleotide (also as the tributylamine salt) to make symmetrical or mixed dinucleotides with two, three, or four bridging phosphates (Figures 1–3, Tables 1–3).
Figure 2.
General synthetic scheme for dinucleoside di- and triphosphates using a monophosphate as the starting nucleotide.
Table 2.
Dinucleoside triphosphates, n = 1 (see Figure 1) EC50 (µM).
| Base 1 | Base 2 | P2Y1 | P2Y2 | P2Y4 | P2Y6 | |
|---|---|---|---|---|---|---|
| ADP | 0.014 | NR | NR | NR | ||
| UDP | NR | 4.2 | 9.5 | 0.5 | ||
| 25 | Adenosine | Adenosine | 0.011 | 28.5 | NR | NR |
| 26 | Adenosine | Uridine | 0.039 | 2.00 | >10 | 0.17 |
| 27 | Adenosine | Cytidine | 0.085 | 6.00 | NR | >10 |
| 28 | Adenosine | Guanosine | 0.12 | 0.23 | 0.52 | 1.53 |
| 29 | Adenosine | Inosine | 0.11 | 1.20 | 2.55 | 1.06 |
| 30 | Uridine | Uridine | NR | 13.0 | SR | 0.92 |
| 31 | Uridine | Cytidine | SR | 2.00 | 16.7 | 0.52 |
| 32 | Uridine | Guanosine | NR | SR | NR | 0.38 |
| 33 | Uridine | Inosine | NR | 4.50 | SR | 1.31 |
| 34 | Uridine | 2′-Deoxyguanosine | NR | 0.47 | 1.98 | 0.28 |
| 35 | Cytidine | Inosine | >10 | >10 | SR | NT |
| 36 | Cytidine | 2′-Deoxyuridine | 17.0 | 3.1 | 40.0 | 0.81 |
| 37 | Inosine | Guanosine | SR | >10 | 6.41 | >10 |
| 38 | Guanosine | Guanosine | NR | NR | NR | NR |
SR = slight response at 100 µM, NR = no response, NT = not tested.
Figure 1.
General structure of dinucleoside polyphosphates.
Figure 3.
General methods for synthesis of dinucleoside tetraphosphates.
Table 1.
Dinucleoside diphosphates, n = 0 (see Figure 1) EC50 (µM).
| Base 1 | Base 2 | P2Y1 | P2Y2 | P2Y4 | P2Y6 | |
|---|---|---|---|---|---|---|
| 13 | Adenosine | Adenosine | 4.25 | NR | NR | NR |
| 14 | Adenosine | Uridine | 2.85 | 23.4 | >10 | NR |
| 15 | Adenosine | Cytidine | 2.11 | 6.9 | SR | 18.8 |
| 16 | Adenosine | Guanosine | 26.5 | 1.3 | 1.5 | 9.14 |
| 17 | Adenosine | 2′-Deoxyguanosine | 24.6 | SR | NR | >10 |
| 4 | Uridine | Uridine | NR | NR | NR | 33.0 |
| 18 | Uridine | Guanosine | SR | 2.1 | 4.64 | 7.54 |
| 19 | Uridine | 2′-Deoxyuridine | NR | NR | NR | NR |
| 20 | Uridine | 2′-Deoxy guanosine | >10 | 29.0 | >10 | >10 |
| 21 | Cytidine | Cytidine | NR | NR | NR | NR |
| 22 | Inosine | Inosine | SR | 4.8 | 5.06 | >10 |
| 23 | 4-Thiouridine | 4-Thiouridine | >10 | 29.1 | 33.9 | 25.2 |
| 24 | 2′-Deoxycytidine2 | 2′-Deoxycytidine | NR | NR | NR | SR |
SR = slight response at 100 µM, NR = no response, NT = not tested.
Table 3.
Dinucleoside tetraphosphates, n = 2 (see Figure 1) EC50 (µM).
| Base 1 | Base 2 | P2Y1 | P2Y2 | P2Y4 | P2Y6 | |
|---|---|---|---|---|---|---|
| ATP | 0.65 | 0.015 | NR | NR | ||
| UTP | NR | 0.015 | 0.07 | 1.47 | ||
| 39 | Adenosine | Adenosine | 0.32 | 0.054 | NR | SR |
| 40 | Adenosine | Uridine | 4.75 | 0.35 | 1.45 | SR |
| 41 | Adenosine | Cytidine | 0.75 | 0.15 | NR | SR |
| 42 | Adenosine | Inosine | 0.55 | 0.17 | NR | NR |
| 43 | Uridine | Uridine | NR | 0.06 | 0.20 | 24.8 |
| 44 | Uridine | Cytine | SR | 0.46 | 0.85 | 12.1 |
| 45 | Uridine | Inosine | NR | 0.52 | 0.28 | >10 |
| 46 | Uridine | Thymidine | NR | 0.18 | 0.35 | NR |
| 47 | Uridine | 4-Thiouridine | NR | 0.04 | 0.17 | 190.0 |
| 48 | Uridine | 2′-Deoxyinosine | NR | 0.23 | 0.47 | >10 |
| 49 | Uridine | 2′-Deoxy guanosine | SR | 0.06 | 0.14 | 1.68 |
| 50 | Uridine | Aracytidine | 6.13 | 0.59 | 0.22 | 3.32 |
| 51 | Uridine | 2-Deoxycytidine | NR | 0.27 | 1.22 | 16.0 |
| 52 | Uridine | 2′Deoxyadenosine | 3.49 | 0.05 | 0.07 | 4.20 |
| 53 | Uridine | Xanthosine | NR | 0.11 | 0.24 | 5.52 |
| 54 | Cytidine | Cytidine | SR | SR | NR | SR |
| 55 | Cytidine | 4-Thiouridine | SR | 0.036 | 0.11 | 5.22 |
| 56 | 4-Thiouridine | 4-Thiouridine | SR | 0.082 | 0.09 | 6.82 |
SR = slight response at 100 µM, NR = no response, NT = not tested.
