Abstract
We have previously reported that ATPγS, a slowly hydrolyzed analog of ATP, inhibits the activation of human CD4+ T lymphocytes by anti-CD3 and anti-CD28 mAb. In this report we have partially characterized the signaling mechanisms involved in this immunosuppressive effect. ATPγS had no inhibitory effect on CD4+ T-cell activation induced by PMA and anti-CD28, indicating that it acts proximally to the TCR. It had no effect on the calcium rise induced by CD3/CD28 stimulation, but inhibited the phosphorylation of three kinases, ERK2, p38 MAPK and PKB, that play a key role in the activation of T cells. The receptor involved in these actions remains unidentified.
Key words: ATP, CD4+, ERK, immunosuppression, lymphocyte, MAPK, PKB
Introduction
The appearance of adenine nucleotides in the extracellular fluids can result from cell necrosis, exocytosis of secretory granules or efflux through membrane transport proteins. Extracellular nucleotides are rapidly degraded by ectonucleotidases ubiquitously present on the cell surface, in particular E-NTPDases such as CD39 [1]. The actions of extracellular nucleotides are mediated by two families of receptors: the P2X receptors that are ligand-gated ion channels and the P2Y receptors that are coupled to G-proteins [1]. So far eight genuine human P2Y receptors have been identified and characterized [2]. According to sequence homology they belong to two distinct subgroups. On the one hand, P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors are all coupled to phospholipase C via a Gq/11 protein, while the P2Y11 receptor is additionally coupled to Gs and adenylyl cyclase stimulation. On the other hand, the P2Y12, P2Y13 and P2Y14 receptors are coupled to Gi.
Many authors have studied the effects of extracellular nucleotides on human leukocytes [reviewed in 3], such as chemotaxis, differentiation, and release of cytokines. Discrepant effects of adenine nucleotides on lymphocyte proliferation have been reported in the past. Gregory and Kern [4] showed that extracellular ATP stimulated the proliferation of murine thymocytes, whereas Fishman et al. [5] observed that ATP suppressed the proliferation of human peripheral lymphocytes, presumably as a result of its degradation into adenosine. Ikehara et al. observed both inhibitory and stimulatory effects of ATP on murine lymphocyte proliferation, depending on the origin of the cells [6]. ATP had a synergistic effect on the proliferation of human peripheral blood T cells stimulated by phytohemagglutinin, an effect apparently mediated by a P2X receptor [7]. It is well known that T-cell responses may greatly differ according to the subpopulation studied. Recently, we showed that extracellular adenine nucleotides such as ATPγS inhibit CD4+ T-cell activation, as reflected by decreased proliferation and secretion of both Th1 and Th2 cytokines in response to CD3 and CD28 ligation [8]. This inhibitory effect might represent a negative feedback mechanism at the level of the immunological synapse, since Mizumoto et al. showed that in CD39−/− mice ATP accumulates in the pericellular fluid of T cells following CD3/TCR- and CD28-mediated activation [9].
The inhibitory effect of ATPγS was associated with an increase in cAMP mediated by an unidentified P2Y receptor, but was independent of PKA inhibition [8]. Langston et al. have recently reproduced the inhibitory effect of ATPγS in mouse T cells, but suggested that it results from the inhibition of ATP hydrolysis by CD39 and not from the activation of a P2Y receptor [10]. On the other hand Loomis et al. have recently reported a stimulatory effect of ATP and ATPgS on the Jurkat T cells, possibly mediated via the P2X7 receptor and p38 MAPK activation [11].
In order to clarify these apparent discrepancies, we have investigated the signalling mechanisms of ATPγS in human CD4+ T cells.
Materials and methods
Reagents
ATP, adenosine 5′-O-(3-thiotriphosphate) (ATPγS) and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma Chemicals (St. Louis, MO, USA). The anti- CD3 monoclonal antibody (mAb) OKT3 (Orthoclone OKT3®) was provided by Janssen-Cilag (Berchem, Belgium) and the anti-CD28 mAb (clone CD28.2) by BD Pharmingen (San Diego, CA, USA).
Isolation of resting CD4+ T cells from peripheral blood
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats of healthy blood donors by density gradient centrifugation on Lymphoprep (Nycomed, Oslo, Norway). After five washes in Hank's balanced salt solution (HBSS) (Gibco Life Technologies, Paisley, UK) CD4+ T cells were isolated from PBMC using the MACS negative depletion system (Miltenyi Biotec, Auburn, CA). No contaminating CD8+ T cells, B cells, monocytes, or natural killer cells were detected.
CD4+ T-cell activation
Purified CD4+ T cells were cultured in RPMI 1640 medium (Gibco Life Technologies, Paisley, UK) supplemented with 10% heat-inactivated fetal bovine serum (FCS) from Hyclone (Logan, Utah), 25 mM Hepes buffer, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 µg ml−1 gentamicin and 50 µM 2-β-mercaptoethanol at 37 °C in 5% CO2. The CD4+ T cells (1 × 105/well) were activated either in flat-bottomed 96-well plates pre-coated with the anti-CD3 mAb (10 µg ml−1) in the presence of soluble anti-CD28 mAb (1 µg ml−1), or with 1 ng ml−1 phorbol 12-myristate 13-acetate (PMA) plus soluble anti-CD28 mAb (1 µg ml−1), in presence or absence of ATPγS. Culture supernatants were harvested after 24 h for measurement of IFN-γ (Biosource International, Camarillo, CA). Each experimental condition was tested in triplicate.
Calcium measurements
Intracellular calcium ([Ca2+]i) was measured in human CD4+ T lymphocytes using the fluorescent Ca2+ indicator fura 2-AM. Purified cells were concentrated at 107 ml−1 in HBSS (supplemented with 0.1% BSA) and incubated at 37 °C in 5% CO2-humidified atmosphere with 0.25 mM sulfinpyrazone (Sigma), 100 µg ml−1 pluronic acid F-127 (Molecular Probes, Leiden, The Netherlands) and 10 µM of fura 2-AM (Molecular Probes), for 30 min at 37 °C. Cells were then washed once in complete medium or HBSS-BSA with 0.25 mM sulfinpyrazone and resuspended at a final concentration of 5 × 105 ml−1 and placed in a 37 °C water bath for 5 min before stimulation with nucleotide or antibodies. The cells were excited alternatively at wavelengths of 340 and 380 nM, and the emission fluorescence intensity was monitored at 505 nM. The ratio of the two fluorescence intensities induced by excitation at the two wavelengths (F340/F380) after substraction of background fluorescence was used to monitor changes in [Ca2+]i.
Immunoprecipitation and Western blot analysis of ERK1/2, p38 and PKB
Purified CD4+ T cells (8 × 106 cells) were pre-incubated with 100 µM ATPγS for 10 min and then stimulated for different times in flat-bottomed plates with 10 µg ml−1 anti-CD3 mAb plus 5 µg ml−1 soluble anti-CD28 mAb plus 40 µg ml−1 goat antibody against mouse immunoglobulins (Sigma). Cells were rapidly pelleted, washed with HBSS and lysed on ice in 20 mM TrisYHCl pH 7.6, 1% NP-40, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM sodium fluoride and protease inhibitor cocktail from Roche Diagnostics. Lysates were cleared by centrifugation at 10,000 g for 20 min and incubated for 2 h at 4 °C with 30 µl of protein G immobilized on Sepharose 4B fast flow (Sigma). The supernatants were recovered after one centrifugation step at 10,000 g for 10 min. The supernatants were then mixed with concentrated Laemmli buffer (final concentration: 10% (w/v) glycerol, 5% (v/v) β-mercaptoethanol, 2.3% (w/v) SDS, 62.5 mM TrisYHCl pH 6.8) with proteinase inhibitors (Roche Diagnostics): 1 µg ml−1 Leupeptin, 60 µg ml−1 Pefabloc, 1 µg ml−1 Aprotinin, and phosphatase inhibitors (Sigma): 1 mM sodium orthovanadate and 10 mM sodium fluoride. The protein concentration was determined using the method of Minamide and Bamburg [12]. The same amount of protein (20 µg) for each condition was electrophoresed on a 12% SDS-polyacrylamide gel. Proteins were then transferred overnight at 28 V and 4 °C onto a nitrocellulose membrane using 20 mM Tris, 154 mM glycine, 20% (v/v) methanol as a transfer buffer. Immunodetection was achieved using antibodies specific for total and phosphorylated ERK1/2, p38 or PKB (all from Cell Signaling Technology, Beverly, MA), and chemiluminescence detection with horseradish peroxidase coupled secondary anti-mouse antibody (1:25,000) for ERK1/2 or anti-rabbit antibody (1:50,000) for p38 and PKB (both from Amersham Pharmacia Biotech).
Results
The inhibition of IFN-γ secretion by ATPγS involves signaling events proximal to the TCR
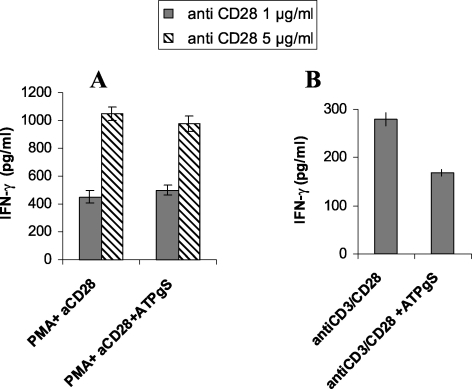
In order to see if the inhibitory action of ATPγS is proximal to the TCR stimulation, we have tested other activators that bypass it, PMA/anti-CD28. As shown in Figure 1, ATPγS inhibited the secretion of IFN-γ in human CD4+ T cells after stimulation with anti-CD3 and anti- CD28, as previously reported [8], but had little inhibitory effect on IFN-γ secretion from T cells activated with PMA plus anti-CD28.
Figure 1.
ATPγS inhibits IFN-γ secretion in primary human CD4+ T cells following activation mediated by anti-CD3 plus anti-CD28 but not by PMA plus anti-CD28. The cells were incubated for 24 h either with PMA (1 ng/ml) and two concentrations of soluble anti-CD28 (1 or 5 µg/ml) (A) or with a combination of pre-coated anti-CD3 and soluble anti-CD28 (1 µg/ml) (B), in the continuous presence or absence of ATPγS, that was added at the same time as PMA/CD28 or anti-CD3/anti-CD28. In the absence of stimulation, no IFN-γ was detectable in the supernatant (data not shown). Data represent the mean ± S.D. of triplicate experimental points obtained in one representative experiment of three.
ATPγS does not affect calcium mobilization in CD4+ T cells
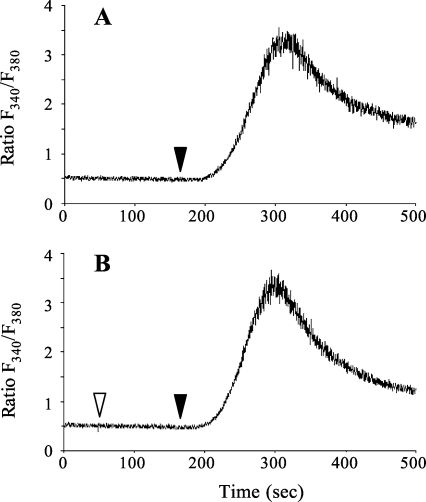
We studied the effect of ATPγS on the calcium response induced by TCR stimulation with anti-CD3 and anti-CD28 mAb crosslinked with goat anti-mouse IgG. ATPγS pretreatment, 2 or 10 min (data not shown), did not modify the calcium response following TCR stimulation (Figure 2). As previously reported, ATPγS did not increase per se [Ca2+]i in human CD4+ T cells [4].
Figure 2.
ATPγS has no effect on the calcium response induced by TCR stimulation with anti-CD3 and anti-CD28. Cells were loaded with FURA 2-AM, and intracellular calcium mobilization was followed on a spectrofluorometer (LS50B, Perkin Elmer). CD4+ T lymphocytes were activated by cross-linking the anti-CD3/anti-CD28 mAb with goat anti-IgG (▾) without (A) or with 100 µM ATPγS added (▿) to the cells 2 min before cross-linking (B). Data are from one representative experiment out of three.
ATPγS inhibits phosphorylation of ERK1/2, p38 and PKB
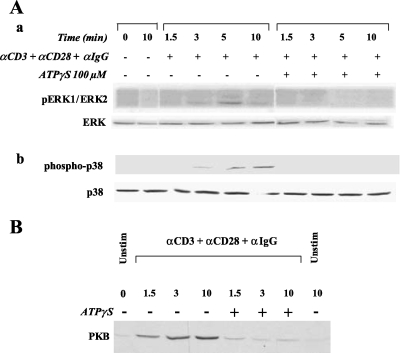
We examined the action of ATPγS on anti-CD3/CD28-induced activation of three known downstream targets, ERK 1/2, p38 and protein kinase B (PKB) in human CD4+ T cells. By itself, ATPγS did not modify the phosphorylation state of p38, ERK1/2 or PKB (data not shown). In primary human CD4+ T cells, as shown in Figures 3A and B, a rapid phosphorylation of ERK2 (p42), p38 and PKB occurred following stimulation by anti-CD3/CD28 antibodies, while no phosphorylation of ERK1 could be detected. ATPγS (100 µM) pretreatment strongly inhibited these phosphorylations (Figure 3).
Figure 3.
(A) ATPγS pre-treatment inhibits the phosphorylation of ERK1/2 (a) and p-38 (b) induced by TCR stimulation with anti-CD3 and anti-CD28 mAb. ATPγS 100 µM was added to the cells 10 min before activation with antibodies. The same amount of protein for each condition was electrophoresed on a 12% SDS-polyacrylamide gel. (B) ATPγS pretreatment inhibits the phosphorylation of PKB induced by TCR stimulation with anti-CD3 and anti-CD28 mAb. ATPγS 100 µM was added to the cells 10 min before activation with antibodies. The same amount of protein (20 µg) for each condition was electrophoresed on a 12% SDS-polyacrylamide gel.
Effect of other adenine nucleotides on IFN-γ secretion
In order to evaluate the hypothesis of Langston et al. [6], we compared the effects of ATPγS, an inhibitor of E-NTPDases, with that of known substrates, ATP and ADP. As shown in Table 1, the secretion of IFN-γ following CD3 and CD28 stimulation was inhibited to a similar extent by ATPγS, ATP and ADP. While these nucleotides induced a significant inhibition of IFN-γ secretion in all the experiments, adenosine had only a small effect (33 ± 4 and 19 ± 5% inhibition) in two experiments out of five (data not shown).
Table 1.
Effect of ATPγs, ATP and ADP on IFN-γ secretion following CD3 and CD28 stimulation.
| Mean % inhibition of IFN-γ secretion | S.D. | n | |
|---|---|---|---|
| ATPγS (100 μM) | 70 | 8 | 12 |
| ATP (100 μM) | 64 | 13 | 3 |
| ADP (100 μM) | 72 | 7 | 5 |
Purified CD4+ T cells were incubated with mAb, pre-coated anti-CD3 and soluble anti-CD28 (1 μg/ml), and nucleotides. After 24 h of incubation, the supernatants were harvested for ELISA. In the absence of stimulation, no IFN-γ was detectable in the supernatant (data not shown). Data collected from triplicate experimental points were normalized to the activated condition (anti-CD3 plus anti-CD28) to obtain a percentage of inhibition of IFN-γ secretion (n = number of experiments with different donors).
Discussion
We have previously reported that ATPγS, a slowly hydrolyzed analog of ATP, inhibits the activation of human CD4+ T lymphocytes by a mixture of anti-CD3 and anti-CD28 mAb [8]. ATPγS inhibited the cell proliferation, secretion of both Th1 (IL-2 and IFN-γ) and Th2 (IL-5 and IL-10) cytokines and expression of CD25. This effect was associated with an increase in cAMP, but was not inhibited by the PKA inhibitor H-89. These results are consistent with several reports indicating that the immunomodulatory action of cAMP is mediated partially by PKA and partially by other mechanisms [13, 14], but it does not allow to determine the precise mechanism of ATPγS action. It the present study we have shown that ATPγS has no inhibitory effect on CD4+ T cells activation induced by a combination of PMA and anti-CD28, indicating that it must act proximally to TCR activation. However ATPγS was unable to reduce the increase of [Ca2+]i induced by the anti-CD3/anti-CD28 combination. It is now well established that MAP kinases play a key role in the activation of T cells during the immune response. Both p38 MAPK and ERK are activated following TCR stimulation [15, 16] and inhibitors of both pathways inhibit the secretion of cytokines [16, 17]. On the other hand the stimulation of CD28 activates phosphatidylinositol 3 kinase (PI3K) and protein kinase B (PKB) which both cooperate with TCR to enhance T-cell proliferation and cytokine secretion [18]. We have now shown that ATPγS inhibits the activation of these three pathways, thus providing an explanation for its immunosuppressive effect. As previously described [15], we detected activation only of ERK2 and not ERK1 in human CD4+ T lymphocytes. These inhibitory effects might result from cAMP elevation, although the contribution of other mechanisms cannot be excluded. Indeed, although it was initially assumed that cAMP interferes with TCR-coupled phospholipase C and intracellular Ca2+ mobilization [19, 20], it now appears that it affects multiple and independent steps along the activation pathway of T cells. In particular cAMP has been associated with inhibition of ERK [21, 22], and PKB [22], but an effect on p38 MAPK has not yet been described.
Langston et al. have reproduced the inhibitory effect of ATPγS in mouse T cells, but suggested that it results from the inhibition of ATP hydrolysis by the E-NTPDase CD39 and not from the activation of a P2Y receptor [10]. That conclusion was mainly based on a similar inhibitory effect of 5′-FSBA, an irreversible inhibitor of E-NTPDases such as CD39 [23]. However 5′-FSBA can modify covalently numerous ATP-binding proteins. The hypothesis of Langston et al. [10] would imply that CD39 substrates, ATP or ADP, have no inhibitory effect or even potentiate the activation of T cells. However, we have now shown that ATP and ADP also inhibited activation of CD4+ T cells. Contrarily to our observations, Loomis et al. recently reported a stimulatory effect of ATP and ATPγS on the Jurkat T cells [11]. In these cells ATP and ATPγS increased both intracellular calcium and p38 MAPK phosphorylation, two effects possibly mediated by the P2X7 receptor [11]. These findings are likely to be a peculiarity of the Jurkat cell line, since in CD4+ T cells isolated from peripheral human blood, we observed that ATPγS had no effect on [Ca2+]i mobilization and p38 MAPK phosphorylation.
The identity of the receptor(s) mediating the effects of ATPγS and other nucleotides on CD4+ T lymphocytes remains unknown. We have previously reported that the lack of ATPγS effect on [Ca2+]i is inconsistent with the involvement of the P2Y11 receptor, the only P2Y receptor coupled to Gs [8]. Furthermore 2′-deoxy-ATP and ARC67085MX, two known agonists of recombinant P2Y11, had no effect on cAMP in these cells [8]. Recently Feng et al. made similar observations in human mast cells: ATPγS inhibited cytokine production and increased cAMP but not [Ca2+]i [24]. They also concluded that this response is unlikely to be mediated by P2Y11. Interestingly various ectonucleotidases expressed in immune cells have a signalling function beside their enzymatic activity : f.i. CD73 [25], an ecto-5′-nucleotidase, and PC-1 [26], an ectophosphodiesterase/pyrophosphatase. In particular, CD73 plays a co-stimulatory role in T-cell activation, independently from its enzymatic activity. It could be speculated that the action of nucleotides on CD4+ T cells is partially mediated by an ectonucleotidase rather than by a classical P2X or P2Y receptor. In that sense, CD39 might also be involved, but via a signalling action, and not as suggested by Langston et al. [10] via its enzymatic activity.
In conclusion, we have further characterized the signalling mechanisms involved in the immunosuppressive effect of ATPγS on human CD4+ T cells. This nucleotide acts by inhibiting the TCR signalling, but has no effect on the calcium rise induced by TCR stimulation. Moreover ATPγS inhibits the activation of three kinases ERK2, p38 MAPK and PKB that play a role downstream of TCR/CD28. The inhibition of ERK2 and p38 MAPK is sufficient to explain inhibition of cytokine secretion. Inhibition of ERK2 and PKB activation is consistent with a role of cAMP although the contribution of other mechanisms cannot be excluded. The receptor involved in the action of ATPγS remains unidentified. Our observations suggest that some nucleotides might be useful as immunosuppressive agents, especially for a topical use.
Acknowledgement
This work was supported by an “Action de Recherche Concertée de la Communauté Française de Belgique,” by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Federal Service for Science, Technology and Culture, and by grants of the Fonds de la Recherche Scientifique Médicale. Xavier Duhant was supported by the Fonds National de la Recherche Scientifique/Télévie, Belgium. Didier Communi is “Chercheur qualifié” of the Fonds National de la Recherche Scientifique. Nathalie Suarez Gonzalez is supported by Euroscreen.
Abbreviations
- 5-FSBA
5-p-(fluorosulfonyl) benzoyl adenosine
- ATPγS
adenosine 5-O-(3-thiotriphosphate)
- E-NTPDase
ecto-nucleoside triphosphate diphosphohydrolase
- ERK
extracellular signal-regulated kinase
- mAb
monoclonal antibody
- MAPK
mitogen activated protein kinase
- PKA
protein kinase A
- PKB
protein kinase B
- PMA
phorbol 12-myristate 13-acetate
- TCR
T-cell receptor
References
- 1.Communi D, Janssens R, Suarez-Huerta N et al. Advances in signalling by extracellular nucleotides: The role and transduction mechanisms of P2Y receptors. Cell Signal 2000; 12: 351-0. [DOI] [PubMed]
- 2.Abbracchio MP, Boeynaems JM, Barnard EA et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 2003; 24: 52-. [DOI] [PMC free article] [PubMed]
- 3.Di Virgilio F, Chiozzi P, Ferrari D et al. Nucleotide receptors: An emerging family of regulatory molecules in blood cells. Blood 2001; 97: 587–600. [DOI] [PubMed]
- 4.Gregory S, Kern M. Adenosine and adenine nucleotides are mitogenic for mouse thymocytes. Biochem Biophys Res Commun 1978; 83: 1111-. [DOI] [PubMed]
- 5.Fishman RF, Rubin AL, Novogrodsky A, Stenzel KH. Selective suppression of blastogenesis induced by different mitogens: Effect of noncyclic adenosine-containing compounds. Cell Immunol 1980; 54: 129-9. [DOI] [PubMed]
- 6.Ikehara S, Pahwa RN, Lunzer DG et al. Adenosine-5-triphosphate-(ATP) mediated stimulation and suppression of DNA synthesis in lymphoid cells. I. Characterization of ATP responsive cells in mouse lymphoid organs. J Immunol 1981; 127: 1834-. [PubMed]
- 7.Baricordi OR, Ferrari D, Melchiorri L et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 1996; 87: 682-0. [PubMed]
- 8.Duhant X, Schandené L, Bruyns C et al. Extracellular adenine nucleotides inhibit the activation of human CD4+ T lymphocytes. J Immunol 2002; 169: 15–21. [DOI] [PubMed]
- 9.Mizumoto N, Kumamoto T, Robson SC et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: Modulatory roles in inflammation and immune responsiveness. Nat Med 2002; 8: 358-5 [DOI] [PubMed]
- 10.Langston HP, Ke Y, Gewirtz AT et al. Secretion of IL-2 and IFN-γ, but not IL-4, by antigen-specific T cells requires extracellular ATP. J Immunol 2003; 170: 2962-0. [DOI] [PubMed]
- 11.Loomis WH, Namiki S, Ostrom RS et al. Hypertonic stress increases T-cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem 2003; 278: 4590-. [DOI] [PubMed]
- 12.Minamide LS, Bamburg JR. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal Biochem 1990; 190: 66–70. [DOI] [PubMed]
- 13.Staples KJ, Bergmann M, Tomita K et al. Adenosine 3-5-cyclic monophosphate (cAMP)-dependent inhibition of IL-5 from human T lymphocytes is not mediated by the cAMP-dependent protein kinase A. J Immunol 2001; 167: 2074-0. [DOI] [PubMed]
- 14.Bryce PJ, Dascombe MJ, Hutchinson IV. Immunomodulatory effects of pharmacological elevation of cyclic AMP in T lymphocytes proceed via a protein kinase A independent mechanism. Immunopharmacology 1999; 41: 139-6. [DOI] [PubMed]
- 15.Whitehurst CE, Boulton TG, Cobb MH, Geppert TD. Extracellular signal-regulated kinases in T cells. Anti-CD3 and 4 beta-phorbol 12-myristate 13-acetate-induced phosphorylation and activation. J Immunol 1992; 148: 3230-. [PubMed]
- 16.Rincon M, Enslen H, Raingeaud J et al. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase-signaling pathway. EMBO J 1998; 17: 2817-9. [DOI] [PMC free article] [PubMed]
- 17.Dumont FJ, Staruch MJ, Fischer P et al. Inhibition of T-cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production.J Immunol 1998; 160: 2579-9. [PubMed]
- 18.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T-cell activation: Pleiotropic pathways downstream of PIP3. Immunol Rev 2003; 192: 7–20. [DOI] [PubMed]
- 19.Lerner A, Jacobson B, Miller RA. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol 1988; 140: 936-0. [PubMed]
- 20.Tamir A, Isakov N. Cyclic AMP inhibits phosphatidylinositol-coupled and -uncoupled mitogenic signals in T lymphocytes. Evidence that cAMP alters PKC-induced transcription regulation of members of the jun and fos family of genes. J Immunol 1994; 152: 3391-. [PubMed]
- 21.Tamir A, Granot Y, Isakov N. Inhibition of T lymphocyte activation by cAMP is associated with down-regulation of two parallel mitogen-activated protein kinase pathways, the extracellular signal-related kinase and c-Jun N-terminal kinase. J Immunol. 1996; 157: 1514-2. [PubMed]
- 22.Grader-Beck T, van Puijenbroek A, Nadler LM, Boussiotis VA. cAMP inhibits both Ras and Rap1 activation in primary human T lymphocytes, but only Ras inhibition correlates with blockade of cell cycle progression. Blood 2003; 101: 998–1006. [DOI] [PubMed]
- 23.Dombrowski KE, Trevillyan JM, Cone JC et al. Identification and partial characterization of an ectoATPase expressed by human natural killer cells. Biochemistry 1993; 32: 6515-2. [DOI] [PubMed]
- 24.Feng C, Mery AG, Beller EM et al. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol 2004; 173: 7539-7. [DOI] [PubMed]
- 25.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev 1998; 161: 95–109. [DOI] [PubMed]
- 26.Goding JW, Terkeltaub R, Maurice M et al. Ecto-phosphodiesterase/ pyrophosphatase of lymphocytes and non-lymphoid cells: Structure and function of the PC-1 family. Immunol Rev 1998; 161: 11–26. [DOI] [PubMed]