Abstract
It is widely accepted that the most important ATP receptors involved in pain transmission belong to the P2X3 and P2X2/3 subtypes, selectively expressed in small diameter dorsal root ganglion (DRG) neurons. However, several types of the metabotropic ATP (P2Y) receptors have also been found in primary afferent neurons; P2Y1 and P2Y2 receptors are typically expressed in small, nociceptive cells. Here we review the results available on the involvement of P2Y receptors in the modulation of pain transmission.
Key words: analgesia, dorsal root ganglion, electrophysiology, immunohistochemistry, P2Y receptor
Introduction
It was observed in the late sixties that the endogenous P2 receptor agonist adenosine 5′-triphosphate (ATP) can evoke pain sensation [1, 2]. The most important ATP-sensitive receptors involved in pain transmission appear to be the ligand-gated P2X3 and P2X2/3 subtypes, selectively expressed by a subpopulation of small diameter dorsal root ganglion (DRG) cells binding isolectin B4 (IB4) and possessing also capsaicin-sensitive vanilloid receptors (TRPV1) [3, 4]. Numerous studies have shown that P2X receptors play a crucial role in facilitating pain transmission at peripheral and spinal sites; a large number of reviews has recently been published on these receptors and nociception [5–10]. However, possible roles for metabotropic P2Y receptors in nociceptive signaling have received limited attention. Here we review the results available on P2Y receptors relating to the sensory pathway and on the available evidence that P2Y receptors are also involved in the modulation of pain transmission.
P2Y receptors
The following G protein-coupled P2Y receptors have been characterized to date in humans: P2Y1, P2Y11, P2Y12, and P2Y13 (sensitive to the adenine nucleotides ADP and ATP); P2Y4 and P2Y6 (sensitive to the pyrimidine nucleotides UDP and UTP); P2Y2 (sensitive to both ATP and UTP); P2Y14 (sensitive to uridine sugars such as UDP-glucose and UDP-galactose) [11–16].
P2Y receptors, in common with other G protein-coupled receptors, have seven transmembrane domains, an extracellular N terminus and an intracellular C terminus. The P2Y1, P2Y2, P2Y4 and P2Y6 receptors are positively coupled to phospholipase C via Gq,11 proteins, while the P2Y12–14 receptors are negatively coupled to adenylyl cyclase via Gi proteins. P2Y11 receptors are dually coupled to Gq,11 and Gs proteins, activating both phospholipase C and adenylyl cyclase [11–16].
The ligand selectivity of different P2Y receptor subtypes has extensively been discussed elsewhere [17–19]. ADP, adenosine 5′-O-(2-thiodiphosphate) (ADP-β-S) and 2-methylthio ADP (2-MeSADP) are agonists at P2Y1, P2Y12 and P2Y13 receptors. Among them, ADP and ADP-β-S act also at P2X1,2,3,4 receptors. UTP is selective for P2Y2 and P2Y4 receptors, whereas UDP preferentially activates the P2Y6 receptor; UDP-glucose acts at P2Y14 receptors. P2Y11 receptors are activated by ATP and 2-MeSATP. Adenosine-3′-phosphate-5′-phosphate (A3P5P), N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS-2179) and (1R,2S,4S,5S)-1-[(phosphato)methyl]-4-(2-chloro-6-aminopurin-9-yl) bicyclo [3.1.0]-hexane-2-phosphate (MRS-2279) are selective antagonists for the P2Y1 receptor. Pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) also shows some preference for P2Y1 receptors over the residual P2Y receptor-types. N6-(2-Methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene ATP (ARC69931MX) is a selective antagonist at P2Y12 and P2Y13 receptors, whereas 2-MeSAMP selectively blocks the P2Y12 receptor-type.
Expression of P2Y receptors in neurons of sensory ganglia
In contrast to P2X3 receptors, which are mostly expressed in small neurons of the DRG and other sensory ganglia, P2Y receptors seem to be equally expressed in small, large and medium-sized sensory neurons. Reverse transcription (RT)-PCR demonstrated the presence of P2Y1 receptor mRNA in rat DRG, nodose ganglion (NG) and trigeminal ganglion (TG) [20–22]. In situ hybridization studies showed the presence of P2Y1 receptor mRNA in about 20% of both rat and mouse DRG neurons [23, 24], with the most intense staining in large, RT-97-immunoractive (IR) neurons, and weaker staining in small, peripherin-IR neurons [25]. Immunostaining studies showed that P2Y1 receptor antibodies stained over 80% of the DRG, NG and TG neurons [22]. In this study, the small-diameter neurons were stained much more intensely than the medium- and large-diameter neurons. The staining was evenly distributed throughout the cytoplasm of these cells and immunoreactive cells were randomly distributed throughout the ganglia. P2Y1 receptor-IR has also been demonstrated in cell bodies of the rat NG [26]. Ligation of the vagus nerve led to accumulation of staining adjacent to the ligature and this accumulation was found central to the proximal ligature and peripheral to the distal ligature, suggesting anterograde and retrograde transport, respectively. These data imply that P2Y1 receptors are transported to and expressed functionally on terminals of sensory nerves.
The co-expession of P2Y1 mRNA with TRPV1 mRNA, thought to be characteristic for nociceptive DRG neurons, was investigated by in situ hybridization. Astonishingly only 2% of TRPV1 mRNA-expressing cells possessed also P2Y1 mRNA and vice versa [24]. However, in another study, where the receptor proteins were identified by immunocytochemistry in combination with confocal laser scanning microscopy, co-staining for P2Y1, TRPV1 and P2X3 receptors was observed in 80% of small-diameter (20–35 µM) neurons in the rat DRG [27]. These data were confirmed by the finding that P2Y1 receptors are often co-localized on the same cells with P2X3 receptors [22]. A high number of P2X3-IR cells were also found to be P2Y1 receptor-IR in DRG, NG and TG; in general, more neurons appeared to express P2Y1 receptors than P2X3 receptors [22]. Double immunofluorescence histochemistry showed that 30% of P2Y1 receptor-IR cells showed also NF200-IR in DRG, NG and TG. NF200 is an anti-neurofilament antibody that stains the large-diameter neurons with myelinated axons [22]. The P2Y1 receptor was also co-localized with the neuropeptide calcitonin gene-related peptide (CGRP) in more than 60% of DRG, NG and TG cells. The P2Y1 receptors were further highly co-expressed in IB4 containing neurons; 80%, 60% and 20% of the IB4 cells were also immunoreactive for the P2Y1 receptor, in DRG, NG and TG cells, respectively [22].
RT-PCR revealed the presence of P2Y2 mRNA in rat DRG, NG and TG [21, 22]. In situ hybridization showed that 80% of adult rat DRG neurons expressed P2Y2 mRNA, but only half of these neurons responded to UTP by phosphorylating the cAMP-responsive element binding protein (CREB) [28]. P2Y2 mRNA was expressed in neurons of all sizes, but was seen less frequently in large cells: 90% of small cells (<30 µm diameter) were positive, as were 40% of larger cells (>40 µm diameter). A similar distribution of P2Y2 expression was observed in neurons of the rat TG [28]. However, another in situ hybridization study showed that only 20% of rat DRG neurons expressed P2Y2 receptors and that a significant population of DRG neurons co-expressed P2Y2 and TRPV1 mRNA [24].
RT-PCR confirmed the presence of P2Y4 mRNA in rat DRG, NG and TG [21, 22]. Immunohistochemistry revealed that the number of neurons staining for the P2Y4 receptor antibody was lower than that staining for the P2Y1 receptor antibody, and the medium- and large-diameter neurons were stained much more frequently than the small-diameter neurons [22]. The co-localization of P2Y4 and P2X3 receptors was less frequent than that of P2Y1 and P2Y4 receptors. Double immunofluorescence histochemistry showed that many P2Y4 receptor-IR cells showed also NF200-immunoreactivity in DRG, NG and TG. CGRP-IR cells co-expressed P2Y4 receptors much less frequently than P2Y1 receptors. Unlike P2Y1 receptor-IR cells, P2Y4-IR neurons lacked IB4 in these sensory ganglia [22]. Considering these findings, it is likely that P2Y4 receptors are preferentially expressed in large, IB4- and CGRP-negative DRG neurons. These neurons are known to be implicated in the transmission of tactile allodynia but not acute pain or thermal hyperalgesia [29] suggesting that P2Y4 receptors may play a role in this process.
RT-PCR demonstrated the presence of P2Y6 mRNA in rat DRG, NG and TG, but the receptor was not confined to sensory neurons [21, 22]. ATP-induced Ca2+-release from inositol 1,4,5-triphosphate (IP3)-sensitive Ca2+ stores in large (30–45 µM) but not in small (20–25 µM) mouse DRG neurons [30]. The effect was thapsigargin-sensitive; however, the involvement of P2Y receptor subtypes other than P2Y6 was not investigated. In another functional study, UTP caused an increase of intracellular calcium concentration ([Ca2+]i) in equal numbers of small (<30 µM), medium (30–40 µM) and large cells (>40 µM), suggesting the existence of P2Y2 and/or P2Y4 receptors at these neurons [21]. ADP-β-S evoked Ca2+ release from intracellular stores of small (20–35 µM) and IB4-IR (nociceptive) DRG cells from the rat and this effect was antagonized by the P2Y1 receptor antagonist MRS 2179 [31]. ATP, ADP and UTP were shown to inhibit voltage-activated calcium channels (VACCs) in cultured small-diameter (20–35 µM) DRG neurons from the rat, indicating the functional presence of P2Y1, P2Y2 and/or P2Y4 receptors on these cells [27].
P2Y receptors in neurons of the dorsal spinal cord
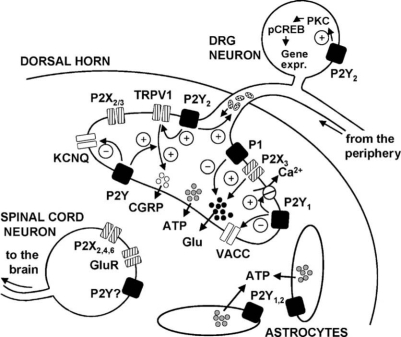
There are only few and relatively controversial data available on the presence of P2Y receptors at dorsal horn neurons. It has been concluded that ATP increases [Ca2+]i in 30% of these neurons via P2Y receptor activation, since the selective P2X1,3 receptor agonist α,β-methylene ATP (α,β-meATP) had no effect [32]. However, ATP also acts via ionotropic P2X receptors of the P2X2,4,6 types which have been shown to be expressed on the somata of dorsal horn neurons [7]. The involvement of these receptors in ATP-induced [Ca2+]i transients was not excluded by selective pharmacological ligands or omission of Ca2+ from the extracellular solution. In another study, the P2Y-selective 2-MeSADP caused no change in [Ca2+]i in spinal cord cultured neurons [33]. The non-selective P2 agonists ATP and 2-MeSATP increased [Ca2+]i in 60% of neurons [34]. The response was not abolished in Ca2+-free extracellular solution, and PPADS blocked the effect of 2-MeSATP. The possible influence of glutamate, released from neighboring cells, was ruled out using diverse glutamate receptor antagonists. In acutely isolated neurons which lost their dendrites, there was no P2Y response [34]. However, further experiments are needed to reliably evaluate the expression of P2Y receptors on spinal cord dorsal horn neurons (Figure 1).
Figure 1.
The role of P2Y receptors in pain pathways. The P2Y receptors are present on DRG cells, spinal cord astrocytes and probably on dorsal horn neurons. In DRG neurons, the P2Y1 receptor stimulates the plasma membrane Ca2+ pump which removes Ca2+ from the cell. The P2Y1 receptor further inhibits VACCs, which play a role in the vesicular release of glutamate from DRG neurons. The P2Y2 receptor potentiates capsaicin-induced membrane currents through the vanilloid receptor 1 (TRPV1), increases the axonal transport of membrane-bound organelles and activates the phosphorylation of CREB in DRG cells. P2Y receptors of unidentified subtypes inhibit the M-type potassium currents through KCNQ channels in DRG cells and potentiate the capsaicin-induced release of CGRP from these cells. Of course, P2X3 and P2X2/3 receptors are also present at the terminals of DRG neurons. P2X3 receptors have been shown to positively modulate the release of glutamate, while P1 receptors of the A1-subtype depress glutamate release. Dorsal horn astrocytes are known to release ATP which is in turn able to activate neighboring cells via P2Y1 and P2Y2 receptors. Finally, spinal cord neurons are endowed with P2X2,4,6 and ionotropic glutamate receptors. The presence of P2Y receptors appears to be likely but has not been confirmed yet. ATP: adenosine-5′-triphosphate; CGRP: calcitonin gene-related peptide; CREB: cAMP-responsive element binding protein; DRG: dorsal root ganglion; Glu: glutamate; GluR: glutamate receptor; KCNQ: potassium voltage-gated channel; pCREB: phosphorylated CREB; PKC: protein kinase C; VACC: voltage-activated calcium channel.
P2Y receptors in glial cells
Sensory ganglia contain, in addition to neurons, glial cells that wrap around the neurons. These cells, also called satellite glial cells (SGCs), are closely apposed to the neurons [35]. There is histological evidence that SGCs are affected in animal models for pain [36–39]. Specific P2Y receptor agonists (UTP, 2-MeSADP and ADP-β-S) but not the specific P2X1,3 receptor agonist α,β-meATP caused an increase in intracellular Ca2+ concentration in SCGs, indicating the functional presence of P2Y1 and P2Y2 or P2Y4 receptors on these cells [40].
ATP, ADP, 2-MeSATP and 2-MeSADP all increased [Ca2+]i in almost all astrocytes and in 20% of oligodendrocytes from cultured dorsal horn, whereas α,β-meATP had no effect [32, 33]. The action of the different P2Y1 receptor agonists were independent of extracellular Ca2+ and sensitive to suramin, A3P5P and thapsigargin, indicating that these cells possess functional P2Y1 receptors. In one of these studies, RT-PCR revealed the presence of P2Y1 mRNA in the spinal cord [33]. Since 2-MeSADP caused no change in [Ca2+]i of spinal cord neurons, it was concluded that the astrocytes are the source of P2Y1 mRNA detected in the whole tissue. UTP was also shown to be able to increase [Ca2+]i, although only in 30% of astrocytes from the dorsal horn [41]. The UTP-induced Ca2+ responses were thapsigargin-sensitive, pertussis toxinin-sensitive, and both PPADS and suramin could antagonize them, demonstrating the functional expression of P2Y2 receptors in dorsal horn astrocytes. Both the P2Y1 and P2Y2 receptors were found to be linked via G protein coupling to the release of [Ca2+]i via the phospholipase C (PLC)/IP3 pathway [42].
There are only sparse data available on the expression of P2Y receptors in spinal cord microglia. Application of the P2X/P2Y agonists ATP and 2-MeSATP on cultured microglia from rat spinal cord in the absence of extracellular Ca2+ induced an increase in [Ca2+]i, whereas the P2X1,3,4-selective β,γ-MeATP had no effect [43]. Therefore, ATP and 2-MeSATP may have increased [Ca2+]i by activating a hitherto uncharacterized P2Y receptor-type.
P2Y receptors and pain
Excitation of DRG cells; possible noxious effects
In an early study on bullfrog DRG, ATP inhibited the M-type potassium current (Figure 1), leading to depolarization and initiation of action potentials [44]. Intracellularly applied guanosine 5′-O-(2-thiobisphosphate) (GDP-β-S), an inhibitor of G protein-mediated reactions, prevented the effect of ATP, suggesting the involvement of P2Y receptors. The M-current is a low-threshold, non-inactivating, voltage-sensitive K+ current, which is weak or undetectable at resting membrane potential and increases with membrane depolarization, inducing spike frequency adaptation and acting as a dynamic brake on neuronal excitability [45, 46]. M-currents emerge from homo- and heteromultimeric assemblies of KCNQ2-5 protein subunits [47]. Considering that agonists of M-currents have been shown to be antinociceptive [48], P2Y receptors inhibiting these currents may be able to stimulate the pain transmission.
In human embryonic kidney-derived HEK-293 cells, ATP, ADP, 2-MeSATP and UTP potentiated the capsaicin-evoked inward currents (Figure 1) [20]. PPADS, reactive blue 2 (RB2) and the protein kinase C (PKC) inhibitor calphostin C inhibited the stimulatory effect of ATP. Moreover, ATP treatment lowered the threshold temperature for TRPV1 activation from 42 to 35 °C, suggesting that TRPV1 activation could trigger the sensation of pain at normal body temperature in the presence of ATP. In further experiments on cultured rat DRG neurons, ATP and ADP caused the same potentiation of TRPV1 as on HEK-293 cells. This effect was independent of P2X receptors, since in cells with P2X receptor-mediated transient currents the magnitude of ATP-induced potentiation of capsaicin-evoked currents was indistinguishable from that seen in neurons lacking P2X responses. A following study from the same group revealed the importance of P2Y2 receptors in nociception through TRPV1 [24]. It was reported that ATP-induced thermal hyperalgesia in vivo is abolished in mice lacking TRPV1, whereas it is preserved in P2Y1 receptor-deficient mice. Moreover, patch-clamp analyses using mouse DRG neurons indicated the involvement of P2Y2 rather than P2Y1 receptors. The potentiation of capsaicin-evoked currents through ATP was still observed in P2Y1-deficient mice, and UTP also potentiated TRPV1 in these cells. Suramin, which blocks P2Y2 but not P2Y4, abolished the effect of UTP.
ATP, but not UTP, accelerates Ca2+ efflux from rat DRG cells by stimulating the plasma membrane Ca2+ pump (Figure 1) [49]. Ca2+ entering the cell during excitation must be removed from the cytoplasm to maintain normal Ca2+ homeostasis; the plasma membrane Ca2+ pump is a key component of the Ca2+ extrusion machinery in neurons [50]. The stimulatory effect of ATP was prevented by PPADS, the P2Y1 antagonist A3P5P, the phospholipase C inhibitor U73122 and by inhibition of PKC through GF109203x. This acceleration of the plasma membrane Ca2+ pump via P2Y1 receptors caused a decrease in amplitude and duration of the action potential after-hyperpolarization and thereby an increase in the excitability of DRG cells.
UTP evoked a slow-onset depolarization and sustained firing in dissociated DRG neurons from the rat that persisted for tens of seconds after removal of UTP from the medium [28]. In a subsequent study, UTP activated sustained action potential firing in 50% of C fibers using the skin nerve preparation from the adult mouse [51]. UDP had no effect, pharmacologically excluding the involvement of P2Y6 receptors. The majority (70%–80%) of UTP-sensitive C- and AM fibers respond strongly to capsaicin, whereas UTP-insensitive fibers were largely capsaicin-insensitive. Most of the UTP-sensitive C- and AM fibers (80%–100%) also responded to the P2X agonist α,β-meATP, indicating that P2Y and P2X receptors are widely co-expressed on these fibers. These data also support the idea that P2Y2 receptors on the terminals of capsaicin-sensitive and P2X3-expressing cutaneous sensory neurons effectively stimulate nociceptive transmission [51].
Furthermore, UTP and ATP, but not the P2Y1,12,13 preferential ADP, increased phosphorylation of the transcription factor CREB in 40% of the DRG neurons (Figure 1), in a PLC-dependent way [28]. Phosphorylated CREB (pCREB) is known to induce the expression of numerous neuropeptides (CGRP, substance P [SP] and brain-derived neurotropic factor [BDNF]) that are upregulated in sensory neurons in response to injury or inflammation [52–54]. Ninety-five percent of the pCREB-IR neurons also displayed peripherin staining, indicating that DRG neurons with unmyelinated axons are involved. One-third of these cells also expressed CGRP and 40% IB4. These data suggest that UTP evokes CREB phosphorylation in a subset of nociceptive DRG neurons. The UTP-induced phosphorylation of CREB was inhibited by suramin, but not by cibacron blue, an antagonist of P2Y4 but not of P2Y2 receptors, indicating an action via P2Y2 receptors. It seems likely that P2Y2 receptors, increasing the excitability of sensory neurons in the short term, may also activate a transcription factor that is likely to foster the expression of nociceptive neurotransmitters in the long term.
UTP has been shown to stimulate CGRP release from rat DRG neurons in the absence of extracellular Ca2+, and suramin abolished this stimulatory action [21]. These data suggest that P2Y2 receptor activation and the subsequent increase of [Ca2+]i may be accompanied by the release of the neuropeptide CGRP from the first order sensory neurons stimulating pain transmission. In contrast, in a more detailed study, neither ATP, UTP nor 2-MeSATP increased the basal release of SP or CGRP from cultured embryonic rat DRG neurons [55]. However, the concentration of UTP used in this study was lower than that used by Sanada et al. [21] (10 vs 100 µM) which can explain the inconsistency between the results. Similarly, ATP had little direct stimulatory effect on the release of CGRP from the nerve terminals in isolated rat dura mater but augmented proton-induced release of CGRP [56]. In embryonic rat DRG neurons, the ability of P2Y receptor agonists to modulate the excitability to capsaicin was also investigated [55]. ATP and UTP, but not 2-MeSATP or α,β-meATP, augmented the release of SP and CGRP evoked by capsaicin (Figure 1). The sensitizing effect of ATP was antagonized by suramin but not by PPADS and was inhibited by the PKC inhibitor bisindolylmaleimide. Together, these data suggest that ATP, acting at P2Y receptors in sensory neurons, can augment their sensitivity to other stimuli in a PKC-dependent manner.
ATP and UTP also increased axonal transport of membrane-bound organelles in anterograde and retrograde directions in cultured adult mouse DRG neurons (Figure 1); suramin completely blocked this effect of ATP [57]. ADP, α,β-meATP and 2-MeSATP were ineffective, suggesting the involvement of P2Y2 receptors. Synaptic functions including neurotransmitter release and up-/downregulation of receptors require axonal transport [58, 59]. In primary sensory neurons from the rat DRG, axonal transport delivers receptors, ion channels, sensory neuropeptides, glutamate and aspartate in both central and peripheral directions [59]. It was suggested that blockade of axonal transport can inhibit the development of neuropathic pain by blocking the signaling of peripheral injury to the central neurons [60]. In this case, P2Y2 receptor activation through agonists would be pronociceptive through stimulation of the axonal transport.
There are several lines of evidence indicating that spinal cord glial cells can create and maintain exaggerated pain states [61–63]. These cells become activated in response to substances released by both the primary afferent terminals (ATP, SP, CGRP, and glutamate) and by spinal cord neurons (nitric oxide, prostaglandins). It is well known that the ATP-induced activation of glial cells is mediated by both P2X and P2Y receptors [64, 65]. Once activated, glial cells can release a variety of neurotransmitters and neuromodulators including ATP, which in turn can further stimulate neighboring glial cells and neurons, and is capable of altering pain transmission. This effect is due both to an enhanced release of SP and glutamate from the central terminals of DRG neurons [66, 67] and to an increased excitability of spinal cord neurons [63]. One important way by which astrocytes regulate their own activity is via Ca2+ signals. A rise in [Ca2+]i limited to one part of an astrocyte may propagate within the rest of the cell, and Ca2+ responses can be transmitted from one astrocyte to others leading to Ca2+ waves that spread within astrocyte networks [68]. There are two main routes by which Ca2+ waves can propagate from one cell to another: Through gap junction channels [69] and through the extracellular space involving the release of pharmacologically active substances from one cell activating surface receptors on neighboring cells. A growing body of evidence indicates that a principal mechanism for the propagation of Ca2+ waves between astrocytes is by release of ATP, which acts as a diffusible extracellular messenger [70–72]. Release of ATP from astrocytes during Ca2+ wave propagation has been demonstrated [72]. In spinal cord astrocytes, Ca2+ wave propagation was found to be mediated by P2Y1 and P2Y2 receptors (Figure 1) [33, 73]. Both receptors were necessary and sufficient for the propagation of Ca2+ waves but characteristics of the propagation were different: Ca2+ waves propagating via P2Y2 receptors travel faster and further than those propagating via P2Y1 receptors. It was suggested that mediation of Ca2+ waves by ATP leading to activation of two subtypes of receptor, P2Y1 and P2Y2, may be a general principle for gliotransmission in the central nervous system [73].
Antinociceptive effects
P2Y receptor agonists were found to inhibit cytokine release from activated spinal cord microglia [43]. The rank order of inhibitory potency on cytokine release was: 2-MeSATP = ATP > ADP-β-S = ADP ≫ UTP > UDP. However, P2Y antagonists or further P2X and P2Y selective substances were not used in this study. It is known that activated microglia and astrocytes in the spinal cord can create and maintain chronic pain states [61, 74]. P2Y receptors inhibiting microglia overstimulation through the reduction of cytokine release from these cells could interrupt chronic pain development and continuation.
Repetitive stimulation of the dorsal root, with an intensity adequate to activate C-fibers but not A-fibers, induced slow depolarization of substantia gelatinosa neurons in the adult rat spinal cord transverse slices with dorsal root attached [75]. This effect was inhibited by the μ-opioid receptor agonist DAMGO, but not by DPDPE and U-50488H, δ- and κ-opioid agonists, respectively, indicating that the repetitive stimulation of C-fibers of the dorsal root is relevant to spinal nociception. ATP and UTP, but not α,β-meATP, were found to inhibit the slow depolarization of the substantia gelatinosa neurons in the dorsal horn [76]. The inhibitory effect of ATP was not reversed by suramin, the only antagonist used in this study.
In a subsequent study from the same laboratory, UTP and UDP were shown to be analgesic when applied intrathecally (i.t.) to rats [77]. In the paw pressure test using normal rats, i.t. administration of UTP and UDP elevated the mechanical nociceptive threshold, whereas ATP and α,β-meATP lowered it. Similarly, in the tail-flick test, UTP and UDP prolonged the thermal nociceptive latency. In the von Frey filament test on rats, while ATP induced a significant and long-lasting tactile allodynia, UTP and UDP were ineffective. In the neuropathic pain model, in which the sciatic nerves of rats were partially ligated, UTP and UDP were found to be antiallodynic. Furthermore, UTP and UDP caused no motor deficit in the inclined plane test, indicating that the elevation of mechanical nociceptive threshold and prolongation of the thermal nociceptive latency were likely due to antinociceptive effects, rather than deficits of motor function. Taken together, i.t. administration of UTP and UDP had antinociceptive effects on acute mechanical and thermal nociception in normal rats via P2Y2, P2Y4, and P2Y6 receptors. The mechanism underlying these effects was not investigated.
In addition to the pyrimidine nucleotides acting on P2Y2, P2Y4, and P2Y6 receptors, the ADP analog ADP-β-S has also been found to cause analgesia [27]. ADP-β-S, applied intrathecally to rats, prolonged the nociceptive threshold in the tail-flick test. Furthermore, in the same study, the possible mechanism of action was also investigated and ATP was found to inhibit the N-type VACCs and the subsequent increase in [Ca2+]i in rat DRG neurons (Figure 1). No other Ca2+ channel types were altered. This effect of ATP was antagonized by the P2Y1 selective antagonists MRS 2179 and PPADS and was abolished by intracellular application of GDP-β-S, a competitive G protein inhibitor, suggesting the participation of P2Y1 receptors. Investigation of the secondary messenger pathway revealed that the inhibition was mediated through the βγ subunit of the G protein, since a strongly depolarizing pre-pulse applied shortly prior to the test pulse and known to dissociate Gβγ from the VACC prevented the inhibitory effect of ATP. Furthermore, ADP-β-S inhibited the dorsal root-evoked polysynaptic population EPSPs in the hemisected spinal cord from rats, indicating that the site of action of the P2Y1 receptors is the central terminal of the DRG cells. It is known that N-type calcium channels play a major role in the release of neurotransmitters and ADP, which is generated by the enzymatic degradation of ATP, may inhibit these channels through P2Y1 receptors resulting in decreased sensory transmitter (glutamate) release at the central terminals and as a consequence in reduced spinal pain transmission.
In the same study, UTP was also tested and was found to inhibit the VACCs [27], suggesting that the analgesic effect of UTP [77] may be mediated by the same mechanism as that of ADP-β-S. It is interesting to note that in vitro UTP has also been found to excite DRG neurons (see above). Hence, these opposing effects of UTP may finally lead to an inhibition of pain transmission.
Conclusion
The demonstration of P2Y receptor gene expression in sensory cells and the presence of functional receptors on the cell surface suggests that these receptors can play a role in the nociceptive transmission. Co-localization of the different P2Y receptor-subtypes with markers for nociceptive neurons showed that the P2Y1 and P2Y2 receptors are typically expressed in small, nociceptive cells, whereas the P2Y4 subtype seems to be present in large neurons involved in the transmission of tactile allodynia. Spinal cord astrocytes known to play a significant role in the modulation of pain transmission also express functional P2Y1 and P2Y2 receptors. The investigation of different P2Y receptor agonists acting selectively on the P2Y1 or P2Y2/4 subtypes showed that these receptors can both stimulate and inhibit the excitability of sensory neurons without significant subtype specificity. On the other hand, in vivo studies revealed that i.t. administration of the P2Y2/4 agonist UTP and of the P2Y1 agonist ADP-β-S inhibits pain transmission. It was suggested that P2Y receptors inhibit the release of glutamate from the central terminal of the DRG neurons via inhibition of the VACCs.
Acknowledgement
Financial support by the Deutsche Forschungsgemeinschaft, Bonn (DFG; IL 20/11-1) is gratefully acknowledged.
Abbreviations
- 2-MeSADP
2-methylthio ADP
- 2-MeSAMP
2-methylthio AMP
- 2-MeSATP
2-methylthio ATP
- α,β-meATP
α,β-methylene ATP
- A3P5P
adenosine-3′-phosphate-5′-phosphate
- ADP
adenosine 5′-diphosphate
- ADP-β-S
adenosine 5′-O-(2-thiodiphosphate)
- AMP
adenosine 5′-monophosphate
- AR-C69931MX
N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene ATP
- ATP
adenosine 5′-triphosphate
- BDNF
brain-derived neurotropic factor
- [Ca2+]i
intracellular Ca2+ concentration
- CGRP
calcitonin gene-related peptide
- CREB
cAMP-responsive element binding protein
- DAMGO
D-Ala2,N-Me-Phe4,Gly-ol5-enkephalin
- DPDPE
D-penicillamine(2,5)-enkephalin
- DRG
dorsal root ganglion
- EPSP
excitatory postsynaptic potential
- GDP-β-S
guanosine 5′-O-(2-thiobisphosphate)
- GF109203x
PKC inhibitor
- HEK
human embryonic kidney-derived
- IB4
isolectin B4
- IP3
inositol 1,4,5-triphosphate
- IR
immunoreactivity, immunoreactive
- KCNQ2
potassium voltage-gated channel, KQT-like subfamily, member 2
- mRNA
messenger ribonucleic acid
- MRS-2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS-2279
(1R,2S,4S,5S)-1-[(phosphato)methyl]-4-(2- chloro-6-aminopurin-9-yl) bicyclo [3.1.0]-hexane-2- phosphate
- NG
nodose ganglion
- P2X
ionotropic ATP receptor
- P2Y
metabotropic ATP receptor
- pCREB
phosphorylated CREB
- PKC
protein kinase C
- PLC
phospholipase C
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid
- RB2
reactive blue 2
- RT-PCR
reverse transcription polymerase chain reaction
- SGC
satellite glial cells
- SP
substance P
- TG
trigeminal ganglion
- TRPV1
vanilloid receptor 1
- U-50488H
trans-(±-3,4-dichloro-N-methyl-N-(2-[1-pyrrolidinyl]-cyclohexyl)- benzeneacetamide
- U73122
PLC inhibitor
- UDP
uridine 5′-diphosphate
- UTP
uridine 5′-triphosphate
- VACC
voltage-activated calcium channel
References
- 1.Collier HO, James GW, Schneider C. Antagonism by aspirin and fenamates of bronchoconstriction and nociception induced by adenosine-5′-triphosphate. Nature. 1966;212:411–412. doi: 10.1038/212411a0. [DOI] [PubMed] [Google Scholar]
- 2.Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;4:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen CC, Akopian AN, Sivilotti L, et al. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 4.Lewis C, Neidhart S, Holy C, North RA, et al. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 5.Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- 6.Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/S0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 8.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy C, Assis TS, Currie AJ, Rowan EG. Crossing the pain barrier: P2 receptors as targets for novel analgesics. J Physiol. 2003;553:683–694. doi: 10.1113/jphysiol.2003.049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 12.Barnard EA, Simon J. An elusive receptor is finally caught: P2Y12, an important drug target in platelets. Trends Pharmacol Sci. 2001;22:388–391. doi: 10.1016/S0165-6147(00)01759-4. [DOI] [PubMed] [Google Scholar]
- 13.Communi D, Gonzalez NS, Detheux M, et al. Identification of a novel human ADP receptor coupled to Gi. J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 14.Chambers JK, Macdonald LE, Sarau HM, et al. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- 15.Abbracchio MP, Boeynaems JM, Barnard EA, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang FL, Luo L, Gustafson E, et al. P2Y13: Identification and characterization of a novel Gαi-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther. 2002;301:705–713. doi: 10.1124/jpet.301.2.705. [DOI] [PubMed] [Google Scholar]
- 17.Kügelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson KA, Costanzi S, Ohno M, et al. Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr Top Med Chem. 2004;4:805–819. doi: 10.2174/1568026043450961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- 20.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanada M, Yasuda H, Omatsu-Kanbe M, et al. Increase in intracellular Ca2+ and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111:413–422. doi: 10.1016/S0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 22.Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- 23.Xiao HS, Huang QH, Zhang FX, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriyama T, Iida T, Kobayashi K, et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: Contribution to touch-induced impulse generation. Proc Natl Acad Sci USA. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong AY, Krstew EV, Barden J, Lawrence AJ. Immunoreactive localisation of P2Y1 receptors within the rat and human nodose ganglia and rat brainstem: Comparison with [α33P]deoxyadenosine 5′-triphosphate autoradiography. Neuroscience. 2002;113:809–823. doi: 10.1016/S0306-4522(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 27.Gerevich Z, Borvendeg SJ, Schröder W, et al. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molliver DC, Cook SP, Carlsten JA, et al. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16:1850–1860. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- 29.Shir Y, Seltzer Z. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neurosci Lett. 1990;115:62–67. doi: 10.1016/0304-3940(90)90518-E. [DOI] [PubMed] [Google Scholar]
- 30.Svichar N, Shmigol A, Verkhratsky A, Kostyuk P. ATP induces Ca2+ release from IP3-sensitive Ca2+ stores exclusively in large DRG neurones. NeuroReport. 1997;8:1555–1559. doi: 10.1097/00001756-199705060-00002. [DOI] [PubMed] [Google Scholar]
- 31.Borvendeg SJ, Gerevich Z, Gillen C, Illes P. P2Y receptor-mediated inhibition of voltage-dependent Ca2+ channels in rat dorsal root ganglion neurons. Synapse. 2003;47:159–161. doi: 10.1002/syn.10156. [DOI] [PubMed] [Google Scholar]
- 32.Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J Neurosci. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruglikov I, Shutov L, Potapenko E, et al. Metabotropic purinoreceptors in rat dorsal horn neurones: Predominant dendritic location. NeuroReport. 2001;12:3503–3507. doi: 10.1097/00001756-200111160-00026. [DOI] [PubMed] [Google Scholar]
- 35.Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- 36.Woodham P, Anderson PN, Nadim W, Turmaine M. Satellite cells surrounding axotomised rat dorsal root ganglion cells increase expression of a GFAP-like protein. Neurosci Lett. 1989;98:8–12. doi: 10.1016/0304-3940(89)90364-9. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson JL, Byers MR. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp Neurol. 1995;131:11–22. doi: 10.1016/0014-4886(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Zhou XF. Pericellular Griffonia simplicifolia I isolectin B4-binding ring structures in the dorsal root ganglia following peripheral nerve injury in rats. J Comp Neurol. 2001;439:259–274. doi: 10.1002/cne.1349. [DOI] [PubMed] [Google Scholar]
- 39.Hanani M, Huang TY, Cherkas PS, et al. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–283. doi: 10.1016/S0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 40.Weick M, Cherkas PS, Hartig W, et al. P2 receptors in satellite glial cells in trigeminal ganglia of mice. Neuroscience. 2003;120:969–977. doi: 10.1016/S0306-4522(03)00388-9. [DOI] [PubMed] [Google Scholar]
- 41.Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Idestrup CP, Salter MW. P2Y and P2U receptors differentially release intracellular Ca2+ via the phospholipase c/inositol 1,4,5-triphosphate pathway in astrocytes from the dorsal spinal cord. Neuroscience. 1998;86:913–923. doi: 10.1016/S0306-4522(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 43.Ogata T, Chuai M, Morino T, et al. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2Y receptors. Brain Res. 2003;981:174–183. doi: 10.1016/S0006-8993(03)03028-2. [DOI] [PubMed] [Google Scholar]
- 44.Tokimasa T, Akasu T. ATP regulates muscarine-sensitive potassium current in dissociated bull-frog primary afferent neurones. J Physiol. 1990;426:241–264. doi: 10.1113/jphysiol.1990.sp018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 46.Selyanko AA, Delmas P, Hadley JK, et al. Dominant-negative subunits reveal potassium channel families that contribute to M-like potassium currents. J Neurosci. 2002;22:RC212. doi: 10.1523/JNEUROSCI.22-05-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jentsch TJ. Neuronal KCNQ potassium channels: Physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 48.Rivera-Arconada I, Martinez-Gomez J, Lopez-Garcia JA. M-current modulators alter rat spinal nociceptive transmission: An electrophysiological study in vitro. Neuropharmacology. 2004;46:598–606. doi: 10.1016/j.neuropharm.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Usachev YM, DeMarco SJ, Campbell C, et al. Bradykinin and ATP accelerate Ca2+ efflux from rat sensory neurons via protein kinase C and the plasma membrane Ca2+ pump isoform 4. Neuron. 2002;33:113–122. doi: 10.1016/S0896-6273(01)00557-8. [DOI] [PubMed] [Google Scholar]
- 50.Miller RJ. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37:255–285. doi: 10.1016/0301-0082(91)90028-Y. [DOI] [PubMed] [Google Scholar]
- 51.Stucky CL, Medler KA, Molliver DC. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44. doi: 10.1016/j.pain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Tao X, Finkbeiner S, et al. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/S0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 53.Freeland K, Liu YZ, Latchman DS. Distinct signalling pathways mediate the cAMP response element (CRE)-dependent activation of the calcitonin gene-related peptide gene promoter by cAMP and nerve growth factor. Biochem J. 2000;345:233–238. doi: 10.1042/0264-6021:3450233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian J, Yehia G, Molina C, et al. Cloning of human preprotachykinin-I promoter and the role of cyclic adenosine 5′-monophosphate response elements in its expression by IL-1 and stem cell factor. J Immunol. 2001;166:2553–2561. doi: 10.4049/jimmunol.166.4.2553. [DOI] [PubMed] [Google Scholar]
- 55.Huang H, Wu X, Nicol GD, et al. ATP augments peptide release from rat sensory neurons in culture through activation of P2Y receptors. J Pharmacol Exp Ther. 2003;306:1137–1144. doi: 10.1124/jpet.103.052951. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann K, Reeh PW, Averbeck B. ATP can enhance the proton-induced CGRP release through P2Y receptors and secondary PGE2 release in isolated rat dura mater. Pain. 2002;97:259–265. doi: 10.1016/S0304-3959(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 57.Sakama R, Hiruma H, Kawakami T. Effects of extracellular ATP on axonal transport in cultured mouse dorsal root ganglion neurons. Neuroscience. 2003;121:531–535. doi: 10.1016/S0306-4522(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 58.Grafstein B, Forman DS. Intracellular transport in neurons. Physiol Rev. 1980;60:1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- 59.Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424:577–587. doi: 10.1002/1096-9861(20000904)424:4<577::AID-CNE2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 60.Sotgiu ML, Biella G, Firmi L, Pasqualucci V. Topical axonal transport blocker vincristine prevents nerve injury-induced spinal neuron sensitization in rats. J Neurotrauma. 1998;15:1077–1082. doi: 10.1089/neu.1998.15.1077. [DOI] [PubMed] [Google Scholar]
- 61.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/S0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 62.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Watkins LR, Maier SF. Glia: A novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 64.Illes P, Nörenberg W, Gebicke-Haerter PJ. Molecular mechanisms of microglial activation. B. Voltage- and purinoceptor-operated channels in microglia. Neurochem Int. 1996;29:13–24. doi: 10.1016/0197-0186(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 65.Hide I, Tanaka M, Inoue A, et al. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 66.Inoue A, Ikoma K, Morioka N, et al. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–2213. [PubMed] [Google Scholar]
- 67.Malcangio M, Bowery NG, Flower RJ, Perretti M. Effect of interleukin-1 beta on the release of substance P from rat isolated spinal cord. Eur J Pharmacol. 1996;299:113–118. doi: 10.1016/0014-2999(95)00845-4. [DOI] [PubMed] [Google Scholar]
- 68.Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. doi: 10.1002/(SICI)1098-1136(199809)24:1<50::AID-GLIA6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 69.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: Long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 70.Haydon PG. GLIA: Listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 71.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 72.Guthrie PB, Knappenberger J, Segal M, et al. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gallagher CJ, Salter MW. Differential properties of astrocyte calcium waves mediated by P2Y1 and P2Y2 receptors. J Neurosci. 2003;23:6728–6739. doi: 10.1523/JNEUROSCI.23-17-06728.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 75.Mizukami K, Kounomi S, Kaneko S, Satoh M. Some physiological and pharmacological properties of slow depolarization of substantia gelatinosa neurons by repetitive stimulation of C-fibers of dorsal root in adult rat spinal cord slices with dorsal root attached. Neurosci Lett. 1999;274:49–52. doi: 10.1016/S0304-3940(99)00677-1. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida K, Nakagawa T, Kaneko S, et al. Adenosine 5′-triphosphate inhibits slow depolarization induced by repetitive dorsal root stimulation via P2Y purinoceptors in substantia gelatinosa neurons of the adult rat spinal cord slices with the dorsal root attached. Neurosci Lett. 2002;320:121–124. doi: 10.1016/S0304-3940(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 77.Okada M, Nakagawa T, Minami M, Satoh M. Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. J Pharmacol Exp Ther. 2002;303:66–73. doi: 10.1124/jpet.102.036079. [DOI] [PubMed] [Google Scholar]