Compound 1 was treated with CDI to give the phosphorimidazolide 2 as the activated species [29], and this was reacted with either 1 or 3 to give diuridine 5′-diphosphate (UP2U, 4) or diuridine 5′-triphosphate (UP3U, 5), respectively.
Diuridine 5′-tetraphosphate (9) was synthesized by activation of 7 with DCC, which gave the known cyclic uridine 5′-trimetaphosphate (8), followed by condensation with 1. This route proved to be the most robust and afforded the best impurity profile for purification. Alternately, 3 could be activated with CDI to give the corresponding imidazolide (6), which was condensed with 3 to give 9. UP4U could also be produced by the reaction of phosphorimidazolide 2 with pyrophosphate.
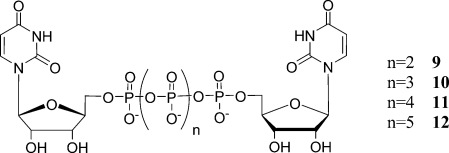
Diuridine 5′-pentaphosphate (UP5U, 10), diuridine 5′-hexaphosphate (UP6U, 11) and diuridine 5′-heptaphosphate (UP7U, 12) were generated as minor byproducts. All three of the described routes give rise to these impurities, in part because of the ability of the tetraphosphate linkage to undergo disproportionation. That is, conditions which enable phosphate linkage formation also enable the linkage to break apart generating mononucleotides which can then react in an unintended manner. Impurities in UTP (7) are most responsible for appearance of these impurities, as the DCC-mediated route is the most facile pathway. Compound 10 was generated by the coupling of 8 with 3, which was present as a small impurity in 7. Compound 11 arose from the condensation of 8 with 7, either as a result of a small amount of hydrolysis of 8 back to UTP (7) in the reaction mixture or by incomplete conversion to the cyclic meta-triphosphate intermediate (8). Compound 12 was likely generated from the reaction between 8 and uridine 5′-tetraphosphate (UP4), which is present as a minor impurity in 7.
Unsymmetrical dinucleotides are prepared in a manner analogous to the procedures described for the preparation of symmetrical dinucleotides. For the cases of dinucleotides with diphosphate or triphosphate linkages, the order of nucleotide addition resulted in little difference in overall reaction yield or ease of synthesis. However, in the case of the tetraphosphates, there was a significant difference in the outcome of the reaction owing to the ease or difficulty in forming the cyclic meta-triphosphate and to the solubility properties of various monophosphates. This was especially evident in the reactions of cytidine and guanosine nucleotides, probably owing to the zwitterionic character of these compounds. The complexity of the unsymmetrical reactions is caused by the same factors that create the di-, tri-, tetra-, penta-, hexa-, and heptaphosphate impurities present in the symmetrical reactions. Again considering the tetraphosphate synthesis, the symmetrical reaction scheme will generate a distribution of these six dinucleotides. However, the unsymmetrical scheme will generate the same set of six dinucleotides with not only the expected set of nucleoside moieties, but all of the possible combinations thereof. These reaction mixtures often gave rise to impurity profiles with 18 peaks observed by HPLC. These impurities were minor components of the reaction, but the broad range of retention times that these various impurities represented created a challenge for purification of the desired material.
Diadenosine polyphosphates are promiscuous ligands, acting upon numerous P2Y and P2X receptors in various tissues throughout the body. An examination of the requirements, or preferences, of the nucleoside components of these dinucleoside polyphosphates in terms of P2Y receptor activity has not been undertaken in a systematic fashion. To accomplish this, reproducible and dynamic synthetic methods were developed to make a relatively complete and representative set of dinucleotides using mostly naturally occurring nucleoside/tide starting materials. Synthetic methods existed for compounds containing adenosine and guanosine but few methods were applicable beyond these purine examples.
The results show that the phosphate chain length was an important determinant for specificity among the receptor subtypes. We have shown that symmetrical dinucleoside tetraphosphates have similar activities and specificities to the analogous nucleoside triphosphate. UTP and ATP are natural substrates for the P2Y2 receptor and dinucleoside tetraphosphates containing either uridine or adenosine are highly active as agonists of the same receptor. This same trend is followed by symmetrical dinucleoside triphosphates which are structural mimics of the analogous nucleoside diphosphates; that is, ADP is a potent agonist for the human P2Y1 receptor and adenosine containing dinucleoside triphosphates are highly active agonists at this receptor. Similarly, UDP is a potent agonist at the P2Y6 receptor as well as uridine containing dinucleoside triphosphates. The identity of the nucleobases in these dinucleotides was not responsible for the presence or lack of activity except for the necessity of adenosine for P2Y1 agonism and uridine for P2Y6 agonism. None of the compounds reported here exhibited any antagonist activity at any of the receptor subtypes tested.
Mixed base dinucleotides represent a unique opportunity to manipulate selectivity and activity on various receptor subtypes. As previously stated, dinucleoside tetraphosphates mimic the analogous mononucleoside triphosphates. As cytidine nucleotides have minimal activity on all receptors, one may expect a cytidine containing dinucleotide to have poor activity. Consistent with this hypothesis, no activity on any receptor was noted when cytidine or deoxycytidine was at both ends of a symmetrical dinucleotide. However, the activity profile of cytidine-containing mixed-base dinucleotides (or dinucleotides containing other less active mononucleotides) is shown to reside with the more active nucleobase. AP4C has good activity primarily at the P2Y1 receptor whereas UP4C has activity primarily at the P2Y2 receptor. Guanosine-containing dinucleotides follow the same trend as the cytidine-containing dinucleotides. The symmetrical guanosine dinucleotides exhibit no agonist activity on any of the receptors tested (GP2G and GP4G data not shown) but as unsymmetrical dinucleotides, some of these guanosine-containing compounds show good activity (UP3G on P2Y6 and UP4dG on P2Y2). AP4U has, as one may expect, activity at P2Y1, P2Y2, and P2Y4 as it contains both adenosine and uridine bases.
One of the advantages dinucleotides have over mononucleotides is an increase in both chemical and metabolic stability. Chemically, these dinucleotides are stable to prolonged dry storage at room temperature. Mononucleotides, and particularly triphosphates, must be stored at or below 0 °C to maintain purity at a high level. High pH (greater than 9) must be maintained for these mononucleotides to exist as relatively pure solutions. At neutral pH or lower, these mononucleotides slowly degrade into the constituent lower nucleotides eventually generating the free nucleoside. Dinucleotides need no such gentle treatment to maintain a high degree of purity in solution.
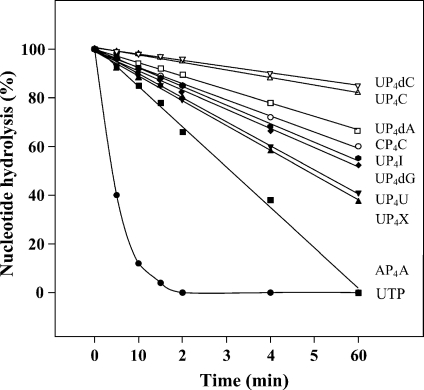
Metabolic instability of mononucleotides is well known [30], and is an important part of their transient endogenous activity. However, this instability of mononucleotides render them unattractive as therapeutic agents. Dinucleotides, on the other hand, have a much higher degree of metabolic stability [31]. Within our series of dinucleotides, there is a fairly wide range of stability on bronchial epithelial cells. As shown in Figure 4, a variety of dinucleotides were tested to determine their relative stabilities compared to the mononucleotide, uridine triphosphate (UTP). Diadenosine tetraphosphate is the least stable of the dinucleosides tested. The most hydrolysis-resistant compounds are the ones which contain a cytosine base. Therefore, selection of a dinucleoside therapeutic should be chosen based on a combination of receptor activity, receptor selectivity, and the metabolic stability in the relevant system.
Figure 4.
Structure of diuridine polyphosphates.
Conclusion
Dinucleoside polyphosphates represent a metabolically and chemically more stable series of nucleotide analogs. Their intrinsic activities on the various purinergic receptors (P2Y1, P2Y2, P2Y4, P2Y6) are as full agonists with a predictive specificity. Adenosine containing dinucleotides have at least minimal activity on P2Y1 while uridine containing dinucleotides have at least minimal activity on P2Y2 and P2Y6. The length of the linking phosphate chain determines a different specificity; diphosphates are most accurately mimicked by dinucleoside triphosphates and triphosphates most resemble dinucleoside tetraphosphates. By making an appropriate choice of phosphate length and nucleobase composition, we have been able to find compounds with necessary stability, efficacy, and specificity to enter into clinical efficacy testing in chronic diseases such as cystic fibrosis and dry eye.
Experimental
Reagents
All starting materials and the dinucleotides AP2A, AP3A, and GP3G were obtained from Sigma (St. Louis, MO) unless otherwise stated. The purity of all nucleotide agonists was established by either reverse phase or anion exchange HPLC (95%–99% purity). Fluo-3-AM was obtained from Molecular Probes (Eugene, OR). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum G-418, and other cell culture reagents were obtained from the Tissue Culture Facility at the University of North Carolina, or from Gibco-BRL Life Technologies (Rockville, MD). 1321N1 human astrocytoma cells stably expressing the P2Y1, P2Y2, P2Y4, or P2Y6 receptors, and wild-type 1321N1 cell were obtained from the University of North Carolina at Chapel Hill.
Intracellular calcium mobilization
1321N1 human astrocytoma cells stably expressing the human P2Y1, P2Y2, P2Y4, and P2Y6 receptors were grown in DMEM containing 4.5 g/l glucose, 5% fetal bovine serum and 600 µg/ml G-418. For intracellular Ca2+ measurements, cells were seeded in 96-well black wall/clear bottom culture plates (#3904 Corning Inc., Corning, NY), at a density of 35,000 cells/well and assays conducted 2 days later when the cells had reached confluence.
On the day of the assay, the growth medium in the culture plates was aspirated and replaced with 2.5 µM Fluo-3-AM in a final volume of 50 µl and incubated for one hour at 25 °C. Then, the dye was replaced with assay buffer (10 mM KCl, 118 mM NaCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 20 mM HEPES, pH 7.4), using a Columbus Plate Washer (Tecan Inc., Research Triangle Park, NC). Intracellular Ca2+ levels in response to P2Y receptor agonists were monitored as changes in fluorescence intensity using a Fluorescent Light Imaging Plate Reader (FLIPR) from Molecular Devices (Sunnyvale, CA). Average fluorescence units (AFU) corresponding to peak height were captured on disk and exported for further analysis. Changes in fluorescence data corresponding to concentrations of intracellular Ca2+ were normalized to the response of the cognate agonists (2MeSADP for P2Y1 receptor, ATP for P2Y2 receptor, UTP for P2Y4 receptor, and UDP for P2Y6 receptor).
Agonist potencies were calculated using a four-parameter logistic equation and the GraphPad software package (San Diego, CA). EC50 values represent the concentration of agonist at which 50% of the maximal effect is achieved. Three experiments using triplicate assays were conducted on separate days for each P2Y receptor subtype.
Airway surface metabolism
Well-differentiated cultures from passage 1 (P1) human airway epithelial cells were grown as previously described [31]. In brief, nasal epithelial cells were harvested from turbinates. Primary cells were isolated by protein digestion and plated on a collagen-coated tissue culture dish (5–10 days) in LHC9 medium containing: 25 ng/ml EGF, 50 nM retinoic acid, 40 µg/ml gentamicin, 0.5 mg/ml bovine serum albumin, 0.8% bovine pituitary extract, 50 U/ml penicillin, 50 µg/µl streptomycin and 0.125 mg/ml amphotericin) termed bronchial epithelial growth medium (BEGM). The cells were trypsinized and subpassaged on porous Transwell Col filters (diameters: well, 24 mm; pore, 0.45 µM) in air-liquid interface (ALI) medium. ALI is similar to BEGM, except for a 50:50 mixture of LHC Basal and DMEM-H as the base, amphotericin and gentamicin are omitted, and EGF concentration is reduced to 0.5 ng/ml. After 4 weeks, the cultures were composed of columnar ciliated cells (>90%) and secretory cells covering a layer of basal-like cells. Enzyme assays were conducted on cultures of transepithelial electrical resistance ≥ 300 Ω/cm2. Extracellular lactate dehydrogenase, employed as a test of cellular integrity, was tested 1 day before the nucleotide assays.
On the day of the experiments, the cell surfaces were rinsed three times with Krebs buffer [KRB (in mM)]: 140 Na+, 120 Cl−, 5.2 K+, 25 HCO−3, 2.4 HPO−4, 1.3 Ca2+, 1.3 Mg2+, 5.2 glucose and 25 HEPES (pH 7.4) and then pre-incubated in KRB (0.35 ml mucosal/2 ml serosal) for 30 min at 37 °C (5% CO2/95% O2). The enzyme reaction was initiated by the addition of 0.1 mM mono- or dinucleotide, dissolved in 35 µl KRB, to the mucosal bath and stopped by transferring 30 µl aliquots to tubes containing 0.3 ml ice-cold water. The samples were boiled 5 min, filtered, and analyzed by reversed-phase paired-ion HPLC (Figure 5).
Figure 5.
Time-course of the metabolism of various nucleotides by human normal bronchial cells. The cells were grown to confluence on an air-liquid interface and differentiated into a ciliated cell sheath over 4 weeks. The cells were pre-incubated 30 min at 37°C in Krebs buffer (0.35 ml apical/2 ml basolateral; pH 7.4). The assays were started with 0.1 mM nucleotide added to the apical buffer. Aliquots of 30 µl were transferred to 0.3 ml ice-cold water and boiled during 5 min. Their content in nucleotides was analyzed by HPLC. Data are expressed as percent of initial peak (SEM < 10%; N = 4–8).
The HPLC system consisted of a Dinamax C-18 column and a mobile phase developed with buffer A (10 mM KH2PO4 and 8 mM TBASH, pH 5.3) from 0 to 15 min, buffer B (100 mM KH2PO4, 8 mM TBASH and 10% MeOH, pH 5.3) from 15 to 35–60 min and buffer A from 35–60 to 45–75 min. Absorbance was monitored at 254 nm with an on-line Model 490 multi-wavelength detector (Shimadzu Sci. Instr. Inc., Maryland, USA). Degradation rates were calculated from the decrease in the amount of substrate monitored by HPLC and presented as nmol/min cm2 of surface area. Values were expressed as means ± standard error of the mean (SEM). Unpaired and paired Student's t-tests were used to assess the significance of differences between means. All linear regressions, curve fits and data transformations were performed with PC computer programs Origin and Sigma plot.
Standard preparation of dinucleoside diphosphates
UP2U (4) Uridine 5′-monophosphate, monotributylammonium salt (1.45 g, 2.85 mmol) was dissolved in dry DMF (12 ml) and CDI (0.346 g, 2.13 mmol) added in a single portion. The reaction mixture was heated at 50 °C for 24 h, after which the solvent was removed. This residue was dissolved in a minimal volume of water and purified by preparative ion exchange HPLC (Hamilton PRP-X100 column, 250 × 50 mm, 10 µm, with a linear gradient from water to 90% 1 M NH4HCO3/10% acetonitrile). The fractions containing the product were pooled and the water removed by lyophilization to give UP2U as the diammonium salt (0.596 g, 63% yield).
Standard preparation of dinucleoside triphosphates
AP3U (26) Uridine 5′-diphosphate, bistributylammonium salt (68.2 mg, 0.088 mmol) and adenosine 5′-phosphomorpholidate (100 mg, 0.141 mmol) were dissolved in 1 ml of anhydrous pyridine. 1H tetrazole (19.7 mg, 0.282 mmol) was added to the solution and stirred at 50 °C overnight. The reaction was quenched with triethylammonium bicarbonate (0.5 M, 1 ml) then stirred for 45 min at room temperature. The aqueous solution was extracted with an equal volume of ethyl acetate to remove excess organics then evaporated to a viscous oil. This residue was dissolved in a minimal volume of water and purified by preparative ion exchange HPLC (Hamilton PRP-X100 column, 250 × 50 mm, 10 µm, with a linear gradient from water to 90% 1 M NH4HCO3/10% acetonitrile). The fractions containing the product were pooled and the water removed by lyophilization to give the product as the triammonium salt (15.07 mg, 21.6% yield).
Standard preparation of dinucleoside tetraphosphates
AP4U (40) Adenosine 5′-triphosphate bistributylammonium salt (2.0 g, 2.28 mmol) was dissolved in dry DMF (15 ml) and DCC (0.517 g, 2.51 mmol) added over 30 s. The solution was stirred at room temperature for 45 min, during which time it became rather heterogeneous. 31P NMR indicated almost complete conversion to the cyclical trimetaphosphate, and uridine 5′-monophosphate, monotributylammonium salt (1.28 g, 2.51 mmol) was added as a solid. The reaction mixture was stirred at 30–35 °C for 60 h, by which time HPLC indicated that the desired product constituted about 40% of the total nucleotide content. The precipitated dicyclohexylurea was filtered, and the solvent was evaporated. This residue was dissolved in a minimal volume of water and purified by preparative ion exchange HPLC (Hamilton PRP-X100 column, 250×50 mm, 10 µm, with a linear gradient from water to 90% 1 M NH4HCO3/10% acetonitrile). The fractions containing the product were pooled and the water removed by lyophilization to give AP4U · 4NH4 (560 mg, 28% yield).
Analytical data
Common naming format is used below instead of IUPAC nomenclature (AP4A = diadenosine tetraphosphate).
UP2U (4) 1H NMR (D2O): δ 7.73 (d, H6), 5.78−5.74 (m, H5, H1′), 4.17−3.98 (m, H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.10 (s, P1, P2)
AP2U (14) 1H NMR (D2O): δ 8.23 (s, A-H8), 7.97 (s, A-H2), 7.42 (d, U-H6), 5.88 (m, U-H1′), 5.6 (m, A-H1′), 5.4 (m, U-H5), 4.4−3.9 (m, A&U-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.14 (m, P1, P2)
AP2C (15) 1H NMR (D2O): δ 8.27(s, A-H8), 8.04 (s, A-H2) 7.59 (d, C-H6), 5.82 (d, C-H5), 5.71−5.47 (m, AC-H1′), 4.41 (m, A&C-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −9.97 (m, P1, P2)
AP2G (16) 1HNMR (D2O): δ 8.05 (s, A-H8), 7.9 (s, A-H2), 7.7 (s, G-H8), 5.78 (d, A-H1′), 5.55 (d, G-H1′), 4.45−3.98 (m, A&G-H2′, H3′, H4′, H′5); 31P NMR (D2O): δ −9.95 (m, P1, P2)
AP2dG (17) 1H NMR (D2O): δ 8.13 (s, A-H8), 7.92 (s, A-H2), 7.77 (s, G-H8), 5.82 (d, A-H1′), 5.61 (d, dG-H1′), 4.47−4.28 (m, G&A-H2′, H3′), 4.2−4.01 (m, G&A-4′, 5′); 31P NMR (D2O): δ −10.07 (d, P1, P2)
UP2G (18) 1H NMR (D2O): δ 7.85 (s, G-H8), 7.58 (d, U-H6), 5.7−5.58 (m, G&U-H1′, U-H5) 4.3−4.05 (m, G&U-2′, 3′, 4′, 5′); 31P NMR (D2O): δ −10.13 (m, P1, P2)
UP2dU (19) 1H NMR (D2O): δ 7.75−7.71 (m, U&dU-H6), 6.14 (t, dU-H1′), 5.76−5.71 (m, U-H1′, U&dU-H5), 4.64−4.07 (m, U-2′, U&dU, 3′, 4′, 5′), 2.18 (dU-2′); 31P NMR (D2O): δ −10.15 (m1, P1, P2)
UP2dG (20) 1H NMR (D2O): δ 7.8 (s, G-H8), 7.56 (d, U-H6), 5.8−5.75 (m, U-H5), 5.77−5.6 (m, U&dG-H1′), 4.3−3.93 (m, U-H2′, U&dG-H3′, H4′, H5′), 2.6−2.2 (m, dG-H2′); 31P NMR (D2O): δ −10.16 (d, P1, P2)
CP2C (21) 1H NMR (D2O): δ 7.96 (d, H6), 6.1 (d, H5), 5.7 (d, H1′), 4.13−4.00 (m, H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −11.38 (m, P1, P2)
IP2I (22) 1H NMR (D2O): δ 7.75 (d, H6), 6.16 (m, H1′), 5.88 (d, H5), 4.39 (s, H3′), 4.02 (m, H5′), 2.26−2.1(m, H2′); 31P NMR (D2O): δ −10.14 (m, P1, P2)
4-thioUP24-thioU (23) 1H NMR (D2O): δ 7.6 (d, H6), 6.4 (d, H5), 5.8 (s, H3), 4.7−4.02 (m, H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −11.30 (m, P1, P2)
dCP2dC (24) 1H NMR (D2O): δ 7.73 (d, H6), 6.14 (d, H1′), 5.88 (d, H5), 4.3−3.96 (m, H3′, H4′, H5′), 2.27−2.06 (m, H2′); 31P NMR (D2O): δ −10.11 (m, P1, P2)
AP3U (26) 1H NMR (D2O): δ.3 (s, A-H8), 8.02 (s, AH2), 7.62 (d, U-H6), 5.93−5.52 (m, U-H5, UA-H1′), 4.63−4.07 (m, U&A-2′, 3′, 4′, 5′); 31P NMR (D2O): δ −10.20, 10.30 (dd, P1, P3), −21.85 (t, P2)
AP3C (27) 1H NMR (D2O): % 8.2 (s, A-H8), 8.02 (s, A-H2) 7.6 (d, C-H6), 5.8 (d, C-H5), 5.7−5.45 (m, A&CH1′), 4.4 (m, A&C-H2′, H3, H4′, H5′); 31P NMR (D2O): % −10.02, −10.21 (dd, P1, P3),−21.6 (t, P2)
AP3G (28) 1H NMR (D2O): δ 8.2 (s, A-H8), 8.00 (s, A-H2), 7.9 (s, G-H8), 5.8 (d, A-H1′), 5.6 (d, G-H1′), 4.8−4.02 (m, G&A-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −11.28, j10.37 (dd, P1, P3), −22.94 (t, P2)
AP3I (29) 1H NMR (D2O): δ 8.17 (s, A-H8), 8.16 (s, I-H8), 8.01 (s, A-H2), 7.98 (s, I-H2), 5.89−5.82 (m, A&I-H1′), 4.55−4.11 (m, A&I-H2′, H30, H4′, H5′); 31P NMR (D2O): δ −10.28, −10.37 (dd, P1, P3), −21.94 (t, P2)
UP3U (30) 1H NMR (D2O): δ 7.82 (d, H6), 5.85−5.80 (m, H5, H1′), 4.25 (m, H2′, H30), 4.10 (m, H4′, H5′); 31P NMR (D2O): δ −10.45 (d, P1, P3), −22.20 (t, P2)
UP3C (31) 1H NMR (D2O): δ 7.83−7.78 (m, U&C-H6), 5.97 (d, C-H5), 5.83−5.78 (m, U-H5, H1′, C-H1′), 4.3 (m, C&U-2′, 3′, 4′, 5′); 31P NMR (D2O): δ −10.28, −10.37 (dd, P1, P3), ′21.94 (t, P2)
UP3G (32) 1H NMR (D2O): δ 7.93 (s, G-H8), 7.70 (d, U-H6), 5.7−5.64 (m, G&U-H1′), 5.61 (d, U-H5), 4.3−4.01 (m, G&U-2′, 3′, 4′, 5′); 31P NMR (D2O): δ −10.26, −10.34 (dd, P1, P3), −21.87 (t, P2)
UP3I (33) 1H NMR (D2O): δ 8.3; (s, I-H8), 8.02 (s, IH2), 7.77 (d, U-H6), 5.95 (d, U-H5), 5.8−5.6 (m, U&IH1′), 4.41−3.93 (m, U&I-2′, 3′, 4′, 5′); 31P NMR (D2O): δ −10.31, −10.9 (dd, P1, P3), −21.81 (s, P2)
UP3dG (34) 1H NMR (D2O): δ 7.8 (s, G-H8), 7.6 (d, U-H6), 5.8 (d, U-H5), 5.77−5.6 (m, U&dG-H1′), 4.3−3.93 (m, U-H2′, U&dG-H3′, H4′, H5′), 2.6−2.2 (m, dG-H2′); 31P NMR (D2O): δ −10.20, −10.4 (dd, P1, P3), −21.9 (t, P2)
CP3I (35) 1H NMR (D2O): δ 8.3 (s, I-H8), 8.02 (s, I-H2), 7.8 (d, C-H6), 5.95 (d, C-H5), 5.8−5.6 (m, C&I-H1′), 4.41−3.93 (m, C&I-2′, 3′, 4′, 5′); 31P NMR (D2O): δ −10.02, −10.3 (dd, P1, P3), −22.43 (t, P2)
CP3dU (36) 1H NMR (D2O): δ 7.6 (d, U-H6), 7.45 (d, C-H6), 6.14 (t, dU-H1′), 5.9−5.57 (m, C-H1′, dU&C-H5), 4.4−3.9 (m, C-H2′, C&dU-H30, H4′, H5′), 2.18 (dU-20); 31P NMR (D2O): δ −10.05, −10.4 (dd, P1, P3), −21.95 (t, P2)
IP3G (37) 1H NMR (D2O): δ 8.1(s, I-H8), 8.00 (s, I-H2), 7.8 (s, G-H8), 5.8 (d, I-H1′), 5.6 (d, G-H1′), 4.8−4.02 (m, G&I-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.2, −10.6 (dd, P1, P3), −21.88 (t, P2)
AP4A (39) 1H NMR (D2O): δ 8.16 (s, H8), 7.9 (s, H2), 5.83 (d, H1′), 4.54−4.05 (m, H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.60 (m, P1, P4), −22.28 (m, P2, P3)
AP4U (40) 1H NMR (D2O): δ 8.36 (s, A-H8), 8.07 (s, A-H2), 7.68 (d, U-H6), 5.94−5.66 (m, U-H5, U&A-H1′), 4.41−4.06 (m, U&A-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.73 (m, P1, P4), −22.34 (m, P2, P3)
AP4C (41) 1H NMR (D2O): δ 8.34 (s, A-H8), 8.05 (s, A-H2), 7.63 (d, C-H6), 5.85 (d, A-H1′), 5.78−5.73 (m, C-H5, H1′), 4.42−4.07 (m, C&A-H2′, H30, H4′, H5′); 31P NMR (D2O): δ −10.23 (m, P1, P4), −21.99 (m, P2, P3)
AP4I (42) 1H NMR (D2O): δ 8.28 (s, A-H8), 8.19 (s, I-H8), 8.05 (s, A-H2), 7.95 (s, I-H2), 5.89−5.82 (m, A&I-H1′), 4.65−4.11 (m, A&I-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.07 (m, P1, P4), −22.00 (m, P2, P3)
UP4U (43) 1H NMR (D2O): δ 7.8 (1H, d, H6), 5.85 (d, H5), 5.83 (d, H1′), 4.26 (m, H2′, H3′), 4.12 (m, H4′, H5′); 31P NMR (D2O): δ −10.75 (m, P1, P4), −22.32 (m1, P2, P3)
UP4C (44) 1H NMR (D2O): δ 7.8−7.73 (m, C&U-H6), 5.94 (d, C-H5), 5.78−5.75 (m, U-H5, U&C-H1′), 4.15−4.02 (m, U&C-H21′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.84 (m, P1, P4), −22.65 (m, P2, P3)
UP4I (45) 1H NMR (D2O): δ 8.43 (s, I-H8), 8.08 (s, I-H2), 7.75 (d, U-H6), 5.98 (d, I-H1′), 5.79−5.73 (m, U-5, 1′), 4.3−4.0 (m, U&I-2′, 3′, 4′, 5′); 31P NMR (D2O): δ −10.86 (m, P1, P4), −22.61 (m, P2, P3)
UP4T (46) 1H NMR (D2O): δ 7.80 (d, U-H6), 7.59 (s, T-H6), 6.17 (t, T-H1′), 5.82−5.79 (m, U-H5, U-H1′), 4.30−4.00 (m, U-H2′, U&T-H3′, H4′, H5′), 2.19 (m, T-H2′), 1.76 (s, T-5CH3); 31P NMR (D2O): δ −10.34 (m, P1, P4), −22.10 (m, P2, P3)
UP44-thioU (47) 1H NMR (D2O): δ 7.71 (d, U-H6), 7.56 (d, thioU-H6), 6.41 (d, thioU-H5), 5.74−5.70 (m, U-H1′, H5, thioU-H1′), 4.64−4.60 (m, thioU&U-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.22 (m, P1, P4), −21.77 (m, P2, P3)
UP4dI (48) 1H NMR (D2O): % 8.27 (s, dI-H8), 8.03 (s, dI-H2), 7.75 (d, U-H6), 6.32 (m, dI-H1′), 5.76 (m, U-H5, H1′) 4.22−4.06 (m, U-H2′, U&dI-H30, H4′, H5′), 2.67−2.42 (m, dI-H2′); 31PNMR(D2O): δ −10.22 (m, P1, P4), −22.17(m, P2, P3)
UP4dG (49) 1H NMR (D2O): δ 7.9 (s, dG-H8), 7.70 (d, U-H6), 6.11(t, U-5), 5.79−5.70 (m, U&dG-H1′), 4.25−4.02 (m, U-H2′, U&dG-H3′, H4′, H5′) 2.6−2.2 (m, dG-H2′); 31P NMR (D2O): δ −10.72 (m, P1, P4), −22.35 (m, P2, P3)
UP4araC (50) 1H NMR (D2O): δ 7.81−7.76 (m, U&CH6), 6.15−6.0 (m, C-H1′), 5.85−5.78 (m, U-H1′, U&C-H5), 4.3−4.0 (m, U&C-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.79 (m, P1, P4), −22.52 (m, P2, P3)
UP4dC (51) 1H NMR (D2O): δ 7.8 (m, dC-H6, U-H6), 6.1 (t, dC-H1′), 5.93 (d, dC-H1′), 5.93 (d, dC-H5), 5.76 (m, U-H1′, H5), 4.2−4.0 (m, U-2′, dC&U-H3′, H4′, H5′), 2.3−2.0 (m, dC-H2′); 31P NMR (D2O): δ −10.81 (m, P1, P4), −22.62 (m, P2, P3)
UP4dA (52) 1H NMR (D2O): δ 8.31(d, dA-H8), 8.06 (d, dA-H2), 7.72 (d, U-H6), 6.33 (d, dA-H1′), 5.74−5.65 (M, U-H1′, H5), 4.20−4.02 (m, U-H2′, dA&U-H3′, H4′, H5′), 2.4−2.6 (m, dA-H2′); 31P NMR (D2O): δ −10.81 (m, P1, P4), −22.6 (m, P2, P3)
UP4X (53) 1H NMR (D2O): δ 7.88 (s, X-H8), 7.70 (d, U-H6), 5.76−5.70 (m, U-H5, H1′, X-H1′), 4.49−3.92 (m, X&U-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.73 (m, P1, P4), ′22.4 (m, P2, P3)
CP4C (54) 1H NMR (D2O): δ 7.77 (d, C-H6), 5.96 (D, CH5), 5.81 (d, C-H1′), 4.21−4.09 (m, C-H1′, H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −10.81 (m, P1, P4), −22.29 (m, P2, P3)
CP44-thioU (55) 1H NMR (D2O): δ 7.79 (d, C-H6), 7.66 (d, U-H6), 6.48 (d, U-H5), 5.98 (d, C-H1′), 5.81 (d, C-H5), 5.76 (d, U-H1′), 4.25−4.0 (m, U&C-H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −11.40 (m, P1, P4), −22.96 (m, P2, P3)
4-thioUP44-thioU (56) 1H NMR (D2O): δ 7.67 (d, H6), 6.48 (d, H5), 5.77 (s, H1′), 4.30−4.05 (m, H2′, H3′, H4′, H5′); 31P NMR (D2O): δ −11.41 (m, P1, P4), −22.79 (m, P2, P3)
Acknowledgement
The authors wish to thank Wendy Anders for her help with manuscript preparation, Aris Ragouzeous for NMR assistance and interpretation, and the technical assistance of Tommie Deese, Katie Travis, and Genny Evans.
Abbreviations
- araC
arabinocytidine
- CDI
carbonyldiimidazole
- DCC
dicylohexylcarbodiimide
- ppi
inorganic pyrophosphate
- 4-thioU
4-thiouridine
References
- 1.Vallejo CG, Lobaton CD, Quintanilla M et al. Dinucleosidasetetraphosphatase in rat liver and Artemia salina. Biochim Biophys Acta 1976; 438: 304-. [DOI] [PubMed]
- 2.Coste H, Brevet A, Plateau P, Blanquet S. Non-adenylylated bis(5-nucleosidyl) tetraphosphates occur in Saccharomyces cerevisiae and in Escherichia coli and accumulate upon temperature shift or exposure to cadmium. J Biol Chem 1987; 262: 12096-03. [PubMed]
- 3.Zamecnik PC, Stephenson ML, Janeway CM, Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun 1966; 24: 91-. [DOI] [PubMed]
- 4.Pintor J, Rotllan P, Torres M, Miras-Portugal MT. Characterization and quantification of diadenosine hexaphosphate in chromaffin cells: Granular storage and secretagogue-induced release. Anal Biochem 1992; 200: 296–300. [DOI] [PubMed]
- 5.Rotllan P, Miras-Portugal MT. Adenosine kinase from bovine adrenal medulla. Eur J Biochem 1985; 151: 365-1. [DOI] [PubMed]
- 6.Hilderman RH, Christensen EF. P1,P4-diadenosine 5-tetraphosphate induces nitric oxide release from bovine aortic endothelial cells. FEBS Lett 1998; 427: 320-. [DOI] [PubMed]
- 7.Zamecnik PC, Kim B, Gao MJ et al. Analogues of diadenosine 5-5′′-P1,P4-tetraphosphate (AP4A) as potential anti-platelet-aggregation agents. Proc Natl Acad Sci USA 1992; 89: 2370-. [DOI] [PMC free article] [PubMed]
- 8.Pintor J, Diaz-Hernandez M, Gualix J et al. Diadenosine polyphosphate receptors. from rat and guinea-pig brain to human nervous system. Pharmacol Ther 2000; 87: 103-5. [DOI] [PubMed]
- 9.Pintor J, Peral A, Hoyle CH et al. Effects of diadenosine polyphosphates on tear secretion in New Zealand white rabbits. J Pharmacol Exp Ther 2002; 300: 291-. [DOI] [PubMed]
- 10.Pintor J, Carracedo G, Alonso MC et al. Presence of diadenosine polyphosphates in human tears. Pflugers Arch 2002; 443: 432-. [DOI] [PubMed]
- 11.Kikuta Y, Ohiwa E, Okada K et al. Clinical application of diadenosine tetraphosphate (Ap4A:F-1500) for controlled hypotension. Acta Anaesthesiol Scand 1999; 43: 82-. [DOI] [PubMed]
- 12.Mundasad MV, Novack GD, Allgood VE et al. Ocular safety of INS365 ophthalmic solution: A P2Y2 agonist, in healthy subjects. J Ocular Pharmacol Ther 2001; 17: 173-. [DOI] [PubMed]
- 13.Yerxa BR, Sabater JR, Davis CW et al. Pharmacology of INS37217, a next generation P2Y2 receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther 2002; 302: 871-0. [DOI] [PubMed]
- 14.Maminishkis A, Jalickee S, Blaug SA et al. The P2Y2 receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci 2002; 43: 3555-6. [PubMed]
- 15.Plateau P, Mayaux JF, Blanquet S. Zinc(II)-dependent synthesis of diadenosine 5-5′′-P(1),P(4) -tetraphosphate by Escherichia coli and yeast phenylalanyl transfer ribonucleic acid synthetases. Biochemistry 1981; 20: 4654-2. [DOI] [PubMed]
- 16.Kitabatake S, Dombou M, Tomioka I, Nakajima H. Synthesis of P1,P4-di(adenosine 5-) tetraphosphate by leucyl-tRNA synthetase, coupled with ATP regeneration. Biochem Biophys Res Commun 1987; 146: 173-. [DOI] [PubMed]
- 17.Lowe G. Stereochemical analysis of the enzymic synthesis and hydrolysis of AP4A. Nucleus 1991; 10: 181-3.
- 18.Ortiz B, Sillero A, Gunther Sillero MA. Specific synthesis of adenosine(5-tetraphospho(5-nucleoside and adenosine(5-oligophospho(5-adenosine (n > 4) catalyzed by firefly luciferase. Eur J Biochem 1993; 212: 263-0. [DOI] [PubMed]
- 19.Dieckmann R, Pavela-Vrancic M, von Dohren H. Synthesis of (di)adenosine polyphosphates by non-ribosomal peptide synthetases (NRPS). Biochim Biophys Acta 2001;1546:234-1. [DOI] [PubMed]
- 20.Ng K-M, Orel L. The action of water-soluble carbodiimide on adenosine-5-polyphosphates. NAR 1987; 15: 3573-0. [DOI] [PMC free article] [PubMed]
- 21.Blackburn G, Guo M-J. Synthesis, physical, chemical, and enzyme studies on bis-2, 6-diaminopurine β-d-ribofuranoside P1, P4-tetraphosphate. Nucleus 1991; 10: 549-1.
- 22.Stepinski J, Bretner M, Jankowska M et al. Synthesis and properties of P1, P2-, P1, P3- and P1, P4-dinucleoside di-, tri-, and tetraphosphate mRNA 5-CAP analogues. Nucleus 1995; 14: 717-1.
- 23.Lesiak K, Watanabe KA, Majumdar A et al. Synthesis of nonhydrolyzable analogues of thiazole-4-carboxamide and benzamide adenine dinucleotide containing fluorine atom at the C2-of adenine nucleoside: Induction of K562 differentiation and inosine monophosphate dehydrogenase inhibitory activity. J Med Chem 1997; 40: 2533-. [DOI] [PubMed]
- 24.Zatorski A, Watanabe KA, Carr SF et al. Chemical synthesis of benzamide adenine dinucleotide: Inhibition of inosine monophosphate dehydrogenase (types I and II). J Med Chem 1996; 39: 2422-. [DOI] [PubMed]
- 25.Lesiak K, Watanabe KA, Majumdar A et al. Synthesis of a methylenebis(phosphonate) analogue of mycophenolic adenine dinucleotide: A glucuronidation-resistant MAD analogue of NAD. J Med Chem 1998; 41: 618-2. [DOI] [PubMed]
- 26.Brevet A, Coste H, Fromant M et al. Yeast diadenosine 5- 5′′-P1,P4-tetraphosphate alpha,beta-phosphorylase behaves as a dinucleoside tetraphosphate synthetase. Biochemistry 1987; 26: 4763-. [DOI] [PubMed]
- 27.Plateau P, Blanquet S. Zinc-dependent synthesis of various dinucleoside 5- 5′′-P1, P3-Tri- or 5′-5′′-P1, P4-tetraphosphates by Escherichia coli lysyl-tRNA synthetase. Biochemistry 1982; 21: 5273-. [DOI] [PubMed]
- 28.Kanavarioti A, Lu J, Rosenbach MT, Hurley TB. Unexpectedly facile synthesis of symmetrical P1, P2-dinucleoside-5′pyrophosphates. Tetrahedron Lett 1991; 32: 6065-. [DOI] [PubMed]
- 29.Hoard DE, Ott DG. Conversion of mono- and oligodeoxyribonucleotides to 5-triphosphates. J Am Chem Soc 1965; 87: 1785-. [DOI] [PubMed]
- 30.Zimmerman H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol 2000; 362: 299–309. [DOI] [PubMed]
- 31.Picher M, Boucher R. Biochemical evidence for an ecto alkaline phosphodiesterase I in human airways. Am J Respir Cell Mol Biol 2000; 23: 255-1. [DOI] [PubMed]
- 32.Tauber J, Davitt WF, Bokosky JE et al. Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea 2004; 23: 784-2. [DOI] [PubMed